Abstract
Clostridioides difficile is a Gram-positive, spore-forming, rod-shaped, obligate anaerobe that is the leading cause of antibiotic-associated diarrhea. Type IV pili (T4P) are elongated appendages on the surface of C. difficile that are polymerized from many pilin proteins. T4P play an important role in C. difficile adherence and particularly in its persistence in the host intestine. Recent studies have shown that T4P promote C. difficile aggregation, surface motility, and biofilm formation, which may enhance its pathogenicity. Additionally, the second messenger cyclic diguanylate increases pilA1 transcript abundance, indirectly promoting T4P-mediated aggregation, surface motility, and biofilm formation of C. difficile. This review summarizes recent advances in C. difficile T4P research and the physiological activities of T4P in the context of C. difficile pathogenesis.
Introduction
The Gram-positive, spore-forming, rod-shaped, obligate anaerobe Clostridioides difficile is the leading cause of antibiotic-associated diarrhea (Abt et al. Citation2016). The occurrence and development of C. difficile infection (CDI) are influenced by environmental, host, and C. difficile factors, with multiple virulence factors playing a synergistic role in the pathogenesis of CDI (). The contamination and germination of spores is the first step in CDI (Sandhu and McBride Citation2018). C. difficile successfully colonizes the gut while continuing to proliferate and subsequently produces toxins including toxin A, toxin B, and binary toxin (Fletcher et al. Citation2021). These toxins individually or synergistically disrupt the intestinal epithelial cytoskeleton and tight junctions between cells, increasing epithelial permeability, which causes spectrum of symptoms ranging from mild diarrhea to severe complications, such as pseudomembranous colitis, toxic megacolon, and death (Fletcher et al. Citation2021).
Table 1. Virulence factors that contribute to C. difficile pathogenesis.
Successful adherence of C. difficile to host epithelial cells is the prerequisite for its colonization and pathogenicity in the host intestine (Chandra et al. Citation2021). Among the factors mediating bacterial adhesion, the role of pili should not be underestimated. Pili are slender, filamentous, non-flagellar appendages on the surface of bacteria (Proft and Baker Citation2009). Type IV pili (T4P) exist widely in Gram-negative bacteria such as Escherichia coli and Pseudomonas aeruginosa. T4P are formed by non-covalent polymerization of many pilin subunits, which are usually found in bundles at polar sites of bacteria and are involved in adherence to host cells, twitching motility, and genetic transfer (Proft and Baker Citation2009).
The presence of T4P in C. difficile was discovered in 2006 after Varga et al. identified the gene encoding T4P in the genome of C. difficile (Varga et al. Citation2006). Advanced research on C. difficile T4P has since enriched our understanding of the structural characteristics and physiological functions of T4P. In C. difficile, T4P are involved in many phenotypes associated with its pathogenesis, including adherence to host cells, aggregation, surface motility, and biofilm formation (Bordeleau et al. Citation2015; Purcell et al. Citation2016; McKee et al. Citation2018). Interestingly, the biosynthesis and physiological function of T4P are positively influenced by the second messenger cyclic diguanylate (c-di-GMP; Bordeleau et al. Citation2015). c-di-GMP is a ubiquitous second messenger that regulates bacterial growth and multiple bacterial physiological activities including motility, toxicity, and biofilm formation (Jenal et al. Citation2017). Herein, we summarize recent advances in C. difficile T4P research with a focus on T4P-mediated phenotypes associated with C. difficile pathogenesis and the role of c-di-GMP in this process. We provide new ideas for the prevention and treatment of CDI from the perspective of T4P.
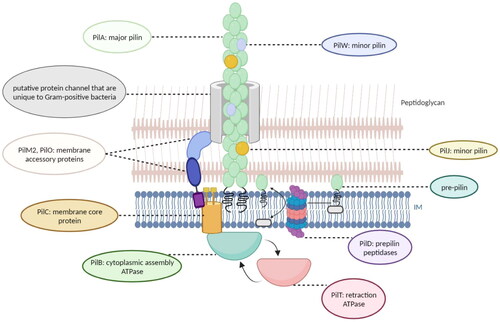
Predicted structural composition of C. difficile T4P
There have been limited comprehensive studies of the T4P structural component protein. We therefore a brief description of its predicted structure based on the T4P-encoding gene identified in C. difficile strain 630 (). Genes encoding T4P in the genome of C. difficile 630 include pilA–D, pilM, pilO, and pilT (Melville and Craig Citation2013). PilA1 proteins are assembled from the inner membrane extending outward through the periplasm. Among all C. difficile pilin genes, pilA1 shows the highest level of mRNA expression, and C. difficile cannot form visible pili after pilA1 mutation (Piepenbrink et al. Citation2015). Reintroduction of pilA1 on a complementary plasmid restores pili formation as well as pilin proteins, indicating that PilA1 is the major and necessary structural component of T4P (Piepenbrink et al. Citation2015). Another important function of PilA1 is to mediate C. difficile biofilm formation. Expression of the pilA1 cluster increases in biofilm cells, and deletion of the pilA1 cluster results in a significant reduction in the mass and thickness of the biofilm (Maldarelli et al. Citation2016; Tremblay et al. Citation2021).
PilB is a cytoplasmic assembly ATPase that drives polymerization and pilus growth. C. difficile has a second “retraction” ATPase, PilT. The amino acid sequences of PilT homologs and PilB are similar, leading to speculation that PilT may be converted to PilB to drive pilus depolymerization (Melville and Craig Citation2013). PilD is a prepilin peptidase that may help maintain the proper ratio of major versus minor proteins (Melville and Craig Citation2013). PilC, also known as the membrane core protein, is responsible for converting the chemical energy generated by ATP binding and hydrolysis into mechanical energy, thus aiding the movement of the pilus (Melville and Craig Citation2013). In Gram-negative bacteria, PilM, PilO, and PilN assemble into membrane accessory proteins that connect the inner membrane to the secretin channel (PilQ) on the outer membrane (Melville and Craig Citation2013). However, most Gram-positive bacteria, including C. difficile, lack an outer membrane and secretin. Thus, PilM and PilO may interact with proteins specific to Gram-positive bacteria to form a channel through the peptidoglycan cell wall, allowing T4P to extend extracellularly (Balasingham et al. Citation2007; Melville and Craig Citation2013; Tammam et al. Citation2013).
PilJ and PilW are minor pilins that are incorporated sporadically throughout the length of the T4P fiber of C. difficile, rather than being present as initiator pilins or in initiation complexes at the fiber tips as in Gram-negative bacteria (Piepenbrink et al. Citation2014; Ronish et al. Citation2022). Ronish et al. found that both PilJ and PilW are involved in the biofilm formation process of C. difficile, but do not play a dominant role (Ronish et al. Citation2022). PilJ and PilW also bind extracellular DNA and thus help to maintain the stability of the C. difficile biofilm (Ronish et al. Citation2022). Moreover, PilJ and PilW mediate twitching motility in C. difficile, and mutation of both pilJ and pilW reduces the twitching area (Ronish et al. Citation2022).
Our current understanding of C. difficile T4P, especially the three-dimensional structure of type IV pilin proteins, is very limited, and the role of PilB, PilC, PilT, and other type IV pilin proteins in the structure and function of T4P needs further investigation. Characterization of the structural domain of C. difficile toxin protein has led to a series of diagnostic methods and therapeutic drugs targeting the structural domain, greatly facilitating the diagnosis and treatment of CDI. Therefore, drawing on the experience with C. difficile toxins, future studies on T4P might pay more attention to the functional structural domains of the type IV pilin proteins.
T4P biosynthesis and multiple physiological functions are regulated by c-di-GMP
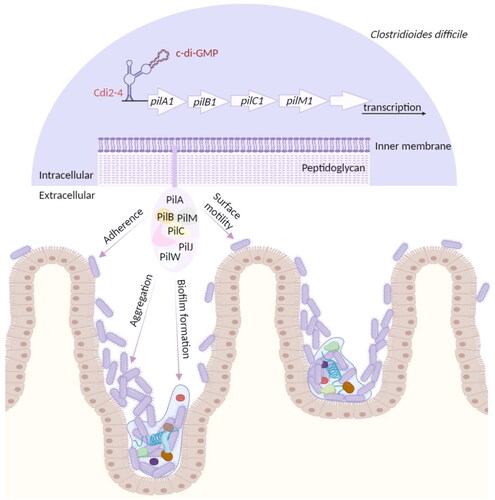
The function of T4P in C. difficile is similar to that in Gram-negative bacteria, including promoting adherence to host intestinal epithelial cells, bacterial aggregation, surface motility, and biofilm formation. c-di-GMP is a nearly ubiquitous bacterial second messenger that plays an important role in many physiological activities (Romling et al. Citation2013). The biosynthesis and degradation of c-di-GMP are regulated by two enzymes, diguanylate cyclases (DGCs) and phosphodiesterases (PDEs), respectively (Sudarsan et al. Citation2008). c-di-GMP is biosynthesized from two GTP molecules by DGCs containing a GGDEF domain and degraded to pGpG or two GMPs by PDEs containing an EAL (PDEA) and HD-GYP domain, respectively (Sudarsan et al. Citation2008). Genomic analysis of C. difficile 630 has revealed 37 proteins containing a GGDEF and/or EAL domain: 18 with a GGDEF domain, one with an EAL domain, and 18 having both GGDEF and EAL domains (Bordeleau et al. Citation2011). In 2012, Purcell et al. constructed a plasmid targeting CD1420 (dccA), the gene encoding DGC in C. difficile 630 (Purcell et al. Citation2012). Introduction of a nisin-inducible pDccA into C. difficile improves intracellular c-di-GMP levels, thus allowing artificial manipulation of intracellular c-di-GMP levels. The function of c-di-GMP depends on its binding to effectors such as PilZ or riboswitches (Romling et al. Citation2013). There are only a few predicted c-di-GMP binding proteins in the genome of C. difficile, but the gene encoding class I and II c-di-GMP riboswitches are abundant, suggesting that c-di-GMP may function primarily by binding to riboswitches in C. difficile (Purcell Citation2022). In C. difficile, elevated intracellular c-di-GMP promotes T4P biosynthesis and gene transcription and enhances T4P-mediated adhesion, aggregation, and biofilm formation ().
T4P biogenesis and the expression of the primary T4P gene cluster were induced by c-di-GMP
The putative T4P genes in the genome of C. difficile 630 are located downstream of a predicted c-di-GMP-II riboswitch, Cdi2_4, a positive transcriptional riboswitch (Bordeleau et al. Citation2015). Binding of c-di-GMP to Cdi2_4 promotes transcriptional elongation of its downstream genes. Under high intracellular c-di-GMP conditions, specific binding of c-di-GMP to the Cdi2_4 aptamer promotes visible formation of pilus-like structures while inhibiting the biosynthesis of flagella in C. difficile 630Δerm wild-type (WT) strains (Bordeleau et al. Citation2015). Mature PilA1 pilin is also detected in WT strains. In sigD (fliA) disruption mutants, the average number of pili/cells is equivalent to that of WT strains (Bordeleau et al. Citation2015). However, under the same high intracellular c-di-GMP induction conditions, pilus-like structures on the surface of both pilA1 and pilB1 mutant strains are not observed, and mature PilA1 pilin cannot be measured, indicating that the pilus-like structures induced by c-di-GMP are pili rather than flagella (Bordeleau et al. Citation2015). In addition, Bordeleau et al. found that the relative expression of the primary T4P gene cluster downstream of Cdi2_4, except pilA2, pilD2, pilJ, and pilW, increased 2- to 10-fold at high intracellular c-di-GMP levels, while the relative expression of pilA1 increased nearly 40-fold (Bordeleau et al. Citation2015).
T4P mediates C. difficile adherence and long-term colonization in the host intestine
Adherence of C. difficile to the host intestinal epithelium appears to be a prerequisite for C. difficile colonization and establishment of CDI. Common adhesion factors on the surface of C. difficile include cell wall proteins, fibronectin-binding proteins flagella, and T4P. Flagella are well-known adhesion factors. A study by Baban et al. showed that mutations in fliC and fliD of C. difficile R20291 decrease adherence to Caco-2 cells (Baban et al. Citation2013). However, other studies have shown that fliC and fliD mutants of C. difficile 630Δerm have increased adherence to Caco-2 cells, suggesting that the factors mediating adhesion vary from strain to strain (Dingle et al. Citation2011).
T4P are also important adhesion factors on the surface of C. difficile. Interestingly, T4P are critical for long-term C. difficile colonization in the host intestine but are dispensable for adhesion to host cells in the early stages of infection. Adherence of pilA1 and pilB1 mutants to HT-29 cells at 1-h post-infection is only lower than that of the parental strain, but pilA1 and pilB1 mutants show significantly reduced adherence to Madin-Darby canine kidney (MDCK) epithelial cells at 24-h post-infection compared with the parental strain (McKee et al. Citation2018). Subsequently, McKee et al. infected mice with spores of the 630Δerm parental strain, the pilA1 mutant, or the pilB1 mutant, respectively, and collected feces from the mice continuously for spore counting (McKee et al. Citation2018). The number of spores recovered from the feces of mice in each group was similar during the first 3 days of infection, but on day 7, spores could only be detected in the feces of mice infected with the 630Δerm parental strain (McKee et al. Citation2018). This study provides strong evidence for the essential role of T4P in C. difficile adherence and especially persistence in the host intestine (McKee et al. Citation2018).
CDI frequently progresses to chronic and recurrent infection, and C. difficile sporulation and biofilm formation are most often thought to be closely associated with recurrent CDI (rCDI). Existing studies suggest that the adherence of C. difficile to host intestinal epithelial cells in the early stage of infection is not dominated by T4P. However, in the later stage of infection, T4P-mediated adherence is conducive to greater C. difficile retention and colonization in the intestine, creating favorable conditions for subsequent biofilm formation and recurrence of infection. Therefore, T4P are a promising target when exploring therapeutic strategies for dealing with complex and problematic rCDI. Notably, although studies show that T4P dominate adherence in the late stage of infection, the exact mechanism behind this remains unknown. Whether C. difficile selectively expresses adherence factors at different stages of infection and how this selective expression is regulated deserve to be explored.
T4P is involved in c-di-GMP-dependent aggregation
Bacterial auto-aggregation refers to the phenomenon whereby bacteria multiply rapidly during culture, resulting in their own aggregation. Aggregation of C. difficile might promote biofilm formation in its early stages (Purcell et al. Citation2012). In Gram-negative bacteria, elevated c-di-GMP inhibits swimming motility by negatively affecting flagellar gene expression or flagellar assembly, thereby promoting bacterial growth in clumps (Jenal et al. Citation2017). Similarly, Purcell et al. observed reduced swimming motility of C. difficile on soft agar and increased cell aggregation after increasing intracellular c-di-GMP by inducing dccA expression with nisin (Purcell et al. Citation2012). High concentrations of c-di-GMP also significantly reduced transcript levels of C. difficile flagellar genes flgB, fliA, and flgM (Purcell et al. Citation2012). This may be responsible for the reduced swimming motility of C. difficile on soft agar, which in turn promotes cell aggregation.
T4P may be another factor that promotes aggregation of C. difficile cells aggregation regulated by c-di-GMP (Bordeleau et al. Citation2015). Increasing the concentration of c-di-GMP in C. difficile 630Δerm by introducing pDccA under nisin induction promotes T4P biosynthesis and causes approximately 90% of cells to aggregate (Bordeleau et al. Citation2015). By contrast, cell aggregation in the uninduced WT strain and the WT strain with pDccAmut is only 0.05% and <2.5%, respectively, under the same conditions (Bordeleau et al. Citation2015). This is consistent with the study of Purcell et al.; high intracellular c-di-GMP levels can strongly promote C. difficile cell aggregation (Purcell et al. Citation2012). Additionally, Bordeleau et al. found that both pilA1 and pilB1 mutant strains harboring pDccA showed a twofold reduction in cell aggregation rate compared with the WT strain harboring pDccA, indicating that pilA1 and pilB1 are significantly involved in c-di-GMP-dependent aggregation (Bordeleau et al. Citation2015).
Increased bacterial aggregation can be attributed to enhanced bacterial adherence. Adherence factors mediate the adherence of bacteria to host cells while also promoting cross-linking and aggregation of bacteria into clusters. C. difficile aggregation expands its habitat in the intestine, facilitating co-metabolism between bacteria, transfer of genetic material such as drug resistance genes, and biofilm formation. Not surprisingly, T4P-mediated increase in C. difficile adhesion and aggregation is a continuous process that ultimately drives C. difficile to form a large and highly resistant infectious complex resembling a biofilm in the host intestine. Hence, inhibition of T4P-mediated adherence and aggregation is also a promising therapeutic strategy for CDI; after all, individual bacteria are easier to cope with than a large complex of infectious agents.
Biofilm formation and surface motility of the epidemic strain C. difficile R20291 is dependent on T4P
Biofilms are primarily composed of bacteria and hydrated extracellular matrices including proteins, polysaccharides, and nucleic acids (Davey and O’Toole Citation2000). Owing to the antibiotic resistance of biofilms, infections in which bacteria form biofilms tend to be persistent, chronic, and extremely difficult to treat (Lebeaux et al. Citation2014; Rahmoun et al. Citation2021). Moreover, biofilm formation facilitates long-term colonization of the host intestine by C. difficile, leading to recurrence of CDI (Rahmoun et al. Citation2021). The biofilm-forming capacity of the epidemic strain R20219 is much stronger than that of the historical strain 630Δerm, with the biofilm formation of R20291 more dependent on T4P (Purcell et al. Citation2016). Disruption of the pilB1 gene in R20291 results in almost complete elimination of biofilm formation with or without pDccA (Purcell et al. Citation2016). At high intracellular concentrations of c-di-GMP, biofilm formation of R20291 is not significantly greater than that of the parental strain R20291 (Purcell et al. Citation2016). By contrast, biofilm formation of 630Δerm is more affected by intracellular c-di-GMP. Biofilm formation of 630Δerm harboring pDccA is significantly higher than that of the parental strain 630Δerm (Purcell et al. Citation2016). However, disruption of pilA1 or pilB1 in 630Δerm does not completely eliminate its biofilm formation, but reduces it to 39 or 54% of that of the parental strain, respectively (Purcell et al. Citation2016).
Differences in reliance on T4P for biofilm formation between the two strains may be attributed to differences in transcription levels. In R20291, pilA1 transcript levels are threefold higher in the biofilm condition than during planktonic growth. However, the transcript abundance of pilA1 in 630Δerm is similar in both states (Purcell et al. Citation2016). High expression of pilA1 may lead to the formation of more T4P on the surface of R20291, resulting in more powerful biofilm formation ability. In addition to T4P genes, the activity of DGCs and PDEs and the abundance of the genes encoding them may also be responsible for this difference. Whether there are differences in c-di-GMP metabolism between R20291 and 630Δerm also needs to be investigated.
Purcell et al. investigated the surface motility of R20291 and 630Δerm on agar surfaces of different solidities (Purcell et al. Citation2016). R20291 expands from its inoculation point on 1.2% (w/v) agar and reaches its maximum range on 1.8% (w/v) agar. R20291 shows stronger surface motility, and greater spread on agars of different solidity than 630Δerm (Purcell et al. Citation2016). The mutation of pilB1 in R20291 significantly reduces surface motility on 1.8% (w/v) agar, but disruption of pilA1 or pilB1 has little effect on the surface motility of 630Δerm on 1.8% agar. High concentrations of c-di-GMP promote the surface motility of R20291, but the effect on 630Δerm is not significant. Disruption of pilB1 also eliminates the enhanced surface motility of R20291 at high intracellular c-di-GMP levels, indicating that T4P are required for surface motility of C. difficile R20291 (Purcell et al. Citation2016). The solidity of healthy intestinal tissue is similar to 1–2% (w/v) agar, and the surface motility of R20291 reaches a maximum of 1.8% agar, suggesting that the high surface motility of R20291 may contribute to bacterial translocation in the host intestine.
To summarize, T4P are indispensable for biofilm formation and surface motility of the epidemic strain C. difficile R20291; however, c-di-GMP may play a more prominent role in biofilm formation of the historical strain 630Δerm. Infection caused by the epidemic strain C. difficile R20291 is a challenging clinical problem owing to high recurrence and mortality rates (Stabler et al. Citation2009). The exact mechanism responsible for the high pathogenicity of epidemic strains remains unclear, but several studies have suggested that it may be related to high toxicity, high drug resistance, and strong sporulation ability (Stabler et al. Citation2009). Given the indispensable role of T4P in mediating biofilm formation and surface motility of the epidemic strain R20291, we suggest that T4P should be included in analyses exploring the reasons for the high pathogenicity of the epidemic strain R20291.
Immunogenicity of C. difficile T4P
Studies in Gram-negative bacteria have shown that T4P pilin subunits are immunogenic. Fernandes et al. found that purified Burkholderia mallei T4P pilin is highly immunogenic (Fernandes et al. Citation2007). Attridge et al. also observed that two monoclonal antibodies against pili isolated from Vibrio cholerae O1 El Tor mediate a protective effect against cholera in mice (Attridge et al. Citation2004). Maldarelli et al. purified six soluble pilin proteins (PilA1, PilA2, PilJ, PilU, PilV, and PilW) in C. difficile and used them to immunize mice (Maldarelli et al. Citation2014). All six pilins induced detectable antibodies. The immune responses induced by PilU, PilV, and PilW were strong, whereas the immune response induced by the major pilin, PilA1, was undetectable (Maldarelli et al. Citation2014). PilJ had the highest antibody specificity, while PilW had the lowest specificity (Maldarelli et al. Citation2014). It is surprising that PilA1, the major structural and functional protein of T4P, induced a lower immune response than the minor pilins PilJ and PilW. Since various pilin subunits induce immune responses that differ in strength and specificity, future studies may consider combining multiple pilin subunits into multivalent vaccines to fully exploit their advantages; the efficacy is expected to be better than that of vaccines against a single protein.
As T4P are immunogenic extracellular appendages involved in C. difficile adhesion and colonization, they are an attractive vaccine target. More importantly, vaccines targeting surface adhesion factors such as T4P prevent bacteria from colonizing the host intestine for long periods of time, thereby reducing recurrent infections, an advantage that toxin-specific vaccines lack (Maldarelli et al. Citation2016). However, the protective effect of a pilin vaccine in C57Bl/6 mice infected with C. difficile did not reach expected levels. Maldarelli et al. immunized mice with PilA1, PilJ, and PilW mixed or separately to observe their efficacy in C57Bl/6 mice infected with C. difficile (Maldarelli et al. Citation2016). Unfortunately, neither the three mixed nor the single antibodies significantly improved weight loss, intestinal tissue damage, or mortality in a mouse model of acute CDI (Maldarelli et al. Citation2016). Notably, previous studies have shown that T4P-mediated adherence predominates in the late stage of CDI. Moreover, biofilms promoted by T4P often cause chronic and recurrent infections. Therefore, it seems inappropriate to evaluate the efficacy of a T4P vaccine in a mouse model of acute CDI. Exploring the protective effects of T4P vaccines in chronic or rCDI may be a wiser choice.
The potential of T4P as vaccine targets should not be underestimated based on poor results in one study alone, as many uncertainties during the experiment might have interfered with the results. Further studies are needed to determine whether T4P are viable vaccine targets for the prevention of C. difficile colonization and infection.
Conclusions and future prospects
Since the twentieth century, CDI has been a challenging problem for healthcare systems worldwide. A comprehensive review of C. difficile T4P has enriched our understanding of this important enteric pathogen. Studies indicate that T4P is involved in and mediates many phenotypes associated with C. difficile pathogenesis, including adherence to host epithelial cells, bacterial self-aggregation, surface motility, and biofilm formation. Since the epidemic strain R20291 has higher T4P gene transcript levels than the historical strain 630, its biofilm formation and surface motility are also more dependent on T4P participation, which makes it difficult not to associate the high pathogenicity of R20291 with T4P. Studies on T4P in C. difficile are still very limited. Whether T4P affects toxin production, sporulation or drug resistance of C. difficile remains to be investigated. In addition, the immunogenicity of T4P makes it an attractive vaccine target. Efforts should continue to develop effective vaccines against T4P, which are important for preventing the pathogenesis and transmission of C. difficile. Moreover, c-di-GMP, an important intracellular second messenger, promotes the biosynthesis of T4P as well as T4P-mediated phenotypes. This suggests that, in addition to direct inhibition of virulence factors such as toxins, flagella, and T4P, regulating the signaling molecules upstream of these virulence factors is also worth studying to determine how to resist C. difficile. Additionally, most of the available research on C. difficile T4P has focused on in vitro assays, and in vivo studies are very limited. The process and mechanism of T4P assisting C. difficile in infecting the host in vivo need to be further explored. Taken together, existing studies support T4P as potential targets for inhibiting CDI and that C. difficile T4P is an important area for further research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abt MC, McKenney PT, Pamer EG. 2016. Clostridium difficile colitis: pathogenesis and host defence. Nat Rev Microbiol. 14(10):609–620. doi: 10.1038/nrmicro.2016.108.
- Antunes A, Camiade E, Monot M, Courtois E, Barbut F, Sernova NV, Rodionov DA, Martin-Verstraete I, Dupuy B. 2012. Global transcriptional control by glucose and carbon regulator CcpA in Clostridium difficile. Nucleic Acids Res. 40(21):10701–10718. doi: 10.1093/nar/gks864.
- Attridge SR, Wallerstrom G, Qadri F, Svennerholm AM. 2004. Detection of antibodies to toxin-coregulated pili in sera from cholera patients. Infect Immun. 72(3):1824–1827. doi: 10.1128/IAI.72.3.1824-1827.2004.
- Baban ST, Kuehne SA, Barketi-Klai A, Cartman ST, Kelly ML, Hardie KR, Kansau I, Collignon A, Minton NP. 2013. The role of flagella in Clostridium difficile pathogenesis: comparison between a non-epidemic and an epidemic strain. PLOS One. 8(9):e73026. doi: 10.1371/journal.pone.0073026.
- Balasingham SV, Collins RF, Assalkhou R, Homberset H, Frye SA, Derrick JP, Tønjum T. 2007. Interactions between the lipoprotein PilP and the secretin PilQ in Neisseria meningitidis. J Bacteriol. 189(15):5716–5727. doi: 10.1128/JB.00060-07.
- Bordeleau E, Fortier LC, Malouin F, Burrus V. 2011. c-di-GMP turn-over in Clostridium difficile is controlled by a plethora of diguanylate cyclases and phosphodiesterases. PLOS Genet. 7(3):e1002039. doi: 10.1371/journal.pgen.1002039.
- Bordeleau E, Purcell EB, Lafontaine DA, Fortier LC, Tamayo R, Burrus V. 2015. Cyclic di-GMP riboswitch-regulated type IV pili contribute to aggregation of Clostridium difficile. J Bacteriol. 197(5):819–832. doi: 10.1128/JB.02340-14.
- Chandra H, Sharma KK, Tuovinen OH, Sun X, Shukla P. 2021. Pathobionts: mechanisms of survival, expansion, and interaction with host with a focus on Clostridioides difficile. Gut Microbes. 13(1):1979882. doi: 10.1080/19490976.2021.1979882.
- Chandrasekaran R, Lacy DB. 2017. The role of toxins in Clostridium difficile infection. FEMS Microbiol Rev. 41(6):723–750. doi: 10.1093/femsre/fux048.
- Davey ME, ‘O’Toole GA. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev. 64(4):847–867. doi: 10.1128/MMBR.64.4.847-867.2000.
- Deakin LJ, Clare S, Fagan RP, Dawson LF, Pickard DJ, West MR, Wren BW, Fairweather NF, Dougan G, Lawley TD, et al. 2012. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect Immun. 80(8):2704–2711. doi: 10.1128/IAI.00147-12.
- Dingle TC, Mulvey GL, Armstrong GD. 2011. Mutagenic analysis of the Clostridium difficile flagellar proteins, FliC and FliD, and their contribution to virulence in hamsters. Infect Immun. 79(10):4061–4067. doi: 10.1128/IAI.05305-11.
- Dupuy B, Govind R, Antunes A, Matamouros S. 2008. Clostridium difficile toxin synthesis is negatively regulated by TcdC. J Med Microbiol. 57(Pt 6):685–689. doi: 10.1099/jmm.0.47775-0.
- Fernandes PJ, Guo Q, Waag DM, Donnenberg MS. 2007. The type IV pilin of Burkholderia mallei is highly immunogenic but fails to protect against lethal aerosol challenge in a murine model. Infect Immun. 75(6):3027–3032. doi: 10.1128/IAI.00150-07.
- Fletcher JR, Pike CM, Parsons RJ, Rivera AJ, Foley MH, McLaren MR, Montgomery SA, Theriot CM. 2021. Clostridioides difficile exploits toxin-mediated inflammation to alter the host nutritional landscape and exclude competitors from the gut microbiota. Nat Commun. 12(1):462. doi: 10.1038/s41467-020-20746-4.
- Gerding DN, Johnson S, Rupnik M, Aktories K. 2014. Clostridium difficile binary toxin CDT: mechanism, epidemiology, and potential clinical importance. Gut Microbes. 5(1):15–27. doi: 10.4161/gmic.26854.
- Jenal U, Reinders A, Lori C. 2017. Cyclic di-GMP: second messenger extraordinaire. Nat Rev Microbiol. 15(5):271–284. doi: 10.1038/nrmicro.2016.190.
- Kevorkian Y, Shirley DJ, Shen A. 2016. Regulation of Clostridium difficile spore germination by the CspA pseudoprotease domain. Biochimie. 122:243–254. doi: 10.1016/j.biochi.2015.07.023.
- Lebeaux D, Ghigo JM, Beloin C. 2014. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev. 78(3):510–543. doi: 10.1128/MMBR.00013-14.
- Maldarelli GA, De Masi L, von Rosenvinge EC, Carter M, Donnenberg MS. 2014. Identification, immunogenicity, and cross-reactivity of type IV pilin and pilin-like proteins from Clostridium difficile. Pathog Dis. 71(3):302–314. doi: 10.1111/2049-632X.12137.
- Maldarelli GA, Matz H, Gao S, Chen K, Hamza T, Yfantis HG, Feng H, Donnenberg MS. 2016. Pilin vaccination stimulates weak antibody responses and provides no protection in a C57Bl/6 murine model of acute Clostridium difficile infection. J Vaccines Vaccin. 7(3):321. doi: 10.4172/2157-7560.1000321.
- Maldarelli GA, Piepenbrink KH, Scott AJ, Freiberg JA, Song Y, Achermann Y, Ernst RK, Shirtliff ME, Sundberg EJ, Donnenberg MS, et al. 2016. Type IV pili promote early biofilm formation by Clostridium difficile. Pathog. Dis. 74(6):ftw061. doi: 10.1093/femspd/ftw061.
- McKee RW, Aleksanyan N, Garrett EM, Tamayo R. 2018. Type IV pili promote Clostridium difficile adherence and persistence in a mouse model of infection. Infect Immun. 86(5):e00943-17. doi: 10.1128/IAI.00943-17.
- Melville S, Craig L. 2013. Type IV pili in gram-positive bacteria. Microbiol Mol Biol Rev. 77(3):323–341. doi: 10.1128/MMBR.00063-12.
- Mori N, Takahashi T. 2018. Characteristics and immunological roles of surface layer proteins in Clostridium difficile. Ann Lab Med. 38(3):189–195. doi: 10.3343/alm.2018.38.3.189.
- Nawrocki KL, Edwards AN, Daou N, Bouillaut L, McBride SM. 2016. CodY-dependent regulation of sporulation in Clostridium difficile. J Bacteriol. 198(15):2113–2130. doi: 10.1128/JB.00220-16.
- Piepenbrink KH, Maldarelli GA, de la Peña CFM, Mulvey GL, Snyder GA, De Masi L, von Rosenvinge EC, Günther S, Armstrong GD, Donnenberg MS, et al. 2014. Structure of Clostridium difficile PilJ exhibits unprecedented divergence from known type IV pilins. J Biol Chem. 289(7):4334–4345. doi: 10.1074/jbc.M113.534404.
- Piepenbrink KH, Maldarelli GA, Martinez de la Peña CF, Dingle TC, Mulvey GL, Lee A, von Rosenvinge E, Armstrong GD, Donnenberg MS, Sundberg EJ, et al. 2015. Structural and evolutionary analyses show unique stabilization strategies in the type IV pili of Clostridium difficile. Structure. 23(2):385–396. doi: 10.1016/j.str.2014.11.018.
- Proft T, Baker EN. 2009. Pili in gram-negative and gram-positive bacteria – structure, assembly and their role in disease. Cell Mol Life Sci. 66(4):613–635. doi: 10.1007/s00018-008-8477-4.
- Purcell EB. 2022. Second messenger signaling in Clostridioides difficile. Curr Opin Microbiol. 65:138–144. doi: 10.1016/j.mib.2021.11.006.
- Purcell EB, McKee RW, Bordeleau E, Burrus V, Tamayo R. 2016. Regulation of type IV pili contributes to surface behaviors of historical and epidemic strains of Clostridium difficile. J Bacteriol. 198(3):565–577. doi: 10.1128/JB.00816-15.
- Purcell EB, McKee RW, McBride SM, Waters CM, Tamayo R. 2012. Cyclic diguanylate inversely regulates motility and aggregation in Clostridium difficile. J Bacteriol. 194(13):3307–3316. doi: 10.1128/JB.00100-12.
- Rahmoun LA, Azrad M, Peretz A. 2021. Antibiotic resistance and biofilm production capacity in Clostridioides difficile. Front Cell Infect Microbiol. 11:683464. doi: 10.3389/fcimb.2021.683464.
- Rohlfing AE, Eckenroth BE, Forster ER, Kevorkian Y, Donnelly ML, Benito de la Puebla H, Doublié S, Shen A. 2019. The CspC pseudoprotease regulates germination of Clostridioides difficile spores in response to multiple environmental signals. PLoS Genet. 15(7):e1008224. doi: 10.1371/journal.pgen.1008224.
- Romling U, Galperin MY, Gomelsky M. 2013. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 77(1):1–52. doi: 10.1128/MMBR.00043-12.
- Ronish LA, Sidner B, Yu Y, Piepenbrink KH. 2022. Recognition of extracellular DNA by type IV pili promotes biofilm formation by Clostridioides difficile. J Biol Chem. 298(10):102449. doi: 10.1016/j.jbc.2022.102449.
- Sandhu BK, McBride SM. 2018. Clostridioides difficile. Trends Microbiol. 26(12):1049–1050. doi: 10.1016/j.tim.2018.09.004.
- Stabler RA, He M, Dawson L, Martin M, Valiente E, Corton C, Lawley TD, Sebaihia M, Quail MA, Rose G, et al. 2009. Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol. 10(9):R102. doi: 10.1186/gb-2009-10-9-r102.
- Sudarsan N, Lee ER, Weinberg Z, Moy RH, Kim JN, Link KH, Breaker RR. 2008. Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science. 321(5887):411–413. doi: 10.1126/science.1159519.
- Tammam S, Sampaleanu LM, Koo J, Manoharan K, Daubaras M, Burrows LL, Howell PL. 2013. PilMNOPQ from the Pseudomonas aeruginosa type IV pilus system form a transenvelope protein interaction network that interacts with PilA. J Bacteriol. 195(10):2126–2135. doi: 10.1128/JB.00032-13.
- Tremblay YDN, Durand BAR, Hamiot A, Martin-Verstraete I, Oberkampf M, Monot M, Dupuy B. 2021. Metabolic adaption to extracellular pyruvate triggers biofilm formation in Clostridioides difficile. ISME J. 15(12):3623–3635. doi: 10.1038/s41396-021-01042-5.
- Varga JJ, Nguyen V, O’Brien DK, Rodgers K, Walker RA, Melville SB. 2006. Type IV pili-dependent gliding motility in the gram-positive pathogen Clostridium perfringens and other clostridia. Mol Microbiol. 62(3):680–694. doi: 10.1111/j.1365-2958.2006.05414.x.
- Zhou Q, Rao F, Chen Z, Cheng Y, Zhang Q, Zhang J, Guan Z, He Y, Yu W, Cui G, et al. 2022. The cwp66 gene affects cell adhesion, stress tolerance, and antibiotic resistance in Clostridioides difficile. Microbiol Spectr. 10(2):e270421. doi: 10.1128/spectrum.02704-21.