Abstract
Chromatin is densely packed with nucleosomes, which limits the accessibility of many chromatin-associated proteins. Pioneer factors (PFs) are usually viewed as a special group of sequence-specific transcription factors (TFs) that can recognize nucleosome-embedded motifs, invade compact chromatin, and generate open chromatin regions. Through this process, PFs initiate a cascade of events that play key roles in gene regulation and cell differentiation. A current debate in the field is if PFs belong to a unique subset of TFs with intrinsic “pioneering activity”, or if all TFs have the potential to function as PFs within certain cellular contexts. There are also different views regarding the key feature(s) that define pioneering activity. In this review, we present evidence from the literature related to these alternative views and discuss how to potentially reconcile them. It is possible that both intrinsic properties, like tight nucleosome binding and structural compatibility, and cellular conditions, like concentration and co-factor availability, are important for PF function.
Introduction
Gene expression is a complex process by which the genetic information encoded in DNA is transcribed and translated into the arsenal of proteins required to orchestrate diverse biological processes in living systems. Gene regulation is crucial to the health of an organism, as misregulation can have severe physiological consequences. The regulation of gene expression occurs at multiple levels, but one of the most important steps is transcriptional initiation.
The gatekeepers of transcriptional initiation are proteins called “transcription factors” (TFs), which engage DNA in a sequence-specific manner to either promote or inhibit transcription of targeted genes through interactions with other co-regulators, general TFs, and transcriptional machinery. The intricacies of how eukaryotic TFs specifically target their DNA motifs within the complex chromatin environment are not fully understood. Genome-wide studies have shown that TFs bind only a small fraction of their putative motifs, and these binding events differ across cell types (Li et al. Citation2011; Arvey et al. Citation2012; Wang et al. Citation2012). Local nucleosome occupancy serves as a major impediment to TF binding because a portion of the nucleosomal DNA surface is sterically occluded by the globular domains of histones, obstructing access to residues within the TF’s recognition motif. A class of TFs called pioneer factors (PFs), however, can overcome this obstacle via their unique ability to bind to their motifs on nucleosomal DNA, promoting DNA accessibility, and allowing non-pioneer TFs (non-PFs) to bind. Accordingly, PFs are critical initiators of cellular differentiation and reprogramming.
A current debate in the field is if the pioneering ability is an intrinsic feature possessed by only a subset of TFs, or if all sequence-specific TFs have the potential to function as PFs within certain cellular contexts. Many studies have reported special properties of PFs, including similar binding affinities toward naked vs nucleosomal DNA, slow dissociation rate off nucleosomes, special structural features that are more compatible with nucleosomes, the ability to scan and access heterochromatin, stronger bookmarking activity through mitosis, etc. However, there are also studies showing that these properties are not necessary or predictive of in vivo pioneering functions, including nucleosome invasion and chromatin opening. In this review, we will first collect evidence from literature that supports each argument above and then discuss how to potentially reconcile these opposing views.
View #1: PFs are a unique subset of TFs
PFs have comparable affinities toward naked vs nucleosomal DNA
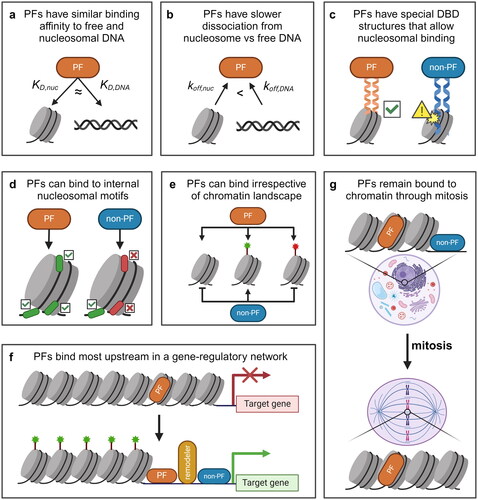
The term “pioneer factor” was originally coined in 2002 to describe a functional class of TFs including FOXA1, which was observed to independently initiate chromatin opening both in vitro and in vivo (Cirillo et al. Citation2002). FOXA1 was found to be the first TF to occupy nucleosomal sites within the enhancer of the serum albumin gene in endodermal cells, before the gene’s activation (Bossard and Zaret Citation1998). This ability of FOXA1 to bind to and open chromatin in vivo has been attributed to its ability to bind nucleosomal DNA in vitro: FOXA1 is observed to bind more stably to nucleosomal target sites within the serum albumin enhancer than to the same sites on free DNA (Cirillo and Zaret Citation1999). One metric used to define PF activity in vitro is comparing the equilibrium dissociation constants (KD) of the factor on a free DNA template vs. the same sequence reconstituted with nucleosomes, with a lower KD corresponding to a stronger binding affinity. For canonical TFs such as Gal4 or LexA, nucleosomes can increase the apparent KD by over 1000-fold relative to free DNA (Luo et al. Citation2014). Similar observations have been made on mammalian non-PFs like c-MYC, C/EBP, and NF-1 (Piña et al. Citation1990; Cirillo et al. Citation2002; Soufi et al. Citation2015; Fernandez Garcia et al. Citation2019). In contrast, many PFs including Reb1, Cbf1, FOXA1, SOX2, GATA4, OCT4, SOX11, P53, ASCL1/E12a, BRN2, HNF1a, PU.1, and Zelda display a similar apparent KD for both nucleosomes and free DNA (Cirillo et al. Citation2002; Soufi et al. Citation2015; McDaniel et al. Citation2019). These studies led to the predominant in vitro definition of PFs as a unique class of TFs that can bind to nucleosome substrates as efficiently as to free DNA ().
Figure 1. Some evidence suggests that PFs are a unique subset of TFs. (a) The apparent binding affinity of PFs for target sites on nucleosomes (KD,nuc) is similar to that of free DNA (KD,DNA). (b) some PFs have a stronger binding affinity for nucleosomal sites due to a dissociation rate compensation mechanism, where the nucleosomal off-rate (koff,nuc) is lower than the off-rate from free DNA (koff,DNA). (c) many PFs possess unique structural features in their DBDs that can better accommodate nucleosome substrates. (d) PFs are able to target internal nucleosomal motifs, like those at the dyad, while non-PFs primarily target motifs on free DNA or at the nucleosome entry-exit site. (e) PFs can target nucleosomal sites irrespective of the underlying chromatin landscape, whereas the binding of non-PFs tends to be inhibited at unmodified and repressive (red) sites but permitted at activating (green) sites. (f) PFs are the first TFs to bind to cis-regulatory elements that initiate gene regulatory events. Non-PFs tend to bind more downstream. (g) PFs can remain bound to chromatin throughout mitosis, whereas non-PFs dissociate from mitotic chromosomes.
PFs use a dissociation rate compensation mechanism to enhance nucleosome binding
How do PFs achieve high affinity on nucleosomal DNA? An early study of FOXA1 binding kinetics provided some clues to address this question (Cirillo and Zaret Citation1999). In this study, FOXA1 binding to both free DNA and nucleosomal DNA was measured by an in vitro competition assay followed by DNase I footprinting. This study found that FOXA1, but not GATA4 or Gal4, has more stable binding to nucleosomes than to free DNA. Interestingly, FOXA1 shows both a slowed association and dissociation rate to nucleosomes relative to free DNA. More recently, the binding kinetics of PFs and non-PFs on nucleosomal templates have been measured more directly using single-molecule assays. Canonical TFs like LexA and Gal4 take advantage of spontaneous nucleosomal DNA unwrapping to access motifs near the entry-exit site, and this “site exposure” process results in a significant decrease in their binding rate on nucleosomal DNA compared to that on naked DNA (Polach and Widom Citation1995; Luo et al. Citation2014). The binding rates of two budding yeast PFs, Reb1 and Cbf1, are also reduced by nucleosomes to a similar extent (Donovan et al. Citation2019), indicating that PFs recognize their nucleosomal motifs near entry-exit with a similar mechanism. PFs and non-PFs, however, have drastically different behavior in terms of dissociation. The nucleosome increases the dissociation rates of LexA and Gal4 by ∼1000-fold relative to naked DNA (Luo et al. Citation2014) but decreases those of Reb1 and Cbf1 by 10-100-fold (Donovan et al. Citation2019). Similar findings have also been reported for the Drosophila PF GAF (Feng et al. Citation2023). These results indicate that PFs use a “dissociation rate compensation” mechanism to achieve high binding affinity to nucleosomes (Donovan et al. Citation2019) (). Such slower dissociation of PFs, in combination with potential faster sliding rates inside nucleosomes, can lead to accelerated target search of PFs for nucleosomal sites (Felipe et al. Citation2022).
The slow dissociation of PFs from nucleosomes in vitro may contribute to their ability to open chromatin in vivo. The increased residence times of these factors may facilitate the recruitment of downstream co-activators or chromatin remodelers for generating accessibility. Additionally, by “sticking” to nucleosomes, PFs may be trapped in nucleosomal regions, promoting the scanning of more buried sites (Lerner et al. Citation2020). In line with this, fluorescent recovery after photobleaching (FRAP) and single-molecule tracking (SMT) experiments have shown that PFs such as FOXA1, Reb1, OCT4, and GAF diffuse more slowly in the nucleus than non-PFs and various chromatin-associated factors (Sekiya et al. Citation2009; Donovan et al. Citation2019; Lerner et al. Citation2020; Plachta et al. Citation2011; Tang et al., Citation2022). Another study of OCT4 in early mouse embryos used fluorescent decay after photoactivation (FDAP) and lineage tracing to show that each cell in the developing embryo displays either a slow or fast OCT4 kinetic pattern. They found that OCT4 kinetics, but not the concentration of OCT4, can predict the lineage patterning of cells in the early mouse embryo. Cells that display slow OCT4 kinetics give rise to pluripotent cell lineages while cells that display fast OCT4 kinetics give rise to an extra-embryonic lineage (Plachta et al. Citation2011). Overall, these experiments suggest that the kinetic properties of PFs on chromatin may be critical for their pioneering activity.
PFs possess special structures compatible with nucleosome binding
What is the structural basis for PFs’ tight nucleosome binding and dissociation compensation? Early studies of FOXA and other forkhead family proteins found that they possess a highly conserved “winged helix” DNA-binding domain (DBD) that resembles the structure of linker histone H1 (Clark et al. Citation1993; Ramakrishnan et al. Citation1993). It was therefore proposed that this structural homology allows FOXA to dock onto nucleosomes and compete off linker histones. Indeed, in vitro studies have found that FOXA proteins can displace H1 from both mononucleosomes and compacted nucleosomal arrays (Cirillo et al. Citation1998, Citation2002). Additionally, FOXA binding results in a decrease in H1 levels at the Afp distal promoter in differentiating ES cells (Taube et al. Citation2010), and FOXA1 occupancy is anti-correlated with H1 enrichment in mouse hepatocytes (Iwafuchi-Doi et al. Citation2016). Although later studies indicate that similarity to H1 is not a universal property among known PFs, many observations support the view that PFs contain special structural features, especially in their DBDs, that make them better suited for nucleosomal targeting and opening.
An in vitro screen of 593 full-length human TFs found that strong nucleosome binders generally possess DBDs containing short recognition α-helices, whereas the DBDs of TFs that weakly bind nucleosomal DNA contain long α-helices, β-sheets, or unstructured regions (Fernandez Garcia et al. Citation2019). DBDs of strong nucleosome binders include basic helix-loop-helix (bHLH), helix-turn-helix (HTH), homeodomains (HD), and zinc fingers (ZNF), which bind either as short scissor-like dimers or through one-sided binding of short α-helices. These structures allow the TFs to interact with one side of DNA, which avoids steric clash with histones (). This is consistent with another observation that PFs may have a special ability to recognize and bind partial motifs on nucleosomes. For example, OCT4, SOX2, KLF4, and FOXA2 can each target a degenerate version of their motifs at nucleosomal genomic sites (Soufi et al. Citation2015; Meers et al. Citation2019). In the case of OCT4, the POUS and POUHD domains within its bipartite POU DBD separately recognize and bind to different halves of the solvent-exposed canonical OCT4 motif (Soufi et al. Citation2015; Dodonova et al. Citation2020). KLF4, whose DBD contains three zinc fingers, is able to recognize a hexameric motif using only two of its zinc fingers to avoid steric hindrance with histone proteins (Soufi et al. Citation2015). Overall, these examples indicate that certain DBD structures that favor binding to short stretches of DNA may be an important feature of PFs.
The nucleosome-binding ability of a TF may also be conferred by structural elements that enable histone contacts. Various PFs, including OCT4, FOXA1, and Cbf1, have been observed to interact with histones (Cirillo et al. Citation2002; Iwafuchi et al. Citation2020; Roberts et al Citation2021; Donovan et al., Citation2023; Michael et al. Citation2023). For example, a scanning mutant library spanning full-length OCT4 found that a deletion within the N-terminal tail of its POUHD DBD abolished its ability to bind nucleosomal DNA without affecting its binding to naked DNA (Roberts et al. Citation2021). FOXA can interact with the H3-H4 tetramer through contacts with an α-helical domain within its C-terminus, and this region is critical for FOXA1 target gene activation and embryonic development in vivo (Cirillo et al. Citation2002; Iwafuchi et al. Citation2020). In another example, the bHLH of Cbf1 makes contacts with H2A and H2B, and substituting residues in this domain leads to a decrease in the affinity of Cbf1 for the nucleosome relative to free DNA (Donovan et al. Citation2023). These interactions can stabilize PFs on the nucleosome surface, resulting in slow dissociation rates and high affinities.
PFs can access internal nucleosomal motifs
The location of a motif within a nucleosome strongly affects TF binding. As mentioned above, the site-exposure mechanism is thought to be the dominant mode for canonical TFs to access nucleosomal DNA (Polach and Widom Citation1995; Li and Widom Citation2004). As unwrapping mostly occurs near the entry-exit sites, TF binding decreases rapidly as the binding site is moved closer to the nucleosome dyad. For example, when the Gal4 motif is moved >30 bp into the nucleosome, passing a major DNA-histone contact point, there is a ∼100-fold reduction in Gal4 binding (Hall et al. Citation2009; Donovan et al. Citation2023). In contrast, there is some evidence that PFs can recognize and bind more internal nucleosomal motifs (). Several PFs, like SOX and FOXA family members, can bind at the nucleosomal dyad in vitro, where interference from histone residues or neighboring DNA is minimized (McPherson et al. Citation1993; Sekiya et al. Citation2009; Zhu et al. Citation2018; Li et al., Citation2019; Michael et al. Citation2020). Other PFs, like OCT4, preferentially bind to entry-exit sites but can still access several internal locations, especially the ones that are solvent-accessible (Zhu et al. Citation2018; Li et al. Citation2019; Michael et al. Citation2020; Tan and Takada Citation2020).
Some in vivo studies have corroborated the finding that PFs can access and displace nucleosomes from internal motif positions. Consistent with in vitro studies showing that FOXA prefers to bind at the nucleosome dyad, some in vivo studies have found that FOXA binds at or very close to the dyad in LNCaP cells and adult mouse liver tissue (Iwafuchi-Doi et al. Citation2016; Ye et al. Citation2016). GATA3 can bind motifs two helical turns from the nucleosome dyad (SHL2), and this binding correlates with productive chromatin remodeling and target enhancer activity in the human mesenchymal to epithelial transition (Tanaka et al. Citation2020). Yeast PFs Abf1, Rap1, Reb1, and Cbf1 can displace an underlying nucleosome to a similar extent regardless of the translational or rotational positioning of the factor’s motif within the nucleosome (Yan et al. Citation2018). Given that nucleosome positioning can be fuzzy due to translational movement over time and/or cell-to-cell variations, it would make sense for PFs to be relatively insensitive to the location of motifs inside nucleosomes. Together, in vitro and in vivo evidence suggests that PFs have a unique ability to target internal nucleosomal motifs.
PFs can scan and bind to heterochromatin
Unlike non-PFs, which predominantly bind to sites within euchromatic regions, some PFs are believed to have the ability to target sites irrespective of the underlying chromatin landscape. Indeed, PFs can bind to closed chromatin lacking histone modifications, which suggests that PFs do not require active histone modifications for binding (Soufi et al. Citation2012; Donaghey et al. Citation2018). PFs may also be able to scan and bind to heterochromatic regions. For example, the PF PAX7 can pioneer enhancers possessing moderate levels of H3K9me3 and high levels of H3K9me2 (Mayran and Drouin Citation2018; Gouhier et al. Citation2024). Notably, within 30 min of ectopic PAX7 expression, PAX7 can scan and weakly bind to H3K9me3-containing sites to a similar extent as sites within less repressive regions (Mayran and Drouin Citation2018). The PFs FOXA1 and SOX2, but not the non-PF HNF4A, can scan and bind to target sites within compact chromatin (Lerner et al. Citation2023). Along these lines, a recent study revealed that FOXA1 can bind and decompact sites within heterochromatin through its intrinsic ability to phase separate into FOXA1 condensates (Ji et al. Citation2024). Using a synthetic ChIP-ISO assay, it was found that FOXA1 binding is not significantly affected by the chromatin context, including H3K9me3 and H3K27me3 marks (Xu et al. Citation2023). Additionally, upon ectopically writing H3K9me3 to a FOXA1-bound region using CRISPRi, FOXA1 binding remains unchanged (Xu et al. Citation2023). Binding of PFs Zelda, Grainy head, and Twist to chromatin remain unaffected after removal of H3K27me3 marks, illustrating that H3K27me3 does not impede PF binding (Gibson et al. Citation2024). Additionally, the binding of OCT4 ectopically expressed in immortalized foreskin fibroblasts is positively correlated with preexisting H3K27me3 modifications and unaffected by preexisting H3K9me3 modifications (Donaghey et al. Citation2018). Many other PFs have been observed to target heterochromatic sites, including ASCL1, EBF1, GATA, GR, KLF4, NRF1, p53, and PU.1 (Mayran and Drouin Citation2018). Together, these results suggest that unmodified and heterochromatic regions may be permissive to the binding of certain PFs ().
PFs are the most upstream factors in the transcription activation pathway
PFs are commonly believed to function as the most upstream factors to interact with chromatin in a sequence-specific manner (). The archetypical PF FOXA1 can bind and open compacted chromatin alone in vitro without additional co-activators or chromatin remodelers (Cirillo et al. Citation2002). Consistent with this property, early studies showed that FOX PFs are the first to bind to the silent Alb1 enhancer in ES cells and undifferentiated gut endoderm cells (Gualdi et al. Citation1996; Xu et al. Citation2007), which leads to subsequent Alb1 activation in liver differentiation (Bossard and Zaret Citation1998). In breast cancer cells, FOXA1 occupies more than half of the estrogen receptor (ER) binding sites prior to estrogen stimulation (Carroll et al. Citation2005, Citation2006; Hurtado et al. Citation2011), which maintains an open chromatin structure to allow for estrogen-induced ER binding and gene expression (Carroll et al. Citation2005; Laganière et al. Citation2005; Hurtado et al. Citation2011). Other PFs have also been observed to prime enhancers for activation in a variety of lineages. For example, PFs PU.1, IRF4, IRF8, and NF-KB bind to a silent intronic Pax5 enhancer and regulate its activation in B-cell development (Decker et al. Citation2009). The PF Grainyhead was shown to prime epithelial enhancers in Drosophila by displacing nucleosomes and increasing chromatin accessibility (Jacobs et al. Citation2018). In budding yeast, PFs Reb1, Mcm1, and RSC bind to the CLN2 promoter, generating a constitutive nucleosome depleted region (NDR) that allows the binding of a non-PF, SBF, in a cell-cycle dependent manner (Bai et al. Citation2010, Citation2011). During zygotic activation in Drosophila, PF Zelda generates chromatin accessibility that enables the binding of patterning TFs and subsequent enhancer activation (Schulz et al. Citation2015; Sun et al. Citation2015; Brennan et al. Citation2023).
There is also evidence that PFs act upstream of chromatin remodelers. A high-throughput study in yeast systematically studied the interplay between all known yeast PFs and four major chromatin remodelers, RSC, SWI/SNF, ISWI, and INO80 (Chen et al. Citation2022). It was found that chromatin remodelers are largely dispensable for nucleosome invasion by PFs and instead function downstream to modulate the length of NDRs. Similarly, the Drosophila PF GAF interacts with chromatin remodelers NURF and pBAP, and it was recently shown that neither NURF nor pBAP is required for the binding of GAF to chromatin (Judd et al. Citation2021; Tang et al. Citation2022). This is in striking contrast to non-PFs, like SBF, which binds downstream of SWI/SNF and SAGA to the HO promoter (Cosma et al. Citation1999; Zhang et al. Citation2013). These studies support a hierarchical model in which PFs act upstream of chromatin remodelers and non-PFs to initiate chromatin opening.
PFs remain bound to chromatin through mitosis
Cell cycle progression presents multiple challenges to transcriptional programs in cells. Notably, as cells enter mitosis, chromatin is further condensed into higher-order structures, transcription ceases, and many TFs and DNA-binding proteins are displaced from the mitotic chromatin (Prescott and Bender Citation1962; Martínez-Balbás et al. Citation1995; Gottesfeld and Forbes Citation1997). However, multiple studies have shown that chromatin accessibility remains relatively unchanged between interphase and mitosis (Martínez-Balbás et al. Citation1995; Hsiung et al. Citation2015; Blythe and Wieschaus Citation2016; Teves et al. Citation2016). This is at least partly because PFs, like FOXA1, OCT4, SOX2, GATA1, ESRRB, and GAF remain bound to mitotic chromatin (Martínez-Balbás et al. Citation1995; Kadauke et al., Citation2012; Caravaca et al. Citation2013; Deluz et al., Citation2016; Festuccia et al. Citation2016; Bellec et al. Citation2022). These findings suggest that PFs may have special properties that allow them to persist on chromatin throughout the cell cycle. (). The exact molecular properties that enable mitotic retention with respect to bookmarking, however, are not well defined.
It is commonly thought that the retention of PFs at specific genomic sites represents one mechanism of “mitotic bookmarking”, where they facilitate rapid rebinding of mitotically-excluded TFs and reactivation of their target genes in G1 (Kadauke et al., Citation2012; Caravaca et al. Citation2013; Festuccia et al. Citation2016; Liu et al. Citation2017). However, one study compared reporter reactivation in G1 by mitotically retained PFs SOX2/OCT4 and the mitotically excluded factor STAT3 and found very similar reactivation kinetics (Deluz et al. Citation2017). The same study also shows that the expression of most SOX2 target genes are not changed upon mitotic degradation of SOX2. Similarly, although GATA1 can increase transcriptional reactivation of a few bookmarked genes (Kadauke et al. Citation2012), this is not true for most genes where GATA1 is retained (Hsiung et al. Citation2016). More studies are needed to understand if and how PFs can remain associated with mitotic chromatin and the functional significance.
View #2: TFs do not need special intrinsic properties to function as PFs
Non-PFs can have pioneering activity at high concentration
Nucleosome invasion can be driven by a physical competition between histones and TFs for the same genomic site, and this process can be modulated by the TF concentration. Indeed, recent reports have shown that pioneering activity critically depends on TF concentration (). A screening of 104 TFs in yeast found six TFs with strong nucleosome-displacing activity, and these six PFs are distinct from the rest by their high concentration and binding specificity (Yan et al. Citation2018). Overexpression of a weak PF results in increased pioneering activity, and even an overexpressed bacterial TF, TetR, can displace nucleosomes over its binding site (Yan et al. Citation2018). In line with this, another study compared the pioneering activity of the canonical PF FOXA1 with that of the non-pioneering TF HNF4A by measuring the relative concentration of each factor needed to bind to accessible versus inaccessible genomic sites within naive K562 cells (Hansen and Cohen Citation2022). Despite its non-PF classification, overexpressed HNF4A exhibits a greater affinity for inaccessible genomic sites compared to FOXA1. These studies suggest that PFs are not necessarily a unique class of TFs. Instead, any TFs with high binding affinity to DNA may successfully compete with histones given sufficient concentration. However, it should be noted that the concentration needed for a canonical TF to function as a PF may be unattainable in a physiologically relevant context.
Figure 2. Some evidence suggests that PFs are not special and all/many TFs can function as PFs. (a) TF concentration correlates positively with nucleosomal targeting and opening. (b) Some PFs do not exhibit the dissociation rate compensation and have a higher nucleosomal off-rate (koff,nuc) than the off-rate from free DNA (koff,DNA). (c) many PFs have a stronger preference for binding to free DNA or at the nucleosome entry-exit site than to internal nucleosomal motifs. (d) Some evidence suggests that PFs are able to target unmarked and activating (green) sites but unable to target repressive (red) sites. (e) Some PFs are not retained on mitotic chromatin, whilst some TFs not characterized as PFs have been shown to remain bound to mitotic chromatin. (f) Some PFs rely on co-binding with other TFs (green or purple) to direct context-specific binding. (g) Some PFs rely on ATP-dependent chromatin remodelers for binding, despite the definition of PFs as being the most upstream factors in a gene regulatory pathway.
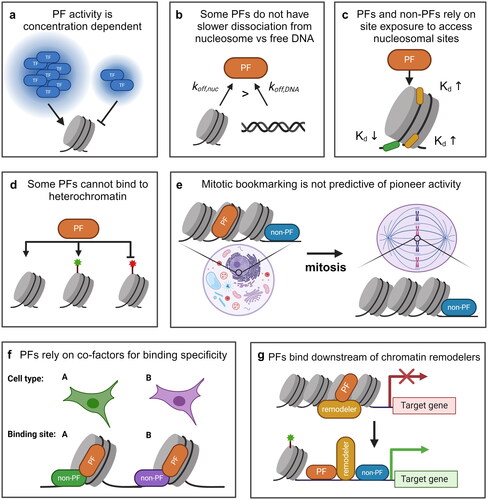
Table 1. Standard assay for PF classification.
Some PFs do not use a dissociation rate compensation mechanism
Although slowed dissociation from nucleosomes has been observed for some PFs in yeast and higher eukaryotes, this property is neither obligatory nor predictive of pioneering activity in vivo. Single-molecule fluorescence experiments have shown that a yeast PF Rap1, in contrast to Reb1 and Cbf1, displays reduced residence times on nucleosomes by about 10-fold relative to naked DNA and is further reduced by around 5-fold on a chromatin fiber relative to mononucleosomes () (Mivelaz et al. Citation2020). Nevertheless, Rap1 can invade chromatin and initiate chromatin opening in vivo (Yan et al. Citation2018; Mivelaz et al. Citation2020; Chen et al. Citation2022). Similarly, the Drosophila PF, Zelda, is unable to maintain long residence times on chromatin in vivo (Dufourt et al. Citation2018; Mir et al. Citation2018), despite its function in initiating chromatin opening (Nien et al. Citation2011; Schulz et al. Citation2015; Sun et al. Citation2015). A yeast bHLH TF, Pho4, does not show dissociation rate compensation in vitro and is usually classified as a non-PF, but it can still displace nucleosomes once overexpressed (Zhou and O’Shea Citation2011; Donovan et al. Citation2023). Interestingly, when the Pho4 basic DNA binding domain is fused to the Cbf1 HLH domain, which contacts histones and likely leads to dissociation rate compensation, the hybrid protein can invade nucleosomes at a lower expression level (Donovan et al. Citation2023). These findings suggest that slow dissociation is not absolutely necessary for nucleosome invasion in vivo, although it may enhance pioneering activities at suboptimal conditions ().
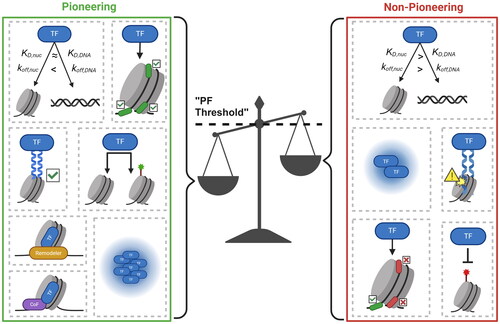
Figure 3. Pioneer binding and activity is determined by a combination of intrinsic protein characteristics and context-dependent factors. A TF will bind to and potentially open a nucleosomal site given sufficient “pioneering” features. Pioneering features can be protein-intrinsic (having a low KD,nuc and koff,nuc, targeting internal nucleosomal motifs, possessing a nucleosome-compatible DBD, engaging in physical histone contacts, binding to unmarked/repressive chromatin, and recruiting chromatin remodelers) or context-specific (high TF concentration and availability of cofactors).
Most PFs still prefer to bind entry-exit
Despite in vitro and in vivo evidence that some PFs can bind to internal nucleosomal sites, most PFs seem to exclusively or preferentially bind to the accessible entry-exit sites of the nucleosome where the DNA can partially unwrap from the histone octamer (). For example, the binding of the PF Rap1 and TP53 is largely limited to the entry-exit sites of nucleosomes (Rossetti et al. Citation2001; Yu and Buck Citation2019; Nishimura et al. Citation2020). PFs OCT4 and GATA3 have some accessibility toward internal nucleosomal sites but still prefer to bind near the edge (Echigoya et al. Citation2020; Huertas et al. Citation2020; Tanaka et al. Citation2020). Even PFs that show dissociation rate compensation, like Reb1 and Cbf1, prefer to bind near the entry-exit site with reduced affinities for sites situated further into the nucleosome core particle (Koerber et al. Citation2009; Donovan et al. Citation2019). These observations are similar to non-PFs, including LexA and Gal4 (Vettese-Dadey et al. Citation1996; Li and Widom Citation2004; Luo et al. Citation2014). These observations suggest that, at least for some PFs, their binding to nucleosomes has strong position sensitivity in vitro, which raises the question of if and how these factors access internal nucleosome positions in vivo.
The binding of some PFs is affected by chromatin landscape
Despite the prevailing belief that PFs are unique from non-PFs because they can bind irrespective of the underlying chromatin landscape, some evidence supports the argument that PFs are sensitive to histone modifications and bind predominantly to open, euchromatic loci (Lupien et al. Citation2008; Hurtado et al. Citation2011; Soufi et al. Citation2012; Wang et al., Citation2015; Chen et al., Citation2016; Donaghey et al. Citation2018; Sinha et al. Citation2023; Gibson et al. Citation2024). Some studies have found that PFs are unable to access binding sites located in heterochromatin regions enriched in H3K9 methylation (). For example, FOXA1 binding has been observed to be anti-correlated with H3K9me2 modifications in MCF7 and LNCaP cells (Lupien et al. Citation2008). Similarly, the binding of FOXA2 ectopically expressed in immortalized foreskin fibroblast cells is depleted within genomic regions containing preexisting H3K9me3 modifications (Donaghey et al. Citation2018). OCT4, SOX2, and KLF4 exhibit reduced binding to certain sites in human fibroblasts compared to stem cells, and these sites tend to be decorated by H3K9me3 in fibroblast cells (Soufi et al. Citation2012). Knocking down H3K9 methyltransferases in fibroblast led to increased binding of the reprogramming factors to these sites (Soufi et al. Citation2012). Additional studies show that knocking down or inhibiting H3K9 methyltransferases or overexpressing H3K9 demethylases can improve the reprogramming efficiency of differentiated cells into pluripotent or totipotent cells, which could be due to improved binding of reprogramming factors to their demethylated target sites (Chen et al. Citation2013; Matoba et al. Citation2014; Chung et al. Citation2015; Huang et al. Citation2016; Liu et al. Citation2018). PF binding may also respond to positive histone marks. A recent study found that the OCT4 POUS DBD interacts with the N-terminal tail of H3, and H3K27ac improves the cooperative binding of OCT4-OCT4 and OCT4-SOX2 to the underlying nucleosome (Sinha et al. Citation2023). However, there are conflicting results showing that H3K27ac does not increase OCT4 binding to nucleosomes (Guan et al. Citation2023; Lian et al. Citation2023). There is also some evidence of PF binding enrichment on nucleosomes previously modified by H3K4me1 and H3K4me2, but it remains unclear if these modifications are absolutely required for PF binding to these sites (Lupien et al. Citation2008; Hurtado et al. Citation2011; Wang et al., Citation2015; Chen et al. Citation2016). It is at least possible that histone modifications may have a direct effect on PF binding and cooperativity, which goes against the notion that PFs bind to target sites independent of the chromatin landscape.
Mitotic bookmarking is not predictive of PF activity
Not all PFs can serve as mitotic bookmarks. The Drosophila PF Zelda, for example, does not persist on mitotic chromatin like many other PFs (Dufourt et al. Citation2018). It’s thought that Zelda can compensate for this dynamic association by continuously binding to chromatin during the S-phase before entering the next round of M-phase (McDaniel et al. Citation2019). Another study monitored the association of 501 mammalian TFs with mitotic chromatin by single-molecule imaging, FRAP, and mapping of TF binding and chromatin accessibility (Raccaud et al. Citation2019). Of the 501 TFs, over 100 are found to be enriched on mitotic chromosomes, and the ability to bind and remain bound to mitotic chromatin is not predictive of pioneer activity (). Additionally, OCT4 is not strongly associated with mitotic chromatin on its own, but its bookmarking capacity was dramatically increased when co-expressed with SOX2 (Raccaud et al. Citation2019), illustrating that even PFs may rely on co-factors to bind chromatin in certain contexts. In summary, although some PFs have been shown to act as mitotic bookmarks, this ability may not be a special property unique to PFs.
Some PFs rely on co-factors to target chromatin
Recent studies have revealed an emerging role of co-factors in modulating PF activity (Luzete-Monteiro and Zaret Citation2022). For example, steroid receptors (SRs), which were originally thought to strictly function downstream of FOXA1 (Carroll et al. Citation2005; Laganière et al. Citation2005; Lupien et al. Citation2008; Hurtado et al. Citation2011), are reported to recruit FOXA1 to a subset of sites in the genome (Swinstead et al. Citation2016). However, these results remain controversial (Glont et al. Citation2019; Paakinaho et al. Citation2019). Several other TFs including CEBPA (Stefflova et al. Citation2013), GATA4 (Donaghey et al. Citation2018; Meers et al. Citation2019), and PDX1 (Geusz et al. Citation2021) have also been found to affect the genome-wide distribution of FOXA1/2. A recent study characterized AP-1 and CEBPB as co-factors that bind cooperatively with FOXA1 and affect its target sites in A549 cells, but not in HepG2 or MCF7 cells (Xu et al. Citation2023). Besides FOXA1, dependency on co-factors to bind was also observed for other PFs. Pluripotency factors OCT4, SOX2, and KLF4 are PFs that participate in the reprogramming of fibroblasts into induced pluripotent stem cells (iPSCs) (Soufi et al. Citation2012), during which a large fraction of each factor’s genomic binding events are dependent on the availability of the other two factors (Chronis et al. Citation2017). In other cell types, OCT4 and SOX2’s genomic occupancy can be directed by PARP-1 (Liu and Kraus Citation2017), OTX2 (Buecker et al. Citation2014), and TFAP2A (Hovland et al. Citation2022). Another putative PF PU.1 (Iwafuchi-Doi and Zaret Citation2014) shows extensive cooperative binding with various lineage-determining TFs during myeloid and lymphoid development (Heinz et al. Citation2010, Citation2013; Gosselin et al. Citation2014; Link et al. Citation2018). In all of these cases, PF-co-factor coordination seems to be cell-type specific, illustrating a potential mechanism of modulating PF targets during differentiation.
Some PFs may also rely on ATP-dependent chromatin remodelers for binding to chromatin (). SWI/SNF is required for the binding of the PFs OCT4, SOX2, and NANOG to a subset of regions and is essential for stabilizing OCT4 on chromatin in ESCs (King and Klose Citation2017). ISWI promotes the binding and stabilization of CTCF on chromatin (Wiechens et al. Citation2016). This is in line with an “assisted loading model” in which PFs rely on chromatin remodelers for stabilizing PF binding and creating an accessible chromatin state to assist the binding of downstream TFs (Swinstead et al. Citation2016). These studies demonstrate that at least some PF binding is affected by a variety of co-factors, in contrast to the traditional view that PFs are independent TFs that bind as the most upstream factor in a transcriptional regulation cascade.
Discussion
How can we reconcile the seemingly opposing views listed above? There may be a few factors that potentially contribute to these discrepancies. First, PFs in literature are defined based on different standards and assays, and therefore, the class of “PFs” may contain different types of TFs. In our opinion, true PFs should be able to invade nucleosomes through internally embedded motifs and eventually lead to nucleosome displacement in vivo. When using assays lacking single nucleosome resolution, e.g. ATAC-seq, bona fide PFs can be hard to differentiate with TFs that bind to narrow linker regions and recruit downstream factors like nucleosome remodelers and histone modifiers to further increase nearby chromatin accessibility. High throughput screening assays for pioneer activities among many TFs in vivo or in vitro are likely to be helpful in categorizing PFs and non-PFs in a standardized manner ().
In addition, PFs may indeed have special properties in vitro, but these properties may not be absolutely necessary or sufficient for nucleosome invasion and chromatin opening in vivo. The PF-nucleosome interaction can be affected by many context-specific factors inside cells, including the PF concentration, altered nucleosome dynamics, co-factor/remodeler availability, etc. (). In particular, we suspect that nucleosome structure is more dynamic in vivo than in vitro (Dion et al. Citation2007; Kubik et al. Citation2019; Iurlaro et al. Citation2021; Kim et al. Citation2021; Schick et al. Citation2021; Chen et al. Citation2022; Tan et al. Citation2023), making it easier for TFs to gain access to nucleosomal DNA. This potentially explains the observation that, although high affinity and slow dissociation between PFs and nucleosomes can enhance PF-nucleosome docking and facilitate the invasion process in vitro, they do not seem to be a requirement for PFs to overcome the nucleosomal barrier in vivo. For instance, Rap1 and Zelda still display pioneer activities in vivo, despite their high off rates from chromatin (Dufourt et al. Citation2018; Mir et al. Citation2018; Mivelaz et al. Citation2020). In vitro, Rap1 binding to nucleosomes is limited to the entry/exit site with significantly decreased binding to internal sites, but can displace an underlying nucleosome to a similar extent regardless of the translational or rotational positioning of the factor’s motif within the nucleosome in vivo (Rossetti et al. Citation2001; Yan et al. Citation2018). Pho4 does not show a dissociation rate compensation in vitro but it can still displace nucleosomes once overexpressed in vivo (Zhou and O’Shea Citation2011; Donovan et al. Citation2023). Similar observations are made with human non-PF, HNF4A (Hansen et al. Citation2022; Hansen and Cohen Citation2022). Finally, even a bacterial TF, TetR, can reposition nucleosomes when overexpressed in yeast (Yan et al. Citation2018). These studies illustrate that the ability to bind nucleosomes in vitro does not always translate to chromatin binding and opening in vivo, and vice versa. It therefore may be inappropriate to directly compare PFs that are predominantly characterized in vitro vs in vivo.
The concentration and co-factor dependence of some PFs also indicate that, instead of an absolute binary classification of PFs and non-PFs, it may be more accurate to define PFs within specific cell contexts. For example, FOXA1 has low expression and relies on cooperativity with AP-1 to bind to many of its targets in the A549 cells, raising the possibility that AP-1 functions as a “co-pioneer” in this case. In contrast, FOXA1 seems to function independently from co-factors in MCF-7 and HepG2 cells, where its expression is significantly higher (Xu et al. Citation2023). Similarly, OCT4 gains pioneer activity and access to inaccessible sites as ESCs transition into epiblast-like cells (EpiLCs) which is mediated by the co-binding of the TF OTX2, suggesting that PF activity can be directed by other TFs (Buecker et al. Citation2014). Together, these observations suggest that the pioneering is highly context-dependent where lower concentrations necessitate the need for co-TF binding for PFs to stably engage and open chromatin, in a cell type-specific manner ().
Ultimately, TFs need to compete with nucleosomes for binding to the same genomic loci. Certain intrinsic properties of TFs can facilitate this process, and these TFs are more likely to behave like PFs. However, even for TFs lacking these intrinsic properties, nucleosome invasion can be achieved by increasing the binding rate kon at high concentration, reducing the dissociation rate of koff by interacting with nucleosome-binding co-factors, or recruiting downstream regulators, like nucleosome remodelers, to destabilize and displace nucleosomes (). This process can also be impacted by local nucleosome dynamics in vivo. For example, chromatin remodeling, histone turnover, DNA replication, and transcription may transiently expose embedded nucleosomal sites and facilitate PF invasion. More studies are needed to systematically identify PFs and non-PFs in various cell types, compare the differences in their molecular features, and elucidate the order of events governing chromatin opening by PFs.
Acknowledgement
The authors acknowledge Bai lab for discussions and advice on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Arvey A, Agius P, Noble WS, Leslie C. 2012. Sequence and chromatin determinants of cell-type-specific transcription factor binding. Genome Res. 22(9):1723–1734. doi: 10.1101/gr.127712.111.
- Bai L, Charvin G, Siggia ED, Cross FR. 2010. Nucleosome-depleted regions in cell-cycle-regulated promoters ensure reliable gene expression in every cell cycle. Dev Cell. 18(4):544–555.
- Bai L, Ondracka A, Cross FR. 2011. Multiple sequence-specific factors generate the nucleosome-depleted region on CLN2 promoter. Mol Cell. 42(4):465–476.
- Bellec M, Dufourt J, Hunt G, Lenden-Hasse H, Trullo A, Zine El Aabidine A, Lamarque M, Gaskill MM, Faure-Gautron H, Mannervik M, et al. 2022. The control of transcriptional memory by stable mitotic bookmarking. Nat Commun. 13(1):1176. doi: 10.1038/s41467-022-28855-y.
- Blythe SA, Wieschaus EF. 2016. Establishment and maintenance of heritable chromatin structure during early Drosophila embryogenesis. Elife. 5. doi: 10.7554/eLife.20148.
- Bossard P, Zaret KS. 1998. GATA transcription factors as potentiators of gut endoderm differentiation. Development. 125(24):4909–4917. doi: 10.1242/dev.125.24.4909.
- Brennan KJ, Weilert M, Krueger S, Pampari A, Liu H-Y, Yang AWH, Morrison JA, Hughes TR, Rushlow CA, Kundaje A, et al. 2023. Chromatin accessibility in the Drosophila embryo is determined by transcription factor pioneering and enhancer activation. Dev Cell. 58(19):1898–1916 e9.
- Buecker C, Srinivasan R, Wu Z, Calo E, Acampora D, Faial T, Simeone A, Tan M, Swigut T, Wysocka J, et al. 2014. Reorganization of enhancer patterns in transition from naive to primed pluripotency. Cell Stem Cell. 14(6):838–853. doi: 10.1016/j.stem.2014.04.003.
- Caravaca JM, Donahue G, Becker JS, He X, Vinson C, Zaret KS. 2013. Bookmarking by specific and nonspecific binding of FoxA1 pioneer factor to mitotic chromosomes. Genes Dev. 27(3):251–260. doi: 10.1101/gad.206458.112.
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, et al. 2005. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 122(1):33–43. doi: 10.1016/j.cell.2005.05.008.
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, et al. 2006. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 38(11):1289–1297. doi: 10.1038/ng1901.
- Chen J, Chen X, Li M, et al. 2016. Hierarchical Oct4 binding in concert with primed epigenetic rearrangements during somatic cell reprogramming. Cell Rep. 14(6):1540–1554.
- Chen H, Kharerin H, Dhasarathy A, Kladde M, Bai L. 2022. Partitioned usage of chromatin remodelers by nucleosome-displacing factors. Cell Rep. 40(8):111250.
- Chen J, Liu H, Liu J, Qi J, Wei B, Yang J, Liang H, Chen Y, Chen J, Wu Y, et al. 2013. H3K9 methylation is a barrier during somatic cell reprogramming into iPSCs. Nat Genet. 45(1):34–42. doi: 10.1038/ng.2491.
- Chronis C, Fiziev P, Papp B, Butz S, Bonora G, Sabri S, Ernst J, Plath K. 2017. Cooperative binding of transcription factors orchestrates reprogramming. Cell. 168(3):442–459 e20. doi: 10.1016/j.cell.2016.12.016.
- Chung YG, Matoba S, Liu Y, Eum JH, Lu F, Jiang W, Lee JE, Sepilian V, Cha KY, Lee DR, et al. 2015. Histone demethylase expression enhances human somatic cell nuclear transfer efficiency and promotes derivation of pluripotent stem cells. Cell Stem Cell. 17(6):758–766. doi: 10.1016/j.stem.2015.10.001.
- Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. 2002. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell. 9(2):279–289.
- Cirillo LA, McPherson CE, Bossard P, Stevens K, Cherian S, Shim EY, Clark KL, Burley SK, Zaret KS. 1998. Binding of the winged-helix transcription factor HNF3 to a linker histone site on the nucleosome. Embo J. 17(1):244–254. doi: 10.1093/emboj/17.1.244.
- Cirillo LA, Zaret KS. 1999. An early developmental transcription factor complex that is more stable on nucleosome core particles than on free DNA. Mol Cell. 4(6):961–969. doi: 10.1016/s1097-2765(00)80225-7.
- Clark KL, Halay ED, Lai E, Burley SK. 1993. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 364(6436):412–420. doi: 10.1038/364412a0.
- Cosma MP, Tanaka T, Nasmyth K. 1999. Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell. 97(3):299–311. doi: 10.1016/s0092-8674(00)80740-0.
- Decker T, Pasca di Magliano M, McManus S, Sun Q, Bonifer C, Tagoh H, Busslinger M. 2009. Stepwise activation of enhancer and promoter regions of the B cell commitment gene Pax5 in early lymphopoiesis. Immunity. 30(4):508–520. doi: 10.1016/j.immuni.2009.01.012.
- Deluz C, Friman ET, Strebinger D, Benke A, Raccaud M, Callegari A, Leleu M, Manley S, Suter DM. 2016. A role for mitotic bookmarking of SOX2 in pluripotency and differentiation. Genes Dev. 30(22):2538–2550. doi: 10.1101/gad.289256.116.
- Deluz C, Strebinger D, Friman ET, Suter DM. 2017. The elusive role of mitotic bookmarking in transcriptional regulation: insights from Sox2. Cell Cycle. 16(7):601–606. doi: 10.1080/15384101.2017.1288332.
- Dion MF, Kaplan T, Kim M, Buratowski S, Friedman N, Rando OJ. 2007. Dynamics of replication-independent histone turnover in budding yeast. Science. 315(5817):1405–1408. doi: 10.1126/science.1134053.
- Dodonova SO, Zhu F, Dienemann C, Taipale J, Cramer P. 2020. Nucleosome-bound SOX2 and SOX11 structures elucidate pioneer factor function. Nature. 580(7805):669–672. doi: 10.1038/s41586-020-2195-y.
- Donaghey J, Thakurela S, Charlton J, Chen JS, Smith ZD, Gu H, Pop R, Clement K, Stamenova EK, Karnik R, et al. 2018. Genetic determinants and epigenetic effects of pioneer-factor occupancy. Nat Genet. 50(2):250–258. doi: 10.1038/s41588-017-0034-3.
- Donovan BT, Chen H, Eek P, Meng Z, Jipa C, Tan S, Bai L, Poirier MG. 2023. Basic helix-loop-helix pioneer factors interact with the histone octamer to invade nucleosomes and generate nucleosome-depleted regions. Mol Cell. 83(8):1251–1263 e6. doi: 10.1016/j.molcel.2023.03.006.
- Donovan BT, Chen H, Jipa C, Bai L, Poirier MG. 2019. Dissociation rate compensation mechanism for budding yeast pioneer transcription factors. Elife. 8. doi: 10.7554/eLife.43008.
- Donovan BT, Luo Y, Meng Z, Poirier MG. 2023. The nucleosome unwrapping free energy landscape defines distinct regions of transcription factor accessibility and kinetics. Nucleic Acids Res. 51(3):1139–1153. doi: 10.1093/nar/gkac1267.
- Dufourt J, Trullo A, Hunter J, Fernandez C, Lazaro J, Dejean M, Morales L, Nait-Amer S, Schulz KN, Harrison MM, et al. 2018. Temporal control of gene expression by the pioneer factor Zelda through transient interactions in hubs. Nat Commun. 9(1):5194. doi: 10.1038/s41467-018-07613-z.
- Echigoya K, Koyama M, Negishi L, Takizawa Y, Mizukami Y, Shimabayashi H, Kuroda A, Kurumizaka H. 2020. Nucleosome binding by the pioneer transcription factor OCT4. Sci Rep. 10(1):11832. doi: 10.1038/s41598-020-68850-1.
- Espinosa JM, Emerson BM. 2001. Transcriptional regulation by p53 through intrinsic DNA/chromatin binding and site-directed cofactor recruitment. Mol Cell. 8(1):57–69. doi: 10.1016/s1097-2765(01)00283-0.
- Felipe C, Shin J, Kolomeisky AB. 2022. How pioneer transcription factors search for target sites on nucleosomal DNA. J Phys Chem B. 126(22):4061–4068.
- Feng XA, Ness KM, Liu C, Ahmed I, Bowman GD, Ha T, Wu C. 2023. GAGA factor overcomes 1D diffusion barrier by 3d diffusion in search of nucleosomal targets. bioRxiv. doi: 10.1101/2023.07.14.549009.
- Fernandez Garcia M, Moore CD, Schulz KN, Alberto O, Donague G, Harrison MM, Zhu H, Zaret KS. 2019. Structural features of transcription factors associating with nucleosome binding. Mol Cell. 75(5):921–932 e6.
- Festuccia N, Dubois A, Vandormael-Pournin S, Gallego Tejeda E, Mouren A, Bessonnard S, Mueller F, Proux C, Cohen-Tannoudji M, Navarro P, et al. 2016. Mitotic binding of Esrrb marks key regulatory regions of the pluripotency network. Nat Cell Biol. 18(11):1139–1148. doi: 10.1038/ncb3418.
- Geusz RJ, Wang A, Lam DK, Vinckier NK, Alysandratos K-D, Roberts DA, Wang J, Kefalopoulou S, Ramirez A, Qiu Y, et al. 2021. Sequence logic at enhancers governs a dual mechanism of endodermal organ fate induction by FOXA pioneer factors. Nat Commun. 12(1):6636. doi: 10.1038/s41467-021-26950-0.
- Gibson TJ, Larson ED, Harrison MM. 2024. Protein-intrinsic properties and context-dependent effects regulate pioneer factor binding and function. Nat Struct Mol Biol. 31(3):548–558. doi: 10.1038/s41594-024-01231-8.
- Glont SE, Chernukhin I, Carroll JS. 2019. Comprehensive genomic analysis reveals that the pioneering function of FOXA1 is independent of hormonal signaling. Cell Rep. 26(10):2558–2565 e3.
- Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, Stender JD, Chun HB, Garner H, Geissmann F, et al. 2014. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 159(6):1327–1340. doi: 10.1016/j.cell.2014.11.023.
- Gottesfeld JM, Forbes DJ. 1997. Mitotic repression of the transcriptional machinery. Trends Biochem Sci. 22(6):197–202. doi: 10.1016/s0968-0004(97)01045-1.
- Gouhier A, Dumoulin-Gagnon J, Lapointe-Roberge V, Harris J, Balsalobre A, Drouin J. 2024. Pioneer factor Pax7 initiates two-step cell-cycle-dependent chromatin opening. Nat Struct Mol Biol. 31(1):92–101. doi: 10.1038/s41594-023-01152-y.
- Gualdi R, Bossard P, Zheng M, Hamada Y, Coleman JR, Zaret KS. 1996. Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev. 10(13):1670–1682. doi: 10.1101/gad.10.13.1670.
- Guan R, Lian T, Zhou B-R, Wheeler D, Bai Y. 2023. Structural mechanism of LIN28B nucleosome targeting by OCT4. Mol Cell. 83(12):1970–1982 e6.
- Hall MA, Shundrovsky A, Bai L, Fulbright RM, Lis JT, Wang MD. 2009. High-resolution dynamic mapping of histone-DNA interactions in a nucleosome. Nat Struct Mol Biol. 16(2):124–129. doi: 10.1038/nsmb.1526.
- Hansen JL, Cohen BA. 2022. A quantitative metric of pioneer activity reveals that HNF4A has stronger in vivo pioneer activity than FOXA1. Genome Biol. 23(1):221. doi: 10.1186/s13059-022-02792-x.
- Hansen JL, Loell KJ, Cohen BA. 2022. A test of the pioneer factor hypothesis using ectopic liver gene activation. Elife. 11. doi: 10.7554/eLife.73358.
- Heinz S, Benner C, Spann N, et al. 2010. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 38(4):576–589.
- Heinz S, Romanoski CE, Benner C, Allison KA, Kaikkonen MU, Orozco LD, Glass CK. 2013. Effect of natural genetic variation on enhancer selection and function. Nature. 503(7477):487–492. doi: 10.1038/nature12615.
- Hovland AS, Bhattacharya D, Azambuja AP, Pramio D, Copeland J, Rothstein M, Simoes-Costa M. 2022. Pluripotency factors are repurposed to shape the epigenomic landscape of neural crest cells. Dev Cell. 57(19):2257–2272 e5.
- Hsiung CC-S, Bartman CR, Huang P, Ginart P, Stonestrom AJ, Keller CA, Face C, Jahn KS, Evans P, Sankaranarayanan L, et al. 2016. A hyperactive transcriptional state marks genome reactivation at the mitosis-G1 transition. Genes Dev. 30(12):1423–1439. doi: 10.1101/gad.280859.116.
- Hsiung CC-S, Morrissey CS, Udugama M, Frank CL, Keller CA, Baek S, Giardine B, Crawford GE, Sung M-H, Hardison RC, et al. 2015. Genome accessibility is widely preserved and locally modulated during mitosis. Genome Res. 25(2):213–225. doi: 10.1101/gr.180646.114.
- Huang J, Zhang H, Yao J, Qin G, Wang F, Wang X, Luo A, Zheng Q, Cao C, Zhao J, et al. 2016. BIX-01294 increases pig cloning efficiency by improving epigenetic reprogramming of somatic cell nuclei. Reproduction. 151(1):39–49. doi: 10.1530/REP-15-0460.
- Huertas J, MacCarthy CM, Schöler HR, Cojocaru V. 2020. Nucleosomal DNA dynamics mediate Oct4 pioneer factor binding. Biophys J. 118(9):2280–2296.
- Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. 2011. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet. 43(1):27–33. doi: 10.1038/ng.730.
- Iurlaro M, Stadler MB, Masoni F, Jagani Z, Galli GG, Schübeler D. 2021. Mammalian SWI/SNF continuously restores local accessibility to chromatin. Nat Genet. 53(3):279–287. doi: 10.1038/s41588-020-00768-w.
- Iwafuchi M, Cuesta I, Donahue G, Takenaka N, Osipovich AB, Magnuson MA, Roder H, Seeholzer SH, Santisteban P, Zaret KS, et al. 2020. Gene network transitions in embryos depend upon interactions between a pioneer transcription factor and core histones. Nat Genet. 52(4):418–427. doi: 10.1038/s41588-020-0591-8.
- Iwafuchi-Doi M, Donahue G, Kakumanu A, Watts JA, Mahony S, Pugh BF, Lee D, Kaestner KH, Zaret KS. 2016. The pioneer transcription factor FoxA maintains an accessible nucleosome configuration at enhancers for tissue-specific gene activation. Mol Cell. 62(1):79–91.
- Iwafuchi-Doi M, Zaret KS. 2014. Pioneer transcription factors in cell reprogramming. Genes Dev. 28(24):2679–2692. doi: 10.1101/gad.253443.114.
- Jacobs J, Atkins M, Davie K, Imrichova H, Romanelli L, Christiaens V, Hulselmans G, Potier D, Wouters J, Taskiran II, et al. 2018. The transcription factor Grainy head primes epithelial enhancers for spatiotemporal activation by displacing nucleosomes. Nat Genet. 50(7):1011–1020. doi: 10.1038/s41588-018-0140-x.
- Ji D, Shao C, Yu J, Hou Y, Gao X, Wu Y, Wang L, Chen P. 2024. FOXA1 forms biomolecular condensates that unpack condensed chromatin to function as a pioneer factor. Mol Cell. 84(2):244–260 e7.
- Judd J, Duarte FM, Lis JT. 2021. Pioneer-like factor GAF cooperates with PBAP (SWI/SNF) and NURF (ISWI) to regulate transcription. Genes Dev. 35(1–2):147–156. doi: 10.1101/gad.341768.120.
- Kadauke S, Udugama MI, Pawlicki JM, Achtman JC, Jain DP, Cheng Y, Hardison RC, Blobel GA. 2012. Tissue-specific mitotic bookmarking by hematopoietic transcription factor GATA1. Cell. 150(4):725–737. doi: 10.1016/j.cell.2012.06.038.
- Kim JM, Visanpattanasin P, Jou V, Liu S, Tang X, Zheng Q, Li KY, Snedeker J, Lavis LD, Lionnet T, et al. 2021. Single-molecule imaging of chromatin remodelers reveals role of ATPase in promoting fast kinetics of target search and dissociation from chromatin. Elife. 10. doi: 10.7554/eLife.69387.
- King HW, Klose RJ. 2017. The pioneer factor OCT4 requires the chromatin remodeller BRG1 to support gene regulatory element function in mouse embryonic stem cells. Elife. 6. doi: 10.7554/eLife.22631.
- Koerber RT, Rhee HS, Jiang C, Pugh BF. 2009. Interaction of transcriptional regulators with specific nucleosomes across the Saccharomyces genome. Mol Cell. 35(6):889–902. doi: 10.1016/j.molcel.2009.09.011.
- Kubik S, Bruzzone MJ, Challal D, Dreos R, Mattarocci S, Bucher P, Libri D, Shore D. 2019. Opposing chromatin remodelers control transcription initiation frequency and start site selection. Nat Struct Mol Biol. 26(8):744–754. doi: 10.1038/s41594-019-0273-3.
- Laganière J, Deblois G, Lefebvre C, Bataille AR, Robert F, Giguère V. 2005. From the cover: location analysis of estrogen receptor alpha target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc Natl Acad Sci U S A. 102(33):11651–11656.
- Lerner J, Gomez-Garcia PA, McCarthy RL, Liu Z, Lakadamyali M, Zaret KS. 2020. Two-parameter mobility assessments discriminate diverse regulatory factor behaviors in chromatin. Mol Cell. 79(4):677–688 e6.
- Lerner J, Katznelson A, Zhang J, Zaret KS. 2023. Different chromatin-scanning modes lead to targeting of compacted chromatin by pioneer factors FOXA1 and SOX2. Cell Rep. 42(7):112748.
- Lian T, Guan R, Zhou BR, et al. 2023. The human LIN28B nucleosome is inherently pre-positioned for efficient binding of multiple OCT4s without H3 K27 acetylation. bioRxiv.
- Link VM, Duttke SH, Chun HB, Holtman IR, Westin E, Hoeksema MA, Abe Y, Skola D, Romanoski CE, Tao J, et al. 2018. Analysis of genetically diverse macrophages reveals local and domain-wide mechanisms that control transcription factor binding and function. Cell. 173(7):1796–1809 e17. doi: 10.1016/j.cell.2018.04.018.
- Li X-Y, Thomas S, Sabo PJ, Eisen MB, Stamatoyannopoulos JA, Biggin MD. 2011. The role of chromatin accessibility in directing the widespread, overlapping patterns of Drosophila transcription factor binding. Genome Biol. 12(4):R34. doi: 10.1186/gb-2011-12-4-r34.
- Liu Z, Kraus WL. 2017. Catalytic-independent functions of PARP-1 determine Sox2 pioneer activity at intractable genomic loci. Mol Cell. 65(4):589–603 e9.
- Liu Y, Pelham-Webb B, Di Giammartino DC, et al. 2017. Widespread mitotic bookmarking by histone marks and transcription factors in pluripotent stem cells. Cell Rep. 19(7):1283–1293.
- Liu X, Wang Y, Gao Y, Su J, Zhang J, Xing X, Zhou C, Yao K, An Q, Zhang Y, et al. 2018. H3K9 demethylase KDM4E is an epigenetic regulator for bovine embryonic development and a defective factor for nuclear reprogramming. Development. 145(4). doi: 10.1242/dev.158261.
- Li G, Widom J. 2004. Nucleosomes facilitate their own invasion. Nat Struct Mol Biol. 11(8):763–769. doi: 10.1038/nsmb801.
- Li S, Zheng EB, Zhao L, Liu S. 2019. Nonreciprocal and conditional cooperativity directs the pioneer activity of pluripotency transcription factors. Cell Rep. 28(10):2689–2703 e4.
- Luo Y, North JA, Rose SD, Poirier MG. 2014. Nucleosomes accelerate transcription factor dissociation. Nucleic Acids Res. 42(5):3017–3027. doi: 10.1093/nar/gkt1319.
- Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, Carroll JS, Liu XS, Brown M. 2008. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell. 132(6):958–970. doi: 10.1016/j.cell.2008.01.018.
- Luzete-Monteiro E, Zaret KS. 2022. Structures and consequences of pioneer factor binding to nucleosomes. Curr Opin Struct Biol. 75:102425. doi: 10.1016/j.sbi.2022.102425.
- Martínez-Balbás MA, Dey A, Rabindran SK, Ozato K, Wu C. 1995. Displacement of sequence-specific transcription factors from mitotic chromatin. Cell. 83(1):29–38. doi: 10.1016/0092-8674(95)90231-7.
- Matoba S, Liu Y, Lu F, Iwabuchi KA, Shen L, Inoue A, Zhang Y. 2014. Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation. Cell. 159(4):884–895. doi: 10.1016/j.cell.2014.09.055.
- Mayran A, Drouin J. 2018. Pioneer transcription factors shape the epigenetic landscape. J Biol Chem. 293(36):13795–13804.
- McDaniel SL, Gibson TJ, Schulz KN, Fernandez Garcia M, Nevil M, Jain SU, Lewis PW, Zaret KS, Harrison MM. 2019. Continued activity of the pioneer factor zelda is required to drive zygotic genome activation. Mol Cell. 74(1):185–195 e4.
- McPherson CE, Shim EY, Friedman DS, Zaret KS. 1993. An active tissue-specific enhancer and bound transcription factors existing in a precisely positioned nucleosomal array. Cell. 75(2):387–398. doi: 10.1016/0092-8674(93)80079-t.
- Meers MP, Janssens DH, Henikoff S. 2019. Pioneer factor-nucleosome binding events during differentiation are motif encoded. Mol Cell. 75(3):562–575 e5.
- Michael AK, Grand RS, Isbel L, Cavadini S, Kozicka Z, Kempf G, Bunker RD, Schenk AD, Graff-Meyer A, Pathare GR, et al. 2020. Mechanisms of OCT4-SOX2 motif readout on nucleosomes. Science. 368(6498):1460–1465. doi: 10.1126/science.abb0074.
- Michael AK, Stoos L, Crosby P, Eggers N, Nie XY, Makasheva K, Minnich M, Healy KL, Weiss J, Kempf G, et al. 2023. Cooperation between bHLH transcription factors and histones for DNA access. Nature. 619(7969):385–393. doi: 10.1038/s41586-023-06282-3.
- Mir M, Stadler MR, Ortiz SA, Hannon CE, Harrison MM, Darzacq X, Eisen MB. 2018. Dynamic multifactor hubs interact transiently with sites of active transcription in Drosophila embryos. Elife. 7. doi: 10.7554/eLife.40497.
- Mivelaz M, Cao A-M, Kubik S, Zencir S, Hovius R, Boichenko I, Stachowicz AM, Kurat CF, Shore D, Fierz B, et al. 2020. Chromatin Fiber Invasion and Nucleosome Displacement by the Rap1 Transcription Factor. Mol Cell. 77(3):488–500 e9.
- Nien C-Y, Liang H-L, Butcher S, Sun Y, Fu S, Gocha T, Kirov N, Manak JR, Rushlow C. 2011. Temporal coordination of gene networks by Zelda in the early Drosophila embryo. PLoS Genet. 7(10):e1002339. doi: 10.1371/journal.pgen.1002339.
- Nishimura M, Arimura Y, Nozawa K, Kurumizaka H. 2020. Linker DNA and histone contributions in nucleosome binding by p53. J Biochem. 168(6):669–675. doi: 10.1093/jb/mvaa081.
- Paakinaho V, Swinstead EE, Presman DM, Grøntved L, Hager GL. 2019. Meta-analysis of chromatin programming by steroid receptors. Cell Rep. 28(13):3523–3534 e2.
- Piña B, Brüggemeier U, Beato M. 1990. Nucleosome positioning modulates accessibility of regulatory proteins to the mouse mammary tumor virus promoter. Cell. 60(5):719–731. doi: 10.1016/0092-8674(90)90087-u.
- Plachta N, Bollenbach T, Pease S, Fraser SE, Pantazis P. 2011. Oct4 kinetics predict cell lineage patterning in the early mammalian embryo. Nat Cell Biol. 13(2):117–123. doi: 10.1038/ncb2154.
- Polach KJ, Widom J. 1995. Mechanism of protein access to specific DNA sequences in chromatin: a dynamic equilibrium model for gene regulation. J Mol Biol. 254(2):130–149.
- Prescott DM, Bender MA. 1962. Synthesis of RNA and protein during mitosis in mammalian tissue culture cells. Exp Cell Res. 26(2):260–268. doi: 10.1016/0014-4827(62)90176-3.
- Raccaud M, Friman ET, Alber AB, Agarwal H, Deluz C, Kuhn T, Gebhardt JCM, Suter DM. 2019. Mitotic chromosome binding predicts transcription factor properties in interphase. Nat Commun. 10(1):487. doi: 10.1038/s41467-019-08417-5.
- Ramakrishnan V, Finch JT, Graziano V, Lee PL, Sweet RM. 1993. Crystal structure of globular domain of histone H5 and its implications for nucleosome binding. Nature. 362(6417):219–223. doi: 10.1038/362219a0.
- Roberts GA, Ozkan B, Gachulincová I, O’Dwyer MR, Hall-Ponsele E, Saxena M, Robinson PJ, Soufi A. 2021. Dissecting OCT4 defines the role of nucleosome binding in pluripotency. Nat Cell Biol. 23(8):834–845. doi: 10.1038/s41556-021-00727-5.
- Rossetti L, Cacchione S, De Menna A, Chapman L, Rhodes D, Savino M. 2001. Specific interactions of the telomeric protein Rap1p with nucleosomal binding sites. J Mol Biol. 306(5):903–913.
- Schick S, Grosche S, Kohl KE, Drpic D, Jaeger MG, Marella NC, Imrichova H, Lin J-MG, Hofstätter G, Schuster M, et al. 2021. Acute BAF perturbation causes immediate changes in chromatin accessibility. Nat Genet. 53(3):269–278. doi: 10.1038/s41588-021-00777-3.
- Schulz KN, Bondra ER, Moshe A, Villalta JE, Lieb JD, Kaplan T, McKay DJ, Harrison MM. 2015. Zelda is differentially required for chromatin accessibility, transcription factor binding, and gene expression in the early Drosophila embryo. Genome Res. 25(11):1715–1726. doi: 10.1101/gr.192682.115.
- Sekiya T, Muthurajan UM, Luger K, Tulin AV, Zaret KS. 2009. Nucleosome-binding affinity as a primary determinant of the nuclear mobility of the pioneer transcription factor FoxA. Genes Dev. 23(7):804–809. doi: 10.1101/gad.1775509.
- Sinha KK, Bilokapic S, Du Y, Malik D, Halic M. 2023. Histone modifications regulate pioneer transcription factor cooperativity. Nature. 619(7969):378–384. doi: 10.1038/s41586-023-06112-6.
- Soufi A, Donahue G, Zaret KS. 2012. Facilitators and impediments of the pluripotency reprogramming factors’ initial engagement with the genome. Cell. 151(5):994–1004. doi: 10.1016/j.cell.2012.09.045.
- Soufi A, Garcia MF, Jaroszewicz A, Osman N, Pellegrini M, Zaret KS. 2015. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell. 161(3):555–568. doi: 10.1016/j.cell.2015.03.017.
- Stefflova K, Thybert D, Wilson MD, Streeter I, Aleksic J, Karagianni P, Brazma A, Adams DJ, Talianidis I, Marioni JC, et al. 2013. Cooperativity and rapid evolution of cobound transcription factors in closely related mammals. Cell. 154(3):530–540. doi: 10.1016/j.cell.2013.07.007.
- Sun Y, Nien C-Y, Chen K, Liu H-Y, Johnston J, Zeitlinger J, Rushlow C. 2015. Zelda overcomes the high intrinsic nucleosome barrier at enhancers during Drosophila zygotic genome activation. Genome Res. 25(11):1703–1714. doi: 10.1101/gr.192542.115.
- Swinstead EE, Miranda TB, Paakinaho V, Baek S, Goldstein I, Hawkins M, Karpova TS, Ball D, Mazza D, Lavis LD, et al. 2016. Steroid receptors reprogram FoxA1 occupancy through dynamic chromatin transitions. Cell. 165(3):593–605. doi: 10.1016/j.cell.2016.02.067.
- Swinstead EE, Paakinaho V, Presman DM, Hager GL. 2016. Pioneer factors and ATP-dependent chromatin remodeling factors interact dynamically: a new perspective: multiple transcription factors can effect chromatin pioneer functions through dynamic interactions with ATP-dependent chromatin remodeling factors. Bioessays. 38(11):1150–1157. doi: 10.1002/bies.201600137.
- Tanaka H, Takizawa Y, Takaku M, Kato D, Kumagawa Y, Grimm SA, Wade PA, Kurumizaka H. 2020. Interaction of the pioneer transcription factor GATA3 with nucleosomes. Nat Commun. 11(1):4136. doi: 10.1038/s41467-020-17959-y.
- Tan ZY, Cai S, Noble AJ, Chen JK, Shi J, Gan L. 2023. Heterogeneous non-canonical nucleosomes predominate in yeast cells in situ. Elife. 12. doi: 10.7554/eLife.87672.
- Tang X, Li T, Liu S, Wisniewski J, Zheng Q, Rong Y, Lavis LD, Wu C. 2022. Kinetic principles underlying pioneer function of GAGA transcription factor in live cells. Nat Struct Mol Biol. 29(7):665–676. doi: 10.1038/s41594-022-00800-z.
- Tan C, Takada S. 2020. Nucleosome allostery in pioneer transcription factor binding. Proc Natl Acad Sci U S A. 117(34):20586–20596.
- Taube JH, Allton K, Duncan SA, Shen L, Barton MC. 2010. Foxa1 functions as a pioneer transcription factor at transposable elements to activate Afp during differentiation of embryonic stem cells. J Biol Chem. 285(21):16135–16144.
- Teves SS, An L, Hansen AS, Xie L, Darzacq X, Tjian R. 2016. A dynamic mode of mitotic bookmarking by transcription factors. Elife. 5. doi: 10.7554/eLife.22280.
- Vettese-Dadey M, Grant PA, Hebbes TR, et al. 1996. Acetylation of histone H4 plays a primary role in enhancing transcription factor binding to nucleosomal DNA in vitro. Embo J. 15(10):2508–2518.
- Wang A, Yue F, Li Y, Xie R, Harper T, Patel NA, Muth K, Palmer J, Qiu Y, Wang J, et al. 2015. Epigenetic priming of enhancers predicts developmental competence of hESC-derived endodermal lineage intermediates. Cell Stem Cell. 16(4):386–399. doi: 10.1016/j.stem.2015.02.013.
- Wang J, Zhuang J, Iyer S, Lin X, Whitfield TW, Greven MC, Pierce BG, Dong X, Kundaje A, Cheng Y, et al. 2012. Sequence features and chromatin structure around the genomic regions bound by 119 human transcription factors. Genome Res. 22(9):1798–1812. doi: 10.1101/gr.139105.112.
- Wiechens N, Singh V, Gkikopoulos T, Schofield P, Rocha S, Owen-Hughes T. 2016. The chromatin remodelling enzymes SNF2H and SNF2L position nucleosomes adjacent to CTCF and other transcription factors. PLoS Genet. 12(3):e1005940. doi: 10.1371/journal.pgen.1005940.
- Xu C, Kleinschmidt H, Yang J, Leith E, Johnson J, Tan S, Mahony S, Bai L. 2023. Systematic dissection of sequence features affecting the binding specificity of a pioneer factor reveals binding synergy between FOXA1 and AP-1. bioRxiv. doi: 10.1101/2023.11.08.566246.
- Xu J, Pope SD, Jazirehi AR, Attema JL, Papathanasiou P, Watts JA, Zaret KS, Weissman IL, Smale ST. 2007. Pioneer factor interactions and unmethylated CpG dinucleotides mark silent tissue-specific enhancers in embryonic stem cells. Proc Natl Acad Sci U S A. 104(30):12377–12382. doi: 10.1073/pnas.0704579104.
- Yan C, Chen H, Bai L. 2018. Systematic study of nucleosome-displacing factors in budding yeast. Mol Cell. 71(2):294–305.e4.
- Ye Z, Chen Z, Sunkel B, Frietze S, Huang TH-M, Wang Q, Jin VX. 2016. Genome-wide analysis reveals positional-nucleosome-oriented binding pattern of pioneer factor FOXA1. Nucleic Acids Res. 44(16):7540–7554. doi: 10.1093/nar/gkw659.
- Yu X, Buck MJ. 2019. Defining TP53 pioneering capabilities with competitive nucleosome binding assays. Genome Res. 29(1):107–115. doi: 10.1101/gr.234104.117.
- Zhang Q, Yoon Y, Yu Y, Parnell EJ, Garay JAR, Mwangi MM, Cross FR, Stillman DJ, Bai L. 2013. Stochastic expression and epigenetic memory at the yeast HO promoter. Proc Natl Acad Sci U S A. 110(34):14012–14017.
- Zhou X, O’Shea EK. 2011. Integrated approaches reveal determinants of genome-wide binding and function of the transcription factor Pho4. Mol Cell. 42(6):826–836.
- Zhu F, Farnung L, Kaasinen E, Sahu B, Yin Y, Wei B, Dodonova SO, Nitta KR, Morgunova E, Taipale M, et al. 2018. The interaction landscape between transcription factors and the nucleosome. Nature. 562(7725):76–81. doi: 10.1038/s41586-018-0549-5.
