Abstract
To stop the antimicrobial resistance crisis, there is an urgent need for increased investment in antimicrobial research and development. Currently, many researchers are focussing on insects and their microbiota in the search for new antimicrobials. This review summarizes recent literature dedicated to the antimicrobial screening of insect symbionts and/or their metabolites to uncover their value in early drug discovery. We summarize the main steps in the methodology used to isolate and identify active insect symbionts and have noted substantial variation among these studies. There is a clear trend in isolating insect Streptomyces bacteria, but a broad range of other symbionts has been found to be active as well. The microbiota of many insect genera and orders remains untargeted so far, which leaves much room for future research. The antimicrobial screening of insect symbionts has led to the discovery of a diverse array of new active biomolecules, mainly peptides, and polyketides. Here, we discuss 15 of these symbiont-produced compounds and their antimicrobial profile. Cyphomycin, isolated from a Streptomyces symbiont of a Cyphomyrmex fungus-growing ant, seems to be the most promising insect symbiont-derived antimicrobial so far. Overall, insect microbiota appears to be a promising search area to discover new antimicrobial drug candidates.
Introduction
Currently, antimicrobial resistance has grown to be one of the biggest challenges to the global health system, causing approximately 700.000 deaths each year. If no urgent action is taken, antimicrobial resistance could lead to a yearly death toll of 10 million people by 2050, nullifying decades of medical progress (World Health Organization Citation2019a, Citation2019b). Only a few new antibiotics have been developed the past 5 years, and most of them belong to structural classes with already established drug resistance (World Health Organization Citation2019a, Citation2019b). New, innovative research approaches are required to halt the emergence of a post-antibiotic era. Despite the increasing popularity of synthetic antimicrobial design, nature remains a valuable source for the discovery of new active compounds. Of all antimicrobials currently on the market, 69% is derived from a natural compound, with 97% of these from microbial origin (Patridge et al. Citation2016). Natural products seem to withstand the test of time when it comes to the development of new antimicrobial agents (Antoraz et al. Citation2015; Herrmann et al. Citation2016; Moloney Citation2016). To increase the chances of finding new active molecules with novel modes of action, focus will need to shift from frequently mined natural sources such as soil bacteria to relatively underexplored natural sources (Donadio et al. Citation2010; Challinor and Bode Citation2015). One such potential source of novel antimicrobials is insects. Although insects have been used in medicine for centuries, for instance, the use of maggots of Lucilia sericata for wound healing, no insect-derived drugs have reached the market so far (Sherman Citation2009). The amount of published papers covering insect-derived antimicrobial compounds, however, has increased steadily over the past decade (Ratcliffe et al. Citation2011; Berasategui et al. Citation2016; Seabrooks and Hu Citation2017; Xie et al. Citation2019). Both compounds produced by the insects themselves, such as insect antimicrobial peptides, as those produced by their symbiotic microorganisms, are a popular topic in drug discovery research (Berasategui et al. Citation2016; Wu et al. Citation2018). The focus of this review is on the symbiotic microorganisms. Insect symbionts are microbes that live in a close, long-term relationship with their insect host (Hoang et al. Citation2019). Symbionts can be found in the insect itself, for example in the gut or in specialized cells called bacteriocytes (Paniagua Voirol et al. Citation2018). Some symbionts proliferate on the insect’s exterior surface or in their habitat, such as their nest or food provisions (Okabe Citation2013; Kaltenpoth and Engl Citation2014; Hoang et al. Citation2019). Not all microbes associated with insects or their environment, however, are involved in a symbiotic relationship. The nature of the symbiosis can be diverse, ranging from a mutual profitable relationship to parasitism (Su et al. Citation2013).
Two arguments support the attention on these symbiotic microorganisms to discover novel drug candidates: first of all, although insects have a well-developed defence system of their own, such as an exoskeleton barrier, humoral, and cellular immune responses including melanization, phagocytosis, and antimicrobial peptide production (Andersen Citation2009; Kaltenpoth and Engl Citation2014; Sheehan et al. Citation2018), many life stages of insects such as the egg and larval stages, are still very vulnerable to environmental threats (Van Arnam et al. Citation2018). For various insects, it has been shown that defensive symbionts play a crucial role during these developmental stages. Solitary wasps, for example, use Streptomyces bacteria to protect their pupae in brood chambers (Van Arnam et al. Citation2018). Apart from brood protection, these microbes can also help to protect the insect’s nutritional resources (Kaltenpoth and Engl Citation2014; Beemelmanns et al. Citation2016). Second, many defensive symbionts have been characterized for adult insects as well (Kaltenpoth Citation2009; Kaltenpoth and Engl Citation2014; Flórez et al. Citation2015; Matarrita-Carranza et al. Citation2017). Insects are remarkably successful in colonizing a variety of ecological niches and are exposed to many different threats during their lifetime, including predatory insects, parasites, and entomopathogens (Van Arnam et al. Citation2018). Symbiotic microorganisms play an important role in the insects’ defence system against these foreign invaders, and help them thrive in challenging environments (Rajagopal Citation2009; Engel and Moran Citation2013; Van Arnam et al. Citation2018).
Various mechanisms that confer this protection against entomopathogens have been identified. The native microbial community can trigger and consecutively increase the insect’s own immune response, but they can also reduce the colonization of the insect by entomopathogens, a mechanism called colonization resistance (Rajagopal Citation2009). In this case, the insect microbiota competes with the microbial invaders for nutrients and space, or they can directly take part in the killing of these entomopathogens (Rajagopal Citation2009; Pickard et al. Citation2017). The latter phenomenon is especially interesting in the light of the current drug-resistance crisis and could be exploited in the search for new antimicrobials. Indeed, more and more insect symbionts are surfacing in drug research articles and produce metabolites that are being described as “potential antimicrobials.” Recent research by Chevrette et al. (Citation2019), for example, highlights the antimicrobial capacity of insect-related Streptomyces bacteria, whereas Van Arnam et al. (Citation2018) list molecules that are important in defensive insect-microbe symbiosis (Van Arnam et al. Citation2018; Chevrette et al. Citation2019). It is clear that there is increasing interest in the diverse world of insect symbionts to find new, active compounds of interest.
Objective and methods
With more and more research focussing on the antimicrobial activity of insect symbionts and their metabolites, there is a need for an in-depth overview of the latest developments and discoveries in this field. Ultimately, the goal of this review is to summarize recent literature that discusses the antimicrobial screening of insect symbionts and/or their metabolites to uncover the value and potential these insect symbionts have to offer in early drug discovery. To this end, we have conducted a PubMed/MEDLINE search to gather relevant articles, dating from January 2000 onwards. Research papers in which the insect symbionts were purposefully isolated as a starting point and screened for antimicrobial activity, were of particular interest in our review. First, this review will take a closer look at commonly used screening methodologies. Then, it will make an overview of the insects that are being researched and their symbionts that have antimicrobial activity, to identify possible research trends and gaps. Finally, special attention is paid to the isolated, active metabolites and their potential as new antimicrobial agents.
Screening methodology for antimicrobial producing microorganisms in insects
A popular method of identifying active insect symbionts relies on microbial isolation and culturing, before performing an antimicrobial screening. This more traditional approach to drug discovery from insect symbionts is still widely used and has led to the discovery of several new molecules of interest. Other techniques that can be complementary to this approach but fall out of the scope of this review, include (i) identification of metabolites from whole insect extracts and linking these compounds to a symbiont later on, or (ii) metagenomic analysis to identify microbial genes or gene clusters responsible for the production of active products (Moya et al. Citation2008; Gomes et al. Citation2013; Masson and Lemaitre Citation2020). Metagenomics can be especially valuable to identify active symbionts that live in an obligate association with the host insect, which makes them difficult to cultivate under standard laboratory conditions (Kikuchi Citation2009). Moreover, the identification of dormant biosynthetic gene clusters can help to find the right conditions to activate their expression or to create heterologous expression systems to study the products (Piel Citation2011).
Significant variation can be observed in how studies approach the identification of antimicrobially active symbionts or metabolites, starting from an isolated microbe (). A number of studies carry out a broad screening of a wide range of isolated microbes, whereas the vast majority of studies selectively focus on a subset of symbionts. The former approach is an extensive and time-consuming approach, but offers a better understanding of the complete antimicrobial potential of an insect’s microbiota. The latter strategy possibly misses microorganisms with antimicrobial secondary metabolites. Those studies usually confine their research to the isolation and screening of antimicrobial-producing Actinobacteria or certain symbionts that have been previously reported for their defensive role in the insect species. Regardless of whether the complete microbiota or a subgroup is studied, five main steps can always be distinguished in the process: isolation of insect symbionts, identification of isolates, fermentation and extraction, antimicrobial screening, and finally, structure elucidation of the active metabolites. As shown in , there is no fixed order when performing these steps. After isolation of the symbionts of interest, studies can immediately carry out an antimicrobial screening, or alternatively, they can perform an extraction of the symbiont culture first. Usually, however, symbionts are identified before further testing. Although the screening step typically occurs before structure elucidation, sometimes only screening of a single purified, identified compound is performed. Below, all steps except for structural identification, are described in more detail.
Figure 1. Graphical overview of the five main steps in the screening methodology of antimicrobial- producing insect symbionts. A popular method of identifying active insect symbionts and metabolites relies on microbial isolation and culturing, before performing an antimicrobial screening. In this traditional screening methodology, various key steps can be identified, indicated by the fields in various colours: isolation of insect symbionts, identification of isolates, fermentation and extraction, antimicrobial screening, and finally structure elucidation of active compounds. There is no fixed order when performing these steps, as indicated by the arrows.
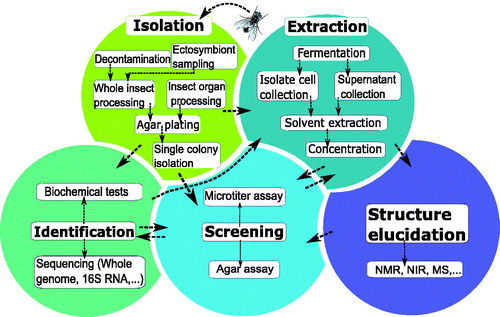
Isolation and identification of microorganisms originating from insects
The starting point of the traditional screening methodology is the isolation of the insect symbionts. Microbial symbionts can be found on the insect cuticle, within their digestive tract and in their cells and tissues (Boucias et al. Citation2018). Apart from the insect itself, defensive symbionts can also be isolated from insect byproducts such as honey or nests (Lee et al. Citation2008a, Citation2008b; Madden et al. Citation2013; Nirma et al. Citation2015). Usually, insects are processed as a whole, but in some cases, insects are dissected to obtain targeted organs such as the gut or salivary glands. To obtain a broader view on the antimicrobial potential of the insect microbiome, ectosymbiont sampling can be performed by rinsing the insect’s cuticle, which has, for example, been performed in studies by Poulsen et al. (Citation2011) and Chevrette et al. (Citation2019), both focussing on Streptomyces bacteria (Poulsen et al. Citation2011; Chevrette et al. Citation2019). The former authors report a higher number of Streptomyces morphotypes found on the cuticle of the wasp Sceliphron caementarium than in the sample of the whole insect body (Poulsen et al. Citation2011).
Finally, serial dilutions are plated on agar, being either a general or a specific medium depending on the goal of the study. Today, 16S rRNA sequence analysis is by far the most applied technique for bacterial identification. When identifying and sequencing isolates, a phylogenetic analysis can be a helpful addition to avoid compound rediscovery from already studied microbial isolates, by distinguishing true insect-associated microbes from those that stem from the insect’s diet or environment. Chevrette et al. (Citation2019), for example, performed an extensive phylogenetic analysis to distinguish insect-associated Streptomyces bacteria from environmental Streptomyces strains (Chevrette et al. Citation2019). Symbionts that have a long history of coevolution with the insect host often show specific insect-related genetic lineages (Ferrari and Vavre Citation2011). Genomic differentiation is especially noticeable for obligate, endocellular symbionts, that have undergone a large genomic size reduction (Kikuchi et al. Citation2009; Lo et al. Citation2016). Some facultative symbionts, however, also display very dynamic genomes, distinctively different from their free-living ancestors (Burke et al. Citation2009; Lo et al. Citation2016).
Antimicrobial screening of microorganisms isolated from insects
A crucial step in the early drug discovery phase is the antimicrobial screening, which ultimately leads to the identification of interesting symbionts and possibly active compounds later on in the study. The screening can be performed on (1) whole cells, (2) crude extracts of a symbiont culture, or (3) purified metabolites. For in vitro antimicrobial screening, two main techniques appear in the selected studies: diffusion and dilution methods. Diffusion screening techniques are agar-based assays and include the cross-streak method, the agar plug diffusion method, and the agar overlay assay. The advantage of such screenings is that they require little equipment and can be used to test a whole isolate without prior extraction (Balouiri et al. Citation2016). Therefore, these techniques are often used in a preliminary screening to narrow down the number of isolates of interest (Wang et al. Citation1999; Lee et al. Citation2008a,Citation2008b; Chouvenc et al. Citation2013; Madden et al. Citation2013). The largest disadvantage of such agar assays is the lack of precise quantification of the antimicrobial effect. Moreover, the diffusion of active compounds throughout the agar is unpredictable and likely influences the end result considerably (Cos et al. Citation2006). Agar-diffusion methods are also difficult to implement in a high-throughput screening set-up (Cos et al. Citation2006). Disc diffusion and well diffusion assays are occasionally carried out as well, but require a fermentation or extraction step beforehand, as the discs or wells cannot be loaded with microbial cells (Promnuan et al. Citation2009; Kroiss et al. Citation2010; Um et al. Citation2013; Moraes et al. Citation2014; Wang et al. Citation2015; Shao et al. Citation2017; Flórez et al. Citation2018). Extracting all collected isolates prior to antimicrobial screening can be very labour-intensive. Apart from agar-diffusion methods, well plate dilution bioassays appear several times as the technique of choice (Oh et al. Citation2009; Barke et al. Citation2010; Carr et al. Citation2012; Wu et al. Citation2014; Arango et al. Citation2016; Van Arnam et al. Citation2016; Akbar et al. Citation2018; Chevrette et al. Citation2019; Heise et al. Citation2019). These tests allow for a more accurate quantification of the antimicrobial effect and can be integrated in a high-throughput screening platform. In the selected studies, read-out of the antimicrobial activity is usually done through optical density measurements. Redox indicators such as resazurin, usually a method of choice in antibacterial screening, were not encountered in the symbiont screening experiments (Cos et al. Citation2006). Well-plate bioassays are typically performed on either a crude extract or a purified antimicrobial agent.
Fermentation and extraction of a symbiont culture
Fermentation and extraction of a symbiont culture can be done before or after the antimicrobial screening. Many parameters in the fermentation process, including fermentation temperature, time of harvesting, and selection of culture medium, determine the metabolic pathways followed by the cultivated strain and are hence decisive for the outcome. For example, the optimal culture medium for fermentation is very strain-dependent and is critical in triggering the microorganism to express its secondary metabolites (Singh et al. Citation2016). To maximize the success ratio, the fermentation culture can be harvested at different time-points in the total fermentation time, as it is difficult to predict at what point the expression of secondary metabolites starts, and along with that, whether metabolite production is growth-associated or not (Heise et al. Citation2019). Prior optimization of these parameters for all symbionts can be laborious, but it leads to a higher yield of secondary metabolites. Next, optimization of the extraction poses a challenge as well, as the compounds of interest are yet unknown, and consequently the extraction parameters as well. Ideally, the extraction procedure is reproducible, able to extract compounds of various structural classes and at the same time preventing degradation of the compounds of interest (Pinu et al. Citation2017). Different extraction solvents can be used to cover a wide range of polarities and to maximize the chance of successfully extracting active metabolites. Methanol and ethyl acetate are most often used and they seem to give satisfying results. Only rarely, when the extraction of a liquid culture fails to deliver results, an agar extraction is carried out, as mentioned by Kim et al. (Citation2014) and Blodgett et al. (Citation2010) (Blodgett et al. Citation2010; Kim et al. Citation2014). In this case, active compounds are extracted from a solid agar base culture instead of a liquid broth culture. After purification of the sample, active compounds can be identified using various molecular identification techniques, such as nuclear magnetic resonance (NMR), near infra-red spectroscopy (NIR), or mass spectrometry (MS).
Insects and symbionts of interest in current research
Insects studied so far
When considering the insects that have been the subject of investigation, it becomes clear that the symbiont antimicrobial activity of many insect genera and orders remains uncharted (). In the 45 studies that we gathered, a total of seven insect orders and 41 genera have been investigated. The Hymenoptera make up the bulk of insects of which the microbiota is examined for antimicrobial activity. Within this order, which encompasses bees, wasps and ants, the fungus-growing ants seem to receive a remarkable amount of attention. To date, 11 genera have been explored. The ant tribe Attini engages in a unique symbiosis with a fungal symbiont, which the ants cultivate for food and in turn protect against invaders (Mehdiabadi et al. Citation2012). Various bacterial symbionts are involved in this symbiosis by producing antimicrobials that kill fungal parasites (Currie et al. Citation1999; Oh et al. Citation2009; Cafaro et al. Citation2011). Bees, who have some of the most limited bacterial communities described in insects, are regularly the topic of interest as well (Paniagua Voirol et al. Citation2018). The fact that honey has some antimicrobial effects can have fuelled the research on their microbiota (Lee et al. Citation2008a, Citation2008b; Johnston et al. Citation2018). Other investigated genera within the Hymenoptera are paper wasps (Polistinae), the beewolf (Philanthus), and the mud dauber wasps of the Sceliphron and Chalybion genera (Kroiss et al. Citation2010; Poulsen et al. Citation2011).
Figure 2. Orders and genera of insects of which microbiota are investigated for antimicrobial activity. The inner-circle represents the orders of the insects, the outer circle represents the insects’ genera.
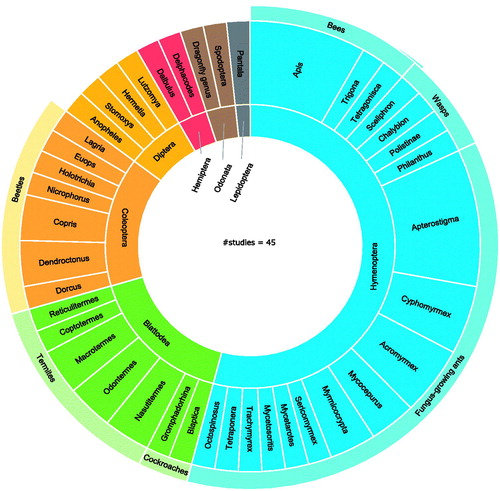
Considering the relatively low species diversity in the Blattodea order (7314 as estimated by Stork Citation2018), compared to that of the Hymenoptera (116,861) and the Coleoptera (386,500), their high ranking seems surprising (Stork Citation2018). The Blattodea order contains cockroaches and termites (Wang et al. Citation2017). The interest in the cockroach microbiota (Akbar et al. Citation2018) is due to their role in the ecosystem as decomposers and recyclers of plant and animal waste (Wang et al. Citation2017; Akbar et al. Citation2018). The symbionts of two types of cockroaches (Gromphadorhina sp. and Blaptica sp.) have been examined so far (Akbar et al. Citation2018). The most intensively explored insect in this order, however, is the termite, with five different genera being considered. Termites have the most extensive bacterial gut community of all insects, and this can explain why their microbiota has undergone frequent antimicrobial screening (Engel and Moran Citation2013). In parallel to the fungus-growing ants, symbionts of the fungus-growing termite Macrotermes natalensis have appeared twice during our investigation (Um et al. Citation2013; Kim et al. Citation2014). Coleoptera, the order of the beetles, ranks third. Interestingly, the fungus-growing beetle Dendroctonus frontalis has appeared in two studies so far (Oh et al. Citation2009; Blodgett et al. Citation2010). Considering the high diversity of the order (386,500 species as estimated by Stork Citation2018) there is room for more symbiont antimicrobial screening within this order (Stork Citation2018).
Strikingly, many of the aforementioned insects, such as cockroaches, termites, bees, wasps, and ants, take part in social behaviour like coprophagy or oral trophallaxis. These practices stimulate the transmission of microorganisms between individual insects, leading to a unique community of host-dependent symbionts (Engel and Moran Citation2013). This too could be a reason for the interest in the antimicrobial potential of their microbiota. The antimicrobial potential of symbionts of other insect orders, such as the Lepidoptera (157,338 species), is considerably less explored and leaves opportunities for future research. A large-scale study published in Nature Communications (Chevrette et al. Citation2019) elucidated the antimicrobial potential of Streptomyces bacteria of 13 different insect orders and included the smaller orders Orthoptera, Mantodea, Neuroptera, Trichoptera, and Thysanura in addition to those already mentioned (Chevrette et al. Citation2019). As details about the insect genera were not mentioned, these orders are not included in . Some insect orders with fewer species, such as Strepsiptera (609 species) or Mecoptera (757), have not appeared in any research of this kind so far.
Antimicrobially active symbiont strains studied so far
The orders and genera of insect microorganisms for which antimicrobial activity is reported can be presented in a similar fashion (). A symbiont strain is included in the graph when either the whole cell, a crude extract or an isolated secondary metabolite has shown activity against either insect or human pathogens. As stated earlier, the summarized studies apply two distinctively different approaches. Those that report on a broad screening of insect symbionts () represent only 20% of the total studies. One of these studies could not be included in the graph, as the identity of the active symbiont strains was not analysed (Lee et al. Citation2008a, Citation2008b). Three other studies only mentioned the most promising active symbiont without reporting all symbiont activity (Lee et al. Citation2008a, Citation2008a; Nirma et al. Citation2015; Correa et al. Citation2019), whereas all the others gave a clear overview of all measured antimicrobial activities. The remaining 80% of studies focussed on either one symbiont or a subset of symbionts, such as Actinobacteria or lactic acid bacteria, for the antimicrobial screening step ().
Figure 3. A graphical overview of the orders and genera of the insect symbionts with reported antimicrobial activity. Graph a shows the symbionts that have been found active through broad screening studies. Graph b shows active insect microbes that have been selected as symbiont of interest prior to antimicrobial screening. Graphs present microbes on genus level, and multiple species within the same genus were counted as one.
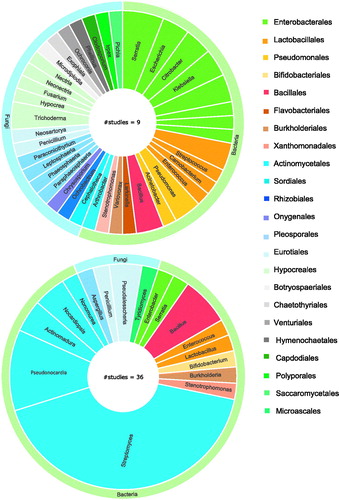
The Actinomycetales order, which encompasses the known antimicrobial-producing genus Streptomyces, has received substantial attention and therefore represents the order with the most known antimicrobially active symbionts. This is not a surprise, as the antimicrobial-producing capacity of Streptomyces bacteria has been studied for a long time, with the first Streptomyces-derived antibiotic, streptomycin, being discovered in 1943. Over two-thirds of all natural antimicrobials that are used clinically are of Streptomyces origin (Engel and Moran Citation2013). It is estimated that only 3% of its antimicrobially active metabolites have been discovered so far, leaving a vast majority still to be discovered (Watve et al. Citation2001). Interestingly, insect-derived Streptomyces strains are stated to have an overall higher antimicrobial activity than the Streptomyces strains found on plant material or soil (Chevrette et al. Citation2019). These findings explain the deep interest in screening with emphasis on Streptomyces strains. Other Actinobacteria symbionts with reported activity are Pseudonocardia sp., Actinomadura sp., Nonomurea sp., Nocardiopsis sp., and Arthrobacter sp. Actinobacteria are only rarely identified in broad screening studies, as their isolation requires specific growth conditions, including a specific nutrient medium, such as a starch-casein agar supplemented with nystatin and nalidixic acid and an incubation period that is longer than traditional periods in microbiology (4–8 weeks at 30 °C) (Promnuan et al. Citation2009).
Another order that is frequently identified is that of the Enterobacterales. Active strains are mostly reported in the broad screening studies, which is to be expected as many insect gut symbionts belong to the Enterobacterales (Yun et al. Citation2014). Within this order, the Serratia genus is well-known for its production of secondary metabolites with a broad antimicrobial spectrum, such as the serrawettins produced by some strains of S. marcescens (Mai Citation2018). An active Serratia symbiont has been identified in four different insect species (Miyashita et al. Citation2015; Akbar et al. 2018; Ganley et al. Citation2018; Heise et al. Citation2019). Escherichia, Citrobacter, and Enterobacter are other genera within this order that are known to produce active lipopeptides, and they all have been identified as an active symbiont twice (Mandal et al. Citation2013). Enterobacter, for example, has been reported as an active symbiont in the sand fly Lutzomya evansi and stag beetles of the Dorcus genus (Miyashita et al. Citation2015; Vivero et al. Citation2019).
Other active symbionts are found in the orders of the Bacillales, Lactobacillales, and Pseudomonadales. Many bacteria that belong to these orders are known for their production of active compounds and their metabolic capacity is of increasing interest in antimicrobial research. For example, a wide variety of active compounds from the Bacillus genus, including bacteriocins and lipopeptides, has been characterized, and some commercial peptide antibiotics, including gramicidin and bacitracin, are of Bacillus origin (Raaijmakers et al. Citation2010; Abriouel et al. Citation2011; Chalasani et al. Citation2015). An active Bacillus insect symbiont has been characterized in four distinct insect species (Lee et al. 2008a, 2008b; Um et al. Citation2013; Toledo et al. Citation2015; Akbar et al. 2018). In parallel, lactic acid bacteria also produce a variety of active molecules, and the Enterococcus-derived bacteriocins specifically are being explored as a potential treatment for drug-resistant infections (Franz et al. Citation2007; Hanchi et al. Citation2018). Lactic acid bacteria are identified as active symbionts in many different insects, such as Enterococcus mundti in the Spodoptera littoralis leafworm (Shao et al. Citation2017). Only a few active symbionts belong to other bacterial orders such as Xanthomonadales and Burkholderiales.
Half of the broad screening studies (i.e. 10% of all studies) selectively deal with the mycobiota and together managed to identify a plethora of active fungal symbionts. In contrast, for the studies that isolate selected symbionts, only a minority focus on fungi. As 42% of the known active compounds of microbial origin are produced by fungi and some of the most frequently used antibiotics, such as amoxicillin, are based on a fungal lead compound, screening of fungi should not be overlooked when performing these studies (Demain Citation2014; World Health Organization Citation2018). The two main represented orders are the Hypocreales and Eurotiales. Both contain fungi known for their antimicrobial secondary metabolites, such as Trichoderma, Penicillium, and Aspergillus (Kawaguchi et al. Citation2013). Interestingly, the promising antimicrobial activity of Pseudoschalleria boydii has been found in two very taxonomically different insect species: Holotrichia parallela and Nasutitermes sp (Nirma et al. Citation2013; Wu et al. Citation2014). Overall, microbial symbionts other than the Actinomycetales can be a potential source of antimicrobial drug leads as well. In this aspect, it can be advised to perform a broad screening of all isolated fungal and bacterial symbionts, combined with a specific isolation and screening of insect Actinobacteria.
Antimicrobial potential of insect symbiont metabolites
This review will now take a closer look at the compounds with antimicrobial activity that have been isolated and identified from insect symbionts through the above-mentioned screening methodology. It is important to mention that this screening approach often leads to a complex mixture of different compounds with diverse molecular structures, from which only a few are further identified. Some active compounds may be missed due to an incomplete characterization of the symbiont extract. From those studies that managed to identify compounds, the majority also further characterized antimicrobially active compounds (27 out of 30 studies). Sometimes, previously characterized active molecules are rediscovered, as was the case for serrawettin W2, isolated from a Serratia symbiont of the Nicrophorus vespilloides beetle (Lee et al. 2008a, 2008b; Heise et al. Citation2019). A total of 14 studies describe previously undescribed metabolites, either entirely new structural molecules or slightly modified versions of known compounds (). Most of the isolated compounds have a polyketide backbone, and only a minority belongs to other molecular classes such as peptides, alkaloids and terpenoids (). Some insects symbionts are also known to produce active small molecules, such as fatty acids or phenolic compounds. In the honeybee Apis mellifera, for example lactic acid bacteria produce organic acids with antimicrobial effects (Olofsson and Vásquez Citation2008; Olofsson et al. Citation2016).
Figure 4. Overview of compounds with antimicrobial activity from insect symbionts. a) Amount of studies that isolated secondary metabolites from the symbiont(s) of interest. b) Visual representation of the structural classes the active molecules belong to.
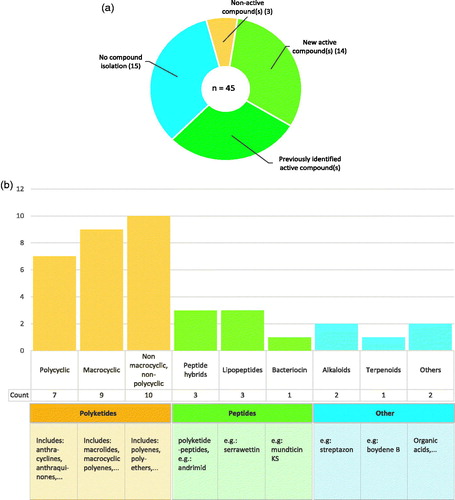
Polyketides are a diverse group of secondary metabolites that includes many different structural families. They are often categorized according to their dominant functional group or to the polyketide synthase type that is involved in their biosynthesis. For simplicity, we have divided the active polyketides into three structural groups. The first group comprises polycyclic polyketides, to which the clinically important tetracyclines belong. The second group contains macrocyclic structures, such as the commercially available macrolide erythromycin. Lastly, the third group contains all other polyketides that do not fit into the previous categories. A total of 26 different active polyketides from insect symbionts have been described.
Next to the polyketides, peptides are another clinically important group of antimicrobials, although less abundant on the market. Structurally, they can contain other moieties including lipids, sugars, or polyketide functional groups. Peptide antimicrobials on the market are synthesized in a non-ribosome-dependent manner, in line with the conventional polyketide antibiotics. These include gramicidin, vancomycin, and daptomycin (Felnagle et al. Citation2008; Lewies et al. Citation2019). Ribosomally synthesized peptides are much less explored and have recently gained increased attention as a promising category of new antimicrobials (Yang et al. Citation2014; Mahlapuu et al. Citation2016). Only one such peptide, referred to as a bacteriocin, has been isolated from an insect symbiont. Mundticin KS, a previously described peptide, was isolated from an Enterococcus mundti strain from Spodoptera littoralis (Shao et al. Citation2017).
In the remainder of this review, the focus is laid upon those compounds that have proven antimicrobial activity and, at the time of their discovery in insect symbionts, had not yet been found in other ecological niches. Their origin and reported antimicrobial activity are summarized in .
Table 1. Overview of compounds with antimicrobial activity isolated from insect symbiont strains.
Lipopeptides
A new family of lipopeptides, stephensiolides A–K, has been characterized from a S. marcescens symbiont of the Anopheles stephensi mosquito. The stephensiolides are cyclic lipodepsipeptides, containing a pentapeptide core (Thr-Ser-Ser-Val/Ile-Ile/Val) and a variable fatty acid side chain () (Ganley et al. Citation2018). An extract containing stephensiolides A–K displays activity against B. subtilis and Plasmodium falciparum (). The recent emergence of new lipopeptide antibiotics on the market, such as daptomycin and caspofungin, has triggered interest in this antimicrobial category (Pirri et al. Citation2009). Several general limitations of lipopeptides have been noted, however, such as their intrinsic toxicity and often low metabolic stability (Raaijmakers et al. Citation2010). Semi-synthetic lipopeptides seem to be the most successful on the market, so newly discovered natural lipopeptides can serve as a lead for further development of more potent and less toxic antimicrobials (Pirri et al. Citation2009).
Figure 5.1. Molecular structures of new active peptides found in insect symbionts. (a) The cyclic lipodepsipeptide stephensiolide H. (b) Dentigerumycin, a polyketide-peptide hybrid. The polyketide fragment is indicated in red (c) The fungal toxin Boydine B, an epipolythiodioxopiperazine derived from a cyclic peptide.
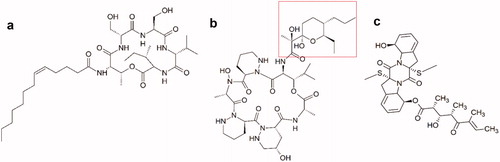
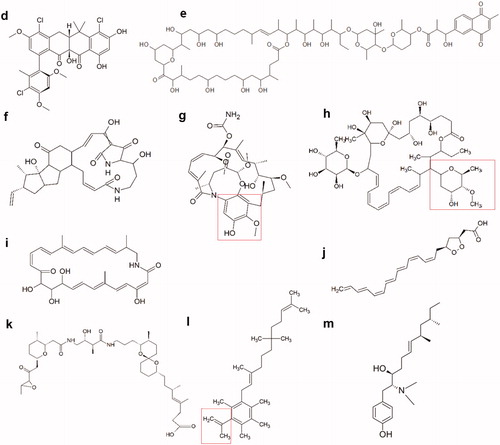
Figure 5.2. Molecular structures of new active polyketides found in insect symbionts. (d) Formicamycin F, one of the new pentacyclic polyketides. (e) Cyphomycin, a macrolide polyketide. (f) Frontalamide B, a polyketide with a macrolactam structure. (g) Natalamycin, a macrolactam polyketide. The substituted phenol function that replaces the benzoquinone function of the geldanamycins, is indicated in red. (h) Selvamicin, a polyene macrolide structurally very similar to the polyene antibiotics nystatin and amphotericin B. Indicated in red, is the sugar function that substitutes the amino sugar group of the regular polyene antibiotics. (i) Sceliphrolactam, a macrocyclic polyene. (j) Mycangimycin, a polyene peroxide with no macrocyclic ring structure. (k) Lagriamide, a polyether polyketide. (l) Ilicicolinic acid C. The carboxylic acid function is indicated in red. (m) N-methyltyroscherin, another non-polycyclic polyketide.
Polyketide-peptides
Dentigerumycin is a previously undiscovered cyclodepsipeptide isolated from a bacterial Pseudonocardia symbiont of Apterostigma dentigerum, a fungus-growing ant. The Pseudonocardia strain plays a role in defending the cultivated fungus against fungal parasites (Oh et al. Citation2009). The ring system of dentigerumycin consists of some unusual amino acids, such as γ-hydroxypiperazic acid (). Aurantimycins and polyoxypeptins are two families of closely related peptides, and both have reported antibacterial activity (Gräfe et al. Citation1995; Oh et al. Citation2009; Du et al. Citation2014). Activity of dentigerumycin has been mainly studied against the parasitic fungus Escovopsis sp. but promising activity against the human pathogenic yeast Candida albicans has been noted as well (Oh et al. Citation2009). So far, no antibacterial activity of dentigerumycin has been reported. Three additional structurally similar peptides, called gerumycin A, B, and C, have been isolated from the same insect species (Sit et al. Citation2015). Interestingly, these peptides do not display the selective antifungal activity against Escovopsis sp. They are slightly smaller molecules than dentigerumycin, missing the polyketide fragment and containing an 18-membered cyclic core instead of a 19-membered one, indicating the importance of these fragments in the antifungal bioactivity of dentigerumycin (Sit et al. Citation2015).
Another structurally interesting polyketide-peptide hybrid is boydine B. It belongs to a group of secondary metabolites, the epipolythiodioxopiperazines (ETP), a class of fungal toxins derived from cyclic peptides (Guo et al. Citation2013). The molecule was found in Pseudoschalleria boydii, a fungal symbiont of the Holotrichia parallela beetle. Boydine B shows potent inhibition of Bifidobacterium sp., Peptostreptococcus sp., and Bacteroides vulgatus among other bacteria () (Wu et al. Citation2014). The molecules are characterized by a diketopiperazine function with an internal disulphide bridge () (Figueroa et al. Citation2012). Aside from their antimicrobial activity, ETPs have well-described cytotoxic effects partly caused by this disulphide group that can bind protein thiol functions and generate reactive oxygen species through redox cycling (Gardiner et al. Citation2005). Although not yet shown for boydine B, this potential cytotoxicity can prevent its use as a future antimicrobial.
Polycyclic polyketides
A range of new interesting pentacyclic polyketides emerged from a Streptomyces symbiont from the African fungus-growing plant-ant Tetraponera penzigi (Qin et al. Citation2017). Three of these components, fasamycin C, D, and E, are structurally closely related to the known fasamycin A and B. The others, called formicamycins, are an entirely new family of polyketides and have some structural deviations, such as a non-aromatic C-ring (). Activity was evaluated for all compounds against B. subtilis, methicillin-resistant S. aureus (MRSA), and vancomycin-resistant Enterococcus faecium (VRE) and was shown to be significantly higher for the formicamycins compared to the fasamycins () (Qin et al. Citation2017).
Macrolides and macrolactams
Both macrolide and macrolactam functional groups are common in the conventional polyketide antibiotics. For example azithromycin is a frequently used macrolide antibiotic, while rifamycin harbours a macrocyclic lactam function. One of the most recently discovered active molecules produced by an insect symbiont is cyphomycin, a polyketide with a macrolide structure (). Cyphomycin is produced by a Streptomyces strain from a fungus-growing ant Cyphomyrmex sp (Chevrette et al. Citation2019). Apart from bioactivity against Escovopsis sp., potent in vitro activity has been reported against Aspergillus fumigatus and C. albicans among other Candida species () (Chevrette et al. Citation2019). Cyphomycin also reduced infection levels in a neutropenic mouse disseminated candidiasis model. Even though cyphomycin shows clear structural similarities with the toxic deplelides, another group of macrolides of Streptomyces origin which are known for their antiproliferative effects, cyphomycin so far showed no significant toxicity during mouse studies (Takeuchi et al. Citation2017; Chevrette et al. Citation2019). Overall, cyphomycin has a very promising profile for further antimicrobial development.
Two new macrolactams of insect symbiont origin are the frontalamides A and B, isolated from a Streptomyces strain of the D. frontalis beetle. These compounds belong to a group of polycyclic tetramate macrolactams (PTMs) that consist of a macrolactam core, a polycyclic ring system, and one tetramic acid function () (Felnagle et al. Citation2008). PTMs are known for their broad bioactivity, including antifungal, antibacterial, and antiprotozoal activities (Felnagle et al. Citation2008; Pirri et al. Citation2009). Frontalamides A and B in particular exhibit activity against Ophiostoma minus, an antagonistic fungus of the beetle. Activity against relevant human pathogens remains to be tested (Flórez et al. Citation2015). The frontalamides share many structural features with other known PTMs, such as ikarugamycin and discodermide, both being investigated for their antiproliferative properties (Pirri et al. Citation2009; Du et al. Citation2014; Flórez et al. Citation2015). Future tests will have to clarify whether the frontalamides have the same antiproliferative activity, which could lead to unwanted side effects. The structural complexity of the PTMs is a challenge for their large-scale production, but given their diverse biological activities it remains a molecular group with potential for future research, with many PTMs still left undiscovered (Gräfe et al. Citation1995; Felnagle et al. Citation2008).
Another new interesting macrolactam is natalamycin A, a geldanamycin analogue from a Streptomyces symbiont of M. natalensis termites. It shares a similar macrolactam core but does not have the benzoquinone function of geldanamycin and has a substituted phenol function instead () (Flórez et al. Citation2018). Geldanamycins are part of the ansamycin family of polyketides, which, aside from their macrolactam function, contain an aromatic ring. In general, the largest potential of this group lies in their antitumoural potential and not in their antimicrobial activity (Sit et al. Citation2015). Natalamycin A itself has been screened against a select set of microorganisms and has activity against both the cultivar fungus of M. natalensis (Termitomyces sp.) and the antagonistic fungus Pseudoxylaria sp (Flórez et al. Citation2018). Future screening will need to elucidate more of the antimicrobial activity as well as the cytotoxicity of natalamycin A.
Macrocyclic polyenes
Polyenes are an important class of antifungals, including nystatin and amphotericin B. Two major shortcomings of these antifungals are their very limited oral bioavailability and their systemic toxicity, limiting their use to mainly topical and parenteral applications (Zotchev Citation2003). Selvamicin, isolated from a Pseudonocardia bacterium of Apterostigma fungus-growing ants, is a polyene polyketide that is structurally very similar to the commercially available polyene antifungals (Van Arnam et al. Citation2016). Some key differences include the absence of the exocyclic carboxyl function and the presence of two regular sugar functions instead of one amino sugar () (Zotchev Citation2003). These molecular differences could explain selvamicin’s less potent antifungal activity against C. albicans compared to the regular polyene antifungals. Its solubility in water, however, is significantly better than that of nystatin (Van Arnam et al. Citation2016).
Analysis of a Streptomyces strain isolated from the mud dauber wasp S. caementarium yielded another active macrocyclic polyene (Poulsen et al. 2011). Sceliphrolactam is a 26-membered macrocyclic lactam, in contrast to the conventional polyene antifungals that consist of a macrolide ring (). Antifungal activity has only been reported against C. albicans (Oh et al. Citation2011). Structurally sceliphrolactam resembles other polyene macrolactams, such as macromonosporin A and salinilactam. This family of compounds remains underexplored and more research is needed to clarify their potential as new bioactive molecules (Skellam et al. Citation2013).
Non-macrocyclic and non-polycyclic polyketides
Mycangimycin is a polyene peroxide from Streptomyces origin, but in contrast to the polyene antifungals it is not macrocyclic. Interestingly, the symbiont stems from D. frontalis, the same beetle that led to the discovery of the frontalamides. Mycangimycin contains a seven-conjugated double bond chain and a five-membered endoperoxide (1,2-dioxolane) ring () and has activity against O. minus (Scott et al. Citation2008; Oh et al. Citation2009). Antifungal activity is also shown against C. albicans, Saccharomyces cerevisiae, and Penicillium sp. (). Antibacterial activity has not been reported yet, but the compound does show antimalarial activity against P. falciparum similar in strength to that of drugs as artemisinin and chloroquine (Oh et al. Citation2009). The 1,2-dioxolane ring of mycangimycin resembles the 1,2,4-trioxane pharmacophore of artemisinin that is essential for its antimalarial activity, while the conjugated heptaene function resembles that of the polyene antifungals (Oh et al. Citation2009; Rudrapal and Chetia Citation2016).
Lagriamide has been isolated from Burkholderia gladioli symbionts found on the eggs and in the accessory glands of the female Lagria villosa beetle (Flórez et al. Citation2018). It has reported activity against the fungus Purpureocillium lilacinum, known to infect the eggs of the beetles () (Flórez et al. Citation2018). Structurally it is closely related to the bistramides (), a group of cyclic polyethers that have been studied for their antiproliferative and anticancer properties, with actin being identified as a cellular target for bistramide A (Biard et al. Citation1994; Statsuk et al. Citation2005). This cytotoxicity makes them less suited for the use as antimicrobial leads. Polyether molecules, however, remain a class of interest within antimicrobial development for their broad antimicrobial activity, acceptable oral bioavailability, and stability, with many opportunities for structural optimization (Kevin Ii et al. Citation2009).
Other new non-polycyclic polyketides are ilicicolinic acids C and D isolated from the fungus Neonectria discophora from a termite nest of Nasutitermes corniger (Nirma et al. Citation2015). Both are structurally almost identical to the previously discovered ilicicolinic acid A (). The ilicicolinic acids are very strongly related to grifolic acid, for which a potent and broad antimicrobial effect has been reported (Asakawa et al. Citation2005). Ilicicolinic acids C and D were found to be moderately active against E. coli with acceptable cytotoxicity () (Nirma et al. Citation2015). However, they were both considerably less active than ilicicolinic acid A, making them less desirable as new antimicrobial leads. The related ilicicolinal did not display the same antimicrobial activity, pointing to the importance of the carboxylic acid function of the ilicicolinic acids (Nirma et al. Citation2015).
N-methyltyroscherin, to some extent similar in structure to the ilicicolinic acids, is a new, methylated tyroscherin derived from the same termite genus, Nasutitermes (Nirma et al. Citation2013). Tyroscherin is a tyrosine-polyketide molecule () (Liaw et al. Citation2015). Interestingly, it was isolated from P. boydii, the same fungal species that yielded the active boydine B. N-methyltyroscherin has antifungal activity against C. albicans and Trichophyton rubrum, in line with that of tyroscherin, yet slightly less active () (Nirma et al. Citation2013).
Overall, a diverse array of new active molecules has been isolated from insect symbionts. Many of these require more in-depth in vitro testing to elucidate more of their antimicrobial capacity, as some molecules, such as the frontalamides and natalamycin A only have been tested against insect pathogens. Apart from the antimicrobial activity, many other preliminary tests have to be conducted to generate insight in the drugability of the molecules. Those tests include the determination of the toxicity of the compound and its physicochemical properties such as solubility in water, as they are equally important in the development of a successful antimicrobial agent (Hughes and Karlén Citation2014). Later on, the target of the molecule also has to be identified. Compounds with a favourable profile can then be used for lead optimization to further improve both the pharmacodynamic and pharmacokinetic parameters, before advancing into preclinical development. Of all investigated compounds, cyphomycin has been tested the most extensively and seems to have the most favourable properties so far.
Concluding remarks
This review has summarized literature that aims to identify insect symbionts with antimicrobial activity, in order to evaluate the potential of these insect symbionts and their secondary metabolites in antimicrobial drug development. In doing so, we have critically analysed both the methodology used and the outcome of the different studies.
Insect symbionts as a source of new antimicrobials have gained increased attention recently, in light of the battle against the growing drug resistance (Berasategui et al. Citation2016). There is a noticeable trend in the research on fungus-growing insects and their symbionts, whereas many other insect species and orders remain largely untargeted. As it is known that the insect microbiota plays an important role in their survival in highly microbiologically contaminated environments (Kaltenpoth and Engl Citation2014), it can be advised to screen more symbionts of insects that are known to colonize microbe-rich substrates.
This review has pointed out that active symbiont strains can be found in very divergent bacterial and fungal classes, although many studies seem to prioritize the analysis of Actinobacteria symbionts. This clear interest in insect Actinobacteria seems justified, as it has been shown that Actinobacteria of insect origin, and specifically Streptomyces strains, are generally more active than those found in plant material and soil (Chevrette et al. Citation2019). To broaden the chances of success and the search area, it is advised to carry out both a broad symbiont screening as well as a specific analysis of Actinobacteria symbionts. A general concern when isolating and analysing microorganisms, is the difficulty to cultivate some strains in a laboratory environment as opposed to their natural habitat, in this case the insect (Shanchez-Contreras and Vlisidou Citation2008; Masson and Lemaitre Citation2020). New, innovative methods, such as metagenomic analysis, can be helpful to maximize the identification of biomolecules of interest and to fully explore the potential of insect symbionts (Gomes et al. Citation2013; Masson and Lemaitre Citation2020). The more traditional isolation methodologies, however, have still proven to be useful to identify new active compounds.
Lastly, we have evaluated compounds with antimicrobial activity found in insect symbionts. A considerable number of studies successfully identified new active molecules, with great structural diversity. This indicates that insect symbionts are a valuable reservoir of new active compounds. Although many of these new molecules are still in early discovery phase and more in-depth tests need to be conducted to uncover their full antimicrobial drugability, insect symbionts seem to have expanded the library of molecules available for antimicrobial development significantly. Extensive testing and structural optimization of these compounds will be key factors in the unfolding of their full antimicrobial potential.
Author contributions
L.V.M., J.D.S, and L.V.C. conceptualized the article, L.V.C. acquired the funding, L.V.M. wrote the article, and J.D.S., P.C., and L.V.C. reviewed and edited the article.
Supplementary_Table_1.docx
Download MS Word (19.7 KB)Disclosure statement
The authors report no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Funding
References
- Abriouel H, Franz CMAP, Ben Omar N, Gálvez A. 2011. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol Rev. 35(1):201–232.
- Akbar N, Siddiqui R, Iqbal M, Sagathevan K, Khan NA. 2018. Gut bacteria of cockroaches are a potential source of antibacterial compound(s). Lett Appl Microbiol. 66(5):416–426.
- Andersen SO. 2009. Chapter 94 - Exoskeleton. In: Resh VH and Cardé RT, editors. Encyclopedia of insects. 2nd ed. San Diego (CA): Academic Press; p. 339–342.
- Antoraz S, Santamaría RI, Díaz M, Sanz D, Rodríguez H. 2015. Toward a new focus in antibiotic and drug discovery from the Streptomyces arsenal. Front Microbiol. 6:461–461.
- Arango RA, Carlson CM, Currie CR, McDonald BR, Book AJ, Green F, Lebow NK, Raffa KF. 2016. Antimicrobial activity of Actinobacteria isolated from the guts of Subterranean Termites. Environ Entomol. 45(6):1415–1423.
- Asakawa Y, Hashimoto T, Ngoc Quang D, Nukada M. 2005. Isolation, synthesis and biological activity of grifolic acid derivatives from the inedible mushroom Albatrellus dispansus. Heterocycles. 65(10):2431–2439.
- Balouiri M, Sadiki M, Ibnsouda SK. 2016. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 6(2):71–79.
- Barke J, Seipke RF, Grüschow S, Heavens D, Drou N, Bibb MJ, Goss RJM, Yu DW, Hutchings MI. 2010. A mixed community of actinomycetes produce multiple antibiotics for the fungus farming ant Acromyrmex octospinosus. BMC Biol. 8:109–109.
- Beemelmanns C, Guo H, Rischer M, Poulsen M. 2016. Natural products from microbes associated with insects. Beilstein J Org Chem. 12:314–327.
- Berasategui A, Shukla S, Salem H, Kaltenpoth M. 2016. Potential applications of insect symbionts in biotechnology. Appl Microbiol Biotechnol. 100(4):1567–1577.
- Biard JF, Roussakis C, Kornprobst JM, Gouiffes-Barbin D, Verbist JF, Cotelle P, Foster MP, Ireland CM, Debitus C. 1994. Bistramides A, B, C, D, and K: a new class of bioactive cyclic polyethers from Lissoclinum bistratum. J Nat Prod. 57(10):1336–1345.
- Blodgett JAV, Oh D-C, Cao S, Currie CR, Kolter R, Clardy J. 2010. Common biosynthetic origins for polycyclic tetramate macrolactams from phylogenetically diverse bacteria. Proc Natl Acad Sci USA. 107(26):11692–11697.
- Boucias DG, Zhou Y, Huang S, Keyhani NO. 2018. Microbiota in insect fungal pathology. Appl Microbiol Biotechnol. 102(14):5873–5888.
- Burke GR, Normark BB, Favret C, Moran NA. 2009. Evolution and diversity of facultative symbionts from the aphid subfamily Lachninae. Appl Environ Microbiol. 75(16):5328–5335.
- Cafaro MJ, Poulsen M, Little AEF, Price SL, Gerardo NM, Wong B, Stuart AE, Larget B, Abbot P, Currie CR, et al. 2011. Specificity in the symbiotic association between fungus-growing ants and protective Pseudonocardia bacteria. Proceedings of the Royal Society. Proc Biol Sci. 278(1713):1814–1822.
- Carr G, Derbyshire ER, Caldera E, Currie CR, Clardy J. 2012. Antibiotic and antimalarial quinones from fungus-growing ant-associated Pseudonocardia sp. J Nat Prod. 75(10):1806–1809.
- Chalasani AG, Dhanarajan G, Nema S, Sen R, Roy U. 2015. An antimicrobial metabolite from Bacillus sp.: significant activity against pathogenic bacteria including multidrug-resistant clinical strains. Front Microbiol. 6:1335–1335.
- Challinor VL, Bode HB. 2015. Bioactive natural products from novel microbial sources. Ann N Y Acad Sci. 1354(1):82–97.
- Chevrette MG, Carlson CM, Ortega HE, Thomas C, Ananiev GE, Barns KJ, Book AJ, Cagnazzo J, Carlos C, Flanigan W, et al. 2019. The antimicrobial potential of Streptomyces from insect microbiomes. Nat Commun. 10(1):516.
- Chouvenc T, Efstathion CA, Elliott ML, Su NY. 2013. Extended disease resistance emerging from the faecal nest of a subterranean termite. Proc Biol Sci. 280(1770):20131885.
- Correa Y, Cabanillas B, Jullian V, Álvarez D, Castillo D, Dufloer C, Bustamante B, Roncal E, Neyra E, Sheen P, et al. 2019. Bioactive compounds from Chrysosporium multifidum. a fungus isolated from Hermetia illucens gut microbiota. bioRxiv. 14:669515.
- Cos P, Vlietinck AJ, Berghe DV, Maes L. 2006. Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol. 106(3):290–302.
- Currie CR, Scott JA, Summerbell RC, Malloch D. 1999. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature. 398(6729):701–704.
- Demain AL. 2014. Importance of microbial natural products and the need to revitalize their discovery. J Ind Microbiol Biotechnol. 41(2):185–201.
- Donadio S, Maffioli S, Monciardini P, Sosio M, Jabes D. 2010. Antibiotic discovery in the twenty-first century: current trends and future perspectives. J Antibiot (Tokyo). 63(8):423–430.
- Du Y, Wang Y, Huang T, Tao M, Deng Z, Lin S. 2014. Identification and characterization of the biosynthetic gene cluster of polyoxypeptin A, a potent apoptosis inducer. BMC Microbiol. 14:30–30.
- Engel P, Moran NA. 2013. The gut microbiota of insects - diversity in structure and function. FEMS Microbiol Rev. 37(5):699–735.
- Felnagle EA, Jackson EE, Chan YA, Podevels AM, Berti AD, McMahon MD, Thomas MG. 2008. Nonribosomal peptide synthetases involved in the production of medically relevant natural products. Mol Pharm. 5(2):191–211.
- Ferrari J, Vavre F. 2011. Bacterial symbionts in insects or the story of communities affecting communities. Philos Trans R Soc Lond B Biol Sci. 366(1569):1389–1400.
- Figueroa M, Graf TN, Ayers S, Adcock AF, Kroll DJ, Yang J, Swanson SM, Munoz-Acuna U, Carcache de Blanco EJ, Agrawal R, et al. 2012. Cytotoxic epipolythiodioxopiperazine alkaloids from filamentous fungi of the Bionectriaceae. J Antibiot (Tokyo). 65(11):559–564.
- Flórez LV, Biedermann PHW, Engl T, Kaltenpoth M. 2015. Defensive symbioses of animals with prokaryotic and eukaryotic microorganisms. Nat Prod Rep. 32(7):904–936.
- Flórez LV, Scherlach K, Miller IJ, Rodrigues A, Kwan JC, Hertweck C, Kaltenpoth M. 2018. An antifungal polyketide associated with horizontally acquired genes supports symbiont-mediated defense in Lagria villosa beetles. Nat Commun. 9(1):2478.
- Franz CMAP, van Belkum MJ, Holzapfel WH, Abriouel H, Gálvez A. 2007. Diversity of enterococcal bacteriocins and their grouping in a new classification scheme. FEMS Microbiol Rev. 31(3):293–310.
- Ganley JG, Carr G, Ioerger TR, Sacchettini JC, Clardy J, Derbyshire ER. 2018. Discovery of antimicrobial lipodepsipeptides produced by a Serratia sp. within mosquito microbiomes. Chembiochem. 19(15):1590–1594.
- Gardiner DM, Waring P, Howlett BJ. 2005. The epipolythiodioxopiperazine (ETP) class of fungal toxins: distribution, mode of action, functions and biosynthesis. Microbiology (Reading). 151(Pt 4):1021–1032.
- Gomes ES, Schuch V, de Macedo Lemos EG. 2013. Biotechnology of polyketides: new breath of life for the novel antibiotic genetic pathways discovery through metagenomics. Braz J Microbiol. 44(4):1007–1034.
- Gräfe U, Schlegel R, Ritzau M, Ihn W, Dornberger K, Stengel C, Fleck WF, Gutsche W, Härtl A, Paulus EF, et al. 1995. Aurantimycins, new depsipeptide antibiotics from Streptomyces aurantiacus IMET 43917. Production, isolation, structure elucidation, and biological activity. J Antibiot (Tokyo). 48(2):119–125.
- Guo CJ, Yeh HH, Chiang YM, Sanchez JF, Chang SL, Bruno KS, Wang CCC. 2013. Biosynthetic pathway for the epipolythiodioxopiperazine acetylaranotin in Aspergillus terreus revealed by genome-based deletion analysis. J Am Chem Soc. 135(19):7205–7213.
- Hanchi H, Mottawea W, Sebei K, Hammami R. 2018. The genus Enterococcus: between probiotic potential and safety concerns-an update. Front Microbiol. 9:1791.
- Heise P, Liu Y, Degenkolb T, Vogel H, Schäberle TF, Vilcinskas A. 2019. Antibiotic-producing beneficial bacteria in the gut of the burying beetle Nicrophorus vespilloides. Front Microbiol. 10:1178–1178.
- Herrmann J, Lukežič T, Kling A, Baumann S, Hüttel S, Petković H, Müller R. 2016. Strategies for the discovery and development of new antibiotics from natural products: three case studies. Curr Top Microbiol Immunol. 398:339–363.
- Hoang KL, Morran LT, Gerardo NM. 2019. Can a symbiont (also) be food? Front Microbiol. 10:2539.
- Hughes D, Karlén A. 2014. Discovery and preclinical development of new antibiotics. Ups J Med Sci. 119(2):162–169.
- Johnston M, McBride M, Dahiya D, Owusu-Apenten R, Nigam PS. 2018. Antibacterial activity of Manuka honey and its components: an overview. AIMS Microbiol. 4(4):655–664.
- Kaltenpoth M. 2009. Actinobacteria as mutualists: general healthcare for insects? Trends Microbiol. 17(12):529–535.
- Kaltenpoth M, Engl T. 2014. Defensive microbial symbionts in Hymenoptera. Funct Ecol. 28(2):315–327.
- Kawaguchi M, Nonaka K, Masuma R, Tomoda H. 2013. New method for isolating antibiotic-producing fungi. J Antibiot (Tokyo). 66(1):17–21.
- Kevin Ii DA, Meujo DA, Hamann MT. 2009. Polyether ionophores: broad-spectrum and promising biologically active molecules for the control of drug-resistant bacteria and parasites. Expert Opin Drug Discov. 4(2):109–146.
- Kikuchi Y. 2009. Endosymbiotic bacteria in insects: their diversity and culturability. Microbes Environ. 24(3):195–204.
- Kikuchi Y, Hosokawa T, Nikoh N, Meng XY, Kamagata Y, Fukatsu T. 2009. Host-symbiont co-speciation and reductive genome evolution in gut symbiotic bacteria of acanthosomatid stinkbugs. BMC Biol. 7(1):2.
- Kim KH, Ramadhar TR, Beemelmanns C, Cao S, Poulsen M, Currie CR, Clardy J. 2014. Natalamycin A, an ansamycin from a termite-associated Streptomyces sp. Chem Sci. 5(11):4333–4338.
- Kroiss J, Kaltenpoth M, Schneider B, Schwinger M-G, Hertweck C, Maddula RK, Strohm E, Svatos A. 2010. Symbiotic streptomycetes provide antibiotic combination prophylaxis for wasp offspring. Nat Chem Biol. 6(4):261–263.
- Lee H, Churey JJ, Worobo RW. 2008a. Antimicrobial activity of bacterial isolates from different floral sources of honey. Int J Food Microbiol. 126(1–2):240–244.
- Lee H, Churey JJ, Worobo RW. 2008b. Purification and structural characterization of bacillomycin F produced by a bacterial honey isolate active against Byssochlamys fulva H25. J Appl Microbiol. 105(3):663–673.
- Lewies A, Du Plessis LH, Wentzel JF. 2019. Antimicrobial peptides: the achilles’ heel of antibiotic resistance? Probiotics Antimicrob Proteins. 11(2):370–381.
- Liaw CC, Chen PC, Shih CJ, Tseng SP, Lai YM, Hsu CH, Dorrestein PC, Yang YL. 2015. Vitroprocines, new antibiotics against Acinetobacter baumannii, discovered from marine Vibrio sp. QWI-06 using mass-spectrometry-based metabolomics approach. Sci Rep. 5:12856.
- Lo WS, Huang YY, Kuo CH. 2016. Winding paths to simplicity: genome evolution in facultative insect symbionts. FEMS Microbiol Rev. 40(6):855–874.
- Madden AA, Grassetti A, Soriano JAN, Starks PT. 2013. Actinomycetes with antimicrobial activity isolated from paper wasp (Hymenoptera: Vespidae: Polistinae) nests. Environ Entomol. 42(4):703–710.
- Mahlapuu M, Håkansson J, Ringstad L, Björn C. 2016. Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol. 6:194–194.
- Mai AGM. 2018. Serratia a novel source of secondary metabolites. Adv Biotechnol Microbiol. 11(3):5.
- Mandal SM, Sharma S, Pinnaka AK, Kumari A, Korpole S. 2013. Isolation and characterization of diverse antimicrobial lipopeptides produced by Citrobacter and Enterobacter. BMC Microbiol. 13(1):152.
- Masson F, Lemaitre B. 2020. Growing ungrowable bacteria: overview and perspectives on. Microbiol Mol Biol Rev. 84(4):e00089–20.
- Matarrita-Carranza B, Moreira-Soto RD, Murillo-Cruz C, Mora M, Currie CR, Pinto-Tomas AA. 2017. Evidence for widespread associations between neotropical hymenopteran insects and Actinobacteria. Front Microbiol. 8:2016.
- Mehdiabadi NJ, Mueller UG, Brady SG, Himler AG, Schultz TR. 2012. Symbiont fidelity and the origin of species in fungus-growing ants. Nat Commun. 3(1):840.
- Miyashita A, Hirai Y, Sekimizu K, Kaito C. 2015. Antibiotic-producing bacteria from stag beetle mycangia. Drug Discov Ther. 9(1):33–37.
- Moloney MG. 2016. Natural products as a source for novel antibiotics. Trends Pharmacol Sci. 37(8):689–701.
- Moraes APR, Videira SS, Bittencourt VREP, Bittencourt AJ. 2014. Antifungal activity of Stenotrophomonas maltophilia in Stomoxys calcitrans larvae. Rev Bras Parasitol Vet. 23(2):194–199.
- Moya A, Peretó J, Gil R, Latorre A. 2008. Learning how to live together: genomic insights into prokaryote-animal symbioses. Nat Rev Genet. 9(3):218–229.
- Nirma C, Eparvier V, Stien D. 2013. Antifungal agents from Pseudallescheria boydii SNB-CN73 isolated from a Nasutitermes sp. termite. J Nat Prod. 76(5):988–991.
- Nirma C, Eparvier V, Stien D. 2015. Antibacterial ilicicolinic acids C and D and ilicicolinal from Neonectria discophora SNB-CN63 isolated from a termite nest. J Nat Prod. 78(1):159–162.
- Oh DC, Poulsen M, Currie CR, Clardy J. 2009. Dentigerumycin: a bacterial mediator of an ant-fungus symbiosis. Nat Chem Biol. 5(6):391–393.
- Oh DC, Poulsen M, Currie CR, Clardy J. 2011. Sceliphrolactam, a polyene macrocyclic lactam from a wasp-associated Streptomyces sp. Org Lett. 13(4):752–755.
- Oh DC, Scott JJ, Currie CR, Clardy J. 2009. Mycangimycin, a polyene peroxide from a mutualist Streptomyces sp. Org Lett. 11(3):633–636.
- Okabe K. 2013. Ecological characteristics of insects that affect symbiotic relationships with mites. Entomol Sci. 16(4):363–378.
- Olofsson TC, Butler È, Markowicz P, Lindholm C, Larsson L, Vásquez A. 2016. Lactic acid bacterial symbionts in honeybees - an unknown key to honey’s antimicrobial and therapeutic activities. Int Wound J. 13(5):668–679.
- Olofsson TC, Vásquez A. 2008. Detection and identification of a novel lactic acid bacterial flora within the honey stomach of the honeybee Apis mellifera. Curr Microbiol. 57(4):356–363.
- Paniagua Voirol LR, Frago E, Kaltenpoth M, Hilker M, Fatouros NE. 2018. Bacterial symbionts in Lepidoptera: their diversity, transmission, and impact on the host. Front Microbiol. 9:556.
- Patridge E, Gareiss P, Kinch MS, Hoyer D. 2016. An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov Today. 21(2):204–207.
- Pickard JM, Zeng MY, Caruso R, Núñez G. 2017. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 279(1):70–89.
- Piel J. 2011. Approaches to capturing and designing biologically active small molecules produced by uncultured microbes. Annu Rev Microbiol. 65:431–453. DOI:10.1146/annurev-micro-090110-102805.
- Pinu FR, Villas-Boas SG, Aggio R. 2017. Analysis of intracellular metabolites from microorganisms: quenching and extraction protocols. Metabolites. 7(4):53.
- Pirri G, Giuliani A, Nicoletto S, Pizzuto L, Rinaldi A. 2009. Lipopeptides as anti-infectives: a practical perspective. Cent Eur J Biol. 4(3):258–273.
- Poulsen M, Oh D-C, Clardy J, Currie CR. 2011. Chemical analyses of wasp-associated streptomyces bacteria reveal a prolific potential for natural products discovery. PLoS One. 6(2):e16763.
- Promnuan Y, Kudo T, Chantawannakul P. 2009. Actinomycetes isolated from beehives in Thailand. World J Microbiol Biotechnol. 25(9):1685–1689.
- Qin Z, Munnoch JT, Devine R, Holmes NA, Seipke RF, Wilkinson KA, Wilkinson B, Hutchings MI. 2017. Formicamycins, antibacterial polyketides produced by Streptomyces formicae isolated from African Tetraponera plant-ants. Chem Sci. 8(4):3218–3227.
- Raaijmakers JM, De Bruijn I, Nybroe O, Ongena M. 2010. Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev. 34(6):1037–1062.
- Rajagopal R. 2009. Beneficial interactions between insects and gut bacteria. Indian J Microbiol. 49(2):114–119.
- Ratcliffe NA, Mello CB, Garcia ES, Butt TM, Azambuja P. 2011. Insect natural products and processes: new treatments for human disease. Insect Biochem Mol Biol. 41(10):747–769.
- Rudrapal M, Chetia D. 2016. Endoperoxide antimalarials: development, structural diversity and pharmacodynamic aspects with reference to 1,2,4-trioxane-based structural scaffold. Drug Des Devel Ther. 10:3575–3590.
- Scott JJ, Oh DC, Yuceer MC, Klepzig KD, Clardy J, Currie CR. 2008. Bacterial protection of beetle-fungus mutualism. Science. 322(5898):63.
- Seabrooks L, Hu L. 2017. Insects: an underrepresented resource for the discovery of biologically active natural products. Acta Pharm Sin B. 7(4):409–426.
- Shanchez-Contreras M, Vlisidou I. 2008. The diversity of insect-bacteria interactions and its applications for disease control. Biotechnol Genet Eng Rev. 25:203–243.
- Shao Y, Chen B, Sun C, Ishida K, Hertweck C, Boland W. 2017. Symbiont-derived antimicrobials contribute to the control of the lepidopteran gut microbiota. Cell Chem Biol. 24(1):66–75.
- Sheehan G, Garvey A, Croke M, Kavanagh K. 2018. Innate humoral immune defences in mammals and insects: the same, with differences? Virulence. 9(1):1625–1639.
- Sherman RA. 2009. Maggot therapy takes us back to the future of wound care: new and improved maggot therapy for the 21st century. J Diabetes Sci Technol. 3(2):336–344.
- Singh V, Haque S, Niwas R, Srivastava A, Pasupuleti M, Tripathi CKM. 2016. Strategies for fermentation medium optimization: an in-depth review. Front Microbiol. 7:2087.
- Sit CS, Ruzzini AC, Van Arnam EB, Ramadhar TR, Currie CR, Clardy J. 2015. Variable genetic architectures produce virtually identical molecules in bacterial symbionts of fungus-growing ants. Proc Natl Acad Sci USA. 112(43):13150–13154.
- Skellam EJ, Stewart AK, Strangman WK, Wright JLC. 2013. Identification of micromonolactam, a new polyene macrocyclic lactam from two marine Micromonospora strains using chemical and molecular methods: clarification of the biosynthetic pathway from a glutamate starter unit. J Antibiot. 66(7):431–441.
- Statsuk AV, Bai R, Baryza JL, Verma VA, Hamel E, Wender PA, Kozmin SA. 2005. Actin is the primary cellular receptor of bistramide A. Nat Chem Biol. 1(7):383–388.
- Stork NE. 2018. How many species of insects and other terrestrial arthropods are there on earth? Annu Rev Entomol. 63(1):31–45.
- Su Q, Zhou X, Zhang Y. 2013. Symbiont-mediated functions in insect hosts. Commun Integr Biol. 6(3):e23804.
- Takeuchi T, Hatano M, Umekita M, Hayashi C, Wada SI, Nagayoshi M, Sawa R, Kubota Y, Kawada M, Igarashi M, et al. 2017. ATP depletion assay led to the isolation of new 36-membered polyol macrolides deplelides A and B from Streptomyces sp. MM581-NF15. Org Lett. 19(16):4207–4210.
- Toledo A, López S, Aulicino M, de Remes-Lenicov AM, Balatti P. 2015. Antagonism of entomopathogenic fungi by Bacillus spp. associated with the Integument of Cicadellids and Delphacids. Int Microbiol. 18:91–97.
- Um S, Fraimout A, Sapountzis P, Oh DC, Poulsen M. 2013. The fungus-growing termite Macrotermes natalensis harbors bacillaene-producing Bacillus sp. that inhibit potentially antagonistic fungi. Sci Rep. 3:3250.
- Van Arnam EB, Currie CR, Clardy J. 2018. Defense contracts: molecular protection in insect-microbe symbioses. Chem Soc Rev. 47(5):1638–1651.
- Van Arnam EB, Ruzzini AC, Sit CS, Horn H, Pinto-Tomás AA, Currie CR, Clardy J. 2016. Selvamicin, an atypical antifungal polyene from two alternative genomic contexts. Proc Natl Acad Sci USA. 113(46):12940–12945.
- Vivero RJ, Mesa GB, Robledo SM, Herrera CXM, Cadavid-Restrepo G. 2019. Enzymatic, antimicrobial, and leishmanicidal bioactivity of gram-negative bacteria strains from the midgut of Lutzomyia evansi, an insect vector of leishmaniasis in Colombia. Biotechnol Rep. 24:e00379.
- Wang L, Feng Y, Tian J, Xiang M, Sun J, Ding J, Yin WB, Stadler M, Che Y, Liu X. 2015. Farming of a defensive fungal mutualist by an attelabid weevil. ISME J. 9(8):1793–1801.
- Wang Y, Mueller UG, Clardy J. 1999. Antifungal diketopiperazines from symbiotic fungus of fungus-growing ant Cyphomyrmex minutus. J Chem Ecol. 25(4):935–941.
- Wang Z, Shi Y, Qiu Z, Che Y, Lo N. 2017. Reconstructing the phylogeny of Blattodea: robust support for interfamilial relationships and major clades. Sci Rep. 7(1):3903.
- Watve MG, Tickoo R, Jog MM, Bhole BD. 2001. How many antibiotics are produced by the genus Streptomyces? Arch Microbiol. 176(5):386–390.
- World Health Organization. 2018. WHO report on surveillance of antibiotic consumption. Geneva, Switzerland: World Health Organization; p. 7.
- World Health Organization. 2019a. 2019 Antibacterial agents in clinical development: an analysis of the antibacterial clinical development pipeline. Geneva, Switzerland: World Health Organization; p. 48.
- World Health Organization. 2019b. No time to wait: securing the future from drug-resistant infections. Geneva, Switzerland: World Health Organization; p. 28.
- Wu Q, Jiang N, Bo Han W, Ning Mei Y, Ming Ge H, Kai Guo Z, Seik Weng N, Xiang Tan R. 2014. Antibacterial epipolythiodioxopiperazine and unprecedented sesquiterpene from Pseudallescheria boydii, a beetle (coleoptera)-associated fungus. Org Biomol Chem. 12(46):9405–9412.
- Wu Q, Patočka J, Kuča K. 2018. Insect antimicrobial peptides, a mini review. Toxins. 10(11):461.
- Xie S, Lan Y, Sun C, Shao Y. 2019. Insect microbial symbionts as a novel source for biotechnology. World J Microbiol Biotechnol. 35(2):25.
- Yang SC, Lin CH, Sung CT, Fang JY. 2014. Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front Microbiol. 5:241–241.
- Yun JH, Roh SW, Whon TW, Jung MJ, Kim MS, Park DS, Yoon C, Nam YD, Kim YJ, Choi JH, et al. 2014. Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl Environ Microbiol. 80(17):5254–5264.
- Zotchev S. 2003. Polyene macrolide antibiotics and their applications in human therapy. Curr Med Chem. 10(3):211–223.
