Abstract
The COVID-19 pandemic has been a disruptive event for cancer patients, especially those with haematological malignancies (HM). They may experience a more severe clinical course due to impaired immune responses. This multi-center retrospective UK audit identified cancer patients who had SARS-CoV-2 infection between 1 March and 10 June 2020 and collected data pertaining to cancer history, COVID-19 presentation and outcomes. In total, 179 patients were identified with a median age of 72 (IQR 61, 81) and follow-up of 44 days (IQR 42, 45). Forty-one percent were female and the overall mortality was 37%. Twenty-nine percent had HM and of these, those treated with chemotherapy in the preceding 28 days to COVID-19 diagnosis had worse outcome compared with solid malignancy (SM): 62% versus 19% died [HR 8.33 (95% CI, 2.56–25), p < 0.001]. Definite or probable nosocomial SARS-CoV-2 transmission accounted for 16% of cases and was associated with increased risk of death (HR 2.47, 95% CI 1.43–4.29, p = 0.001). Patients with haematological malignancies and those who acquire nosocomial transmission are at increased risk of death. Therefore, there is an urgent need to reassess shielding advice, reinforce stringent infection control, and ensure regular patient and staff testing to prevent nosocomial transmission.
Introduction
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease (COVID-19), emerged in Wuhan, China [Citation1,Citation2]. SARS-CoV-2 has since gone on to cause a global pandemic, which has caused significant disruption to routine healthcare.
Individuals with cancer are more prone to respiratory viruses as a result of immunosuppression from either the underlying disease or therapy. For example, the mortality rate from influenza [Citation3,Citation4], and even rhinovirus, can be increased in certain populations [Citation5]. The mortality rate in cancer patients with COVID-19 is higher than the background population and patients with no history of cancer, when adjusted for other potentially explanatory variables, such as age and comorbidities [Citation6].
As a result, patients receiving chemotherapy for malignancy have been classified as high risk in the UK [Citation7]. However, it is not clear whether the risk is the same across all cancer types. Patients with haematological malignancy, who are usually more immunosuppressed than those with solid organ tumors, may have a higher risk of death from COVID-19. To examine the effect of COVID-19 on our patients, we conducted a multicentre retrospective audit. We examined the difference in outcomes between solid organ and haematological malignancy and the outcome effect of recent chemotherapy treatment, to inform management of cancer patients.
Methods
Study design and participants
This multicentre audit collected data from cancer patients who were hospitalized with COVID-19 between 1 March 2020 and 10 June 2020 across six NHS hospitals in England: The Clatterbridge Cancer Center, Liverpool University Hospitals, Arrowe Park Hospital, Royal Albert Edward Infirmary, The Christie and North Middlesex University Hospital. The mortality in patients with cancer in a large prospective cohort UK study (ISARIC WHO CCP-UK) was 44% [Citation6,Citation8], with which we compared our outcomes. To allow comparison with the CCP-UK patient cohort, we only recorded hospitalized patients with COVID-19. Each centre’s governance team approved the audit. All patients included were SARS-CoV-2 positive as determined by each center’s approved PCR testing protocol – irrespective of signs or symptoms [Citation9].
Data collection
Data were collected locally from patients’ medical records using a standard proforma. We collected age, sex, ethnicity, postcode, cancer history, comorbidities (defined in Supplementary Methods), presenting symptoms, results of radiological examinations (Supplementary Methods), and laboratory results. Details of the COVID-19 episode, duration of hospital admission, and any related complications were also collected. Patients were monitored until discharge, death, or last available follow-up. The final date of data collection was 17 June 2020.
Cancer-specific information
Cancer-related data included date of diagnosis, type of primary cancer, treatment history including nature (surgery, radiotherapy, chemotherapy, endocrine therapy, or immunotherapy), and intent of treatment (induce remission, curative, adjuvant, neoadjuvant, radical, watch and wait, non-curative and palliative) as well as the date of last treatment. Where treatments were halted due to COVID-19 and the patient survived, information relating to whether treatment was restarted was sought. Definitions used have been summarized in Supplementary Methods.
Nosocomial and community-acquired transmission
‘Definite’ nosocomial transmission was a COVID-19 diagnosis made >14 days after admission; ‘probable’ if diagnosis was 8–14 days after admission; ‘indeterminate’ if diagnosis was 3–7 days after admission and ‘community-acquired’ if diagnosis was confirmed <3 days since admission [Citation10].
Severity of illness and mortality rate
Definitions of severity of illness were adapted from other publications [Citation9,Citation11–14]. Disease was ‘mild’ if patients did not require oxygen throughout admission; ‘moderate,’ if the oxygen saturations were recorded to be <93% or they required at least 0.35 FiO2 therapy; and ‘severe,’ if they required intensive care unit (ICU) admission or died. Mortality rate was the proportion of patients who died at any point before final data extraction.
Statistical analyses
Continuous data are presented as median (IQR) and categorical data are reported as frequencies of counts and associated percentages. Differences in the distribution of data between haematological and solid malignancy types were assessed using Wilcox test/t-test for continuous data and Fisher’s/Chi-square test for categorical data. The main outcomes were the mortality rate, which was compared to ISARIC WHO CCP-UK and time-to-death from positive SARS-CoV-2 test. Additional methodology has been summarized in Supplementary Methods document.
Results
Clinical characteristics
In total, 179 SARS-CoV-2 positive cancer patients were identified (). The median age of all cancer patients was 72 (IQR 61, 81), 59% (105 of 179) were male and 82% (75 of 92) were White. The median duration of follow-up was 44 days (IQR 42, 45). Twenty-nine percent (52 of 179 patients) had haematological malignancy (HM) and 71% (127 of 179) had solid malignancy (SM), with malignancy subtypes summarized in . Nineteen percent (30 of 161) had received a cancer diagnosis within 3 months of COVID-19. Forty-eight percent (86 of 179) had received anticancer treatment within 4 weeks of COVID-19 [58% (30 of 52) for HM and 44% (56 of 127) for SM].
Table 1. Baseline characteristics of all patients.
Table 2. Cancer specific information.
There were no significant differences in comorbidity between groups. HM patients came from more deprived areas, with an index of multiple deprivation decile score of 4 (IQR 1.75, 8), compared with a score in SM patients of 3 (IQR 1, 5), p = 0.039.
Nosocomial infection
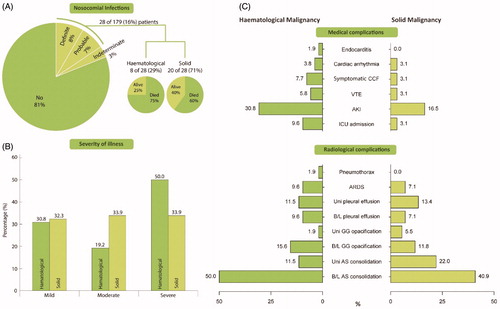
The majority of COVID-19 cases, 81% (145 of 179), were community acquired. Eight percent (15 of 179) of infections were ‘definite’ nosocomial. ‘Probable’ and ‘indeterminate’ cases accounted for 7% (13 of 179) and 3% (6 of 179), respectively (). Together, probable or definite nosocomial infection comprised 16% of cases (29 vs 71% in HM and SM). The mortality of community-acquired COVID-19 was 32% (36 of 145), very similar to that in the indeterminate group of 33% (2 of 6). However, of those with probable nosocomial infection, 69% (9 of 13) died and 60% (9 of 15) with definite nosocomial infection died. In patients with probable or definite nosocomial infection, the mortality rate was 75% in HM and 60% in SM.
Figure 1. Proportions of nosocomial transmission, severity of illness and complications between HM and SM groups. (A) Pie chart with breakdown of patients with nosocomial COVID-19 along with proportion of patients who subsequently died. (B) Bar graph showing severity of illness in HM and SM groups. (C) Differences in complication rate between HM and SM groups, split by ‘medical complications’ and ‘radiological complications.’ CCF: congestive cardiac failure; VTE: venous thromboembolism; AKI: acute kidney injury; ARDS: acute respiratory distress syndrome; B/L: bilateral; Uni: unilateral; GG: ground glass; AS: airspace.
Presenting symptoms of COVID-19
Fever was the commonest presenting symptom (60%, 107 of 179), followed by dry cough (55%, 98 of 179) and shortness of breath (47%, 84 of 179). Respiratory symptoms were the most common set of presenting symptoms (75%, 34 of 179) (Supplementary Figure 1). This was followed by systemic symptoms (71%, 127 of 179) and gastrointestinal (GI) symptoms (21%, 38 of 179). Eleven percent (19 of 179) of patients exhibited all sets of symptoms. In all patients who had GI and respiratory symptoms, systemic symptoms were also present.
Anticancer treatment
Systemic anticancer treatment was administered to 58% (30 of 52) of HM patients and 39% (49 of 127) of SM patients within 4 weeks preceding the COVID-19 episode. Within 4 weeks to 12 months preceding the COVID-19 episode, 14% (18 of 127) of HM patients and 4% (2 of 52) of SM patients received prior treatment, and 9% (12 of 127) vs 11% (6 of 52) received prior therapy more than 12 months of COVID-19 episode in the HM and SM groups, respectively. None of the HM patients underwent prior radiotherapy or surgery with 4 weeks of COVID-19 compared to 7% (9 of 127) of SM patients. All patients receiving anticancer therapy at the time of COVID-19 had their treatment stopped. Of these patients, 27% (8 of 30) and 30% (17 of 56) of HM and SM patients respectively had restarted treatment at the time of the last recorded follow-up. Cancer treatment and the treatment intent are summarized in and Supplementary Table 3.
Laboratory findings and viral load
More patients in the SM group had lymphopaenia with a count <1 × 109/L (78% versus 59% in the HM group, p = 0.003), however median counts were actually lower in HM (Supplementary Table 1). HM patients had both more thrombocytopaenia (62% versus 23% in SM, p < 0.001) and lower counts [116 × 109/L (IQR 80, 228) versus 220 × 109/L (IQR 159, 278) in SM, p = 0.034]. Median hemoglobin was 105 g/dL (IQR 84, 128) in the HM group, compared with 117 g/dL (IQR 101, 132) in the SM group (p = 0.014). There was no significant difference in viral load between HM and SM groups (p = 0.092), or with illness severity or outcome (p = 0.331, Supplementary Table 2), though data were only available for 33% of cases (HM: 50%, 26 of 52 vs SM: 27%, 34 of 127).
Outcomes
Overall 66 patients died (37%), 25 patients with HM and 41 with SM, giving mortality rates of 48% and 32% respectively (p = 0.069). Variables with p < 0.25 in univariable analysis, () were considered as candidates for inclusion in a multivariable Cox regression model and hazard ratios (HR) calculated. In this model, the risk of death was higher in the HM compared with SM group [HR 2.94 (95% CI, 1.59–5.59), p = 0.001, , ].
Figure 2. Overall survival of (A) HM and SM patients; (B) nosocomial COVID-19 transmission (definite or probable) and non-nosocomial infection (community acquired or indeterminate cases); (C) all cancer patients receiving systemic anticancer treatment within 4 weeks of COVID-19 and those who did not and (D) HM and SM patients who received chemotherapy within 4 weeks of COVID-19.
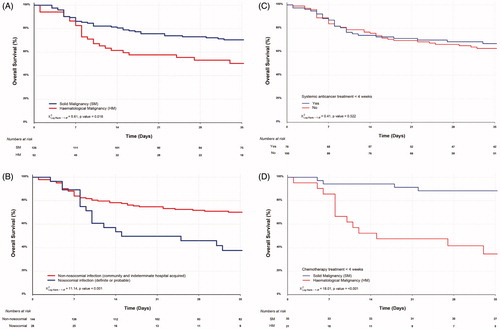
Table 3. Univariable and multivariable analysis of covariates grouped into demographics, cancer type and nosocomial infection, anticancer treatment and investigations with death as an outcome.
Next, we performed sub-group analysis to investigate the potential impact of systemic anticancer treatment on mortality. Forty-four percent (79 of 179) had received systemic anticancer treatment (including chemotherapy, immunotherapy, targeted and/or endocrine therapy) within 4 weeks prior to COVID-19 diagnosis. Of these, 27 died (34%), compared with 39% in those not receiving systemic therapy (p = NS). Although there was no overall effect of chemotherapy on survival (), there was a marked difference seen between HM and SM patients, with HM patients more than twice as likely to die (16 of 30, 53%) then those in the SM group [22% (11 of 49), p = 0.01]. When only patients receiving systemic chemotherapy after exclusion of other systemic anticancer treatment were then analyzed, HM group had an even higher mortality rate of 62% (13 of 21) versus 17% (6 of 36) in the SM group with the risk of death also being much higher [HR 8.33 (95% CI, 2.56–25), p < 0.001]. Two-week survival was 52% (95% CI, 35–79%) in the HM group versus 94% (95% CI, 87–100%) in the SM group ().
A large difference in 14-day survival was also seen in those with nosocomial COVID-19 [53% (95% CI, 38–76%)] than those with non-nosocomial infection [78% (95% CI, 72–85%)] (). Univariable analysis gave a HR comparing the two groups of 2.47 [(95% CI, 1.43–4.29), p = 0.001], although this did not translate into significance in the multivariable model.
Of interest was that ex-smokers appeared to have worse overall survival compared with patients who never smoked and active smokers (Supplementary Figure 2(A)). However, even though this effect was again seen in univariable Cox proportional model for ex-smokers compared with active smokers [HR 3.52 (95% CI, 1.09–11.36), p = 0.035], it was not seen between ex-smokers and nonsmokers (p = 0.386, ).
Furthermore, in the multivariable model, every 1-year increase in age [HR 1.04 (95% CI, 1.01–1.07), p = 0.003] was also seen to be an independent factor associated with increased risk of death. There was a trend toward increasing CRP level being associated with increased risk of death which did not reach statistical significance [HR 1.34 (95% CI, 1.00–1.80), p = 0.052, also see Supplementary Figure 2(B)].
Radiological imaging
Chest radiograph was performed in all but one asymptomatic patient, and 13% (23 of 179) underwent computerized tomography (CT) of the thorax. Bilateral airspace consolidation was the commonest finding with 51% (26 of 51) and 41% (52 of 127) reported in HM and SM groups, respectively (). Unilateral pleural effusion (11.5 vs 13.4% for HM and SM) was seen more frequently than bilateral pleural effusions (9.6 vs 7.1% for HM and SM). ARDS was seen in 9.6 and 7.1% of HM and SM cases, with pneumothorax being a rare finding.
Medical complications during COVID-19 admission
Medical complications during the COVID-19 admission occurred in 28% (51 of 179) of all cancer patients. Acute kidney injury was the commonest complication in cancer patients occurring in 21% (37 of 179). For patients who developed AKI compared with those who did not, the HR was 2.26 (95% CI, 1.34–3.82, p = 0.002). Symptomatic CCF and VTE occurred in 4.5% (8 of 179) and 4% (7 of 179) of cancer patients respectively with breakdown shown between HM and SM in .
Hospital stay and discharge
Fifty percent (26 of 52) of HM patients were discharged compared to 68% (86 of 127) SM patients at the time of last follow-up. The median hospital stay for patients who were discharged was 8 (IQR 3, 14) days whereas the median hospital stay for patients who died was 9 (IQR 5.5, 16) days. When comparing HM and SM groups, the median follow-up time, estimated using the reverse Kaplan–Meier method was 42 (IQR 38, 46) and 45 (IQR 42, 46) days, respectively.
Discussion
Patients with cancer appear to have a higher infection rate with SARS-CoV-2 than the general population [Citation12,Citation14–18], and also higher mortality [Citation6]. In our multicentre audit, the mortality rate was 37% amongst all cancer patients. This compares with a 44% hospital mortality overall in the ISARIC WHO CCP-UK study [Citation6,Citation8] which is the key national characterization protocol for the SARS-CoV-2 pandemic within the UK. This supports the notion that cancer patients with COVID-19 have worse outcomes compared to those without cancer [Citation11–14,Citation17,Citation19–25], although this is not confirmed in all studies [Citation26,Citation27]. Here, we report a significantly higher mortality rate for patients with haematological malignancy (HM, 48%) than with solid malignancy (SM, 32%), also seen in other studies [Citation11,Citation23,Citation28]. Other haematological case series report a high mortality in HM patients of 35.9% [Citation25] and 33% in a study focused on patients with chronic lymphocytic leukemia [Citation24].
In our cohort, the overall nosocomial infection rate was 16%. The outcome from nosocomial infection was markedly worse than from community-acquired infection. Although this difference was not maintained in multivariable analysis, this does not detract from the importance of this result. The reason that nosocomial infection does not remain significant in multivariable analysis is simply that it is likely to be a marker for patients who have a high degree of healthcare interaction, and either have significant co-morbidities, advanced cancer, are immunocompromised from their treatment, or combination of these factors. It is therefore likely that the patient population accounts for this and not the location of SARS-CoV-2 acquisition. This result indicates that the in-patient cancer population is extremely vulnerable to the effects of COVID-19, and underlines the need to avoid nosocomial transmission to these patients at all costs.
Systemic treatments for cancer are heterogeneous, ranging from endocrine therapy right through to high dose chemotherapy. In our cohort, when HM and SM patients were compared, marked differences were seen. The administration of systemic therapy increased the risk of death from COVID-19, whereas in SM patients it appeared to be protective. Given the particular effects of chemotherapy a further comparison was performed where an even greater difference in mortality between HM and SM was seen (62 vs 17%, p < 0.001). The worse outcomes observed in HM patients are likely due to the fact they are severely immunocompromised – the resultant effect of the underlying haematological malignancy and systemic chemotherapy. It is however unclear why outcome improves in the counterpart group of SM patients receiving chemotherapy. Possibilities include: i) greater marrow reserve, ii) differences in the use of granulocyte colony-stimulating factors and iii) chemotherapy-induced reduction in the neutrophil to lymphocyte ratio, leading to improved outcomes, as previously suggested [Citation19].
While a number of studies have reported no impact of chemotherapy on outcome [Citation21,Citation22,Citation28], there are others who have reported otherwise. For example, Zhang et al. [Citation13] reported that anticancer treatment within 14 days of COVID-19 increased the risk of severe events. Similarly, Lee et al. showed that patients with HM receiving recent chemotherapy had worse outcomes, supporting the findings of our study [Citation28,Citation29]. Chemotherapy appeared to be protective in patients with solid organ malignancies, however, perhaps because this delineates a group of patients who are fitter than average, by virtue of being fit enough to receive chemotherapy. Finally, it must be acknowledged that the difference in the absolute number of deaths between these groups in this cohort is small, so these findings may be due to chance alone.
Interestingly, ex-smokers appeared to have an improved survival compared to nonsmokers as suggested in previous reports [Citation21,Citation30]. The reasons for this remain unclear and require further detailed exploration. One possibility is that COPD predominantly affects smokers so these patients receive steroids, which are now known to improve outcome in COVID-19 [Citation31].
Various limitations exist in our study. Firstly, although this is one of the largest cancer cohorts reported with COVID-19, the numbers remain small to infer definite conclusions and therefore larger studies will be required to confirm our findings. Secondly, the retrospective design of the study limited the quality of data collected. Thirdly, an attempt was made to standardize definitions used, however, due to variation in practices across England, complications may have been coded differently in various hospitals. Fourthly, data were incomplete in some cases, further affecting the interpretability of results. Due to the small numbers, subgroup analysis was limited. Since there is no real-time capture of data, current studies are affected by issues of selection and recall bias. Furthermore, absence of non-cancer controls prevents stratification of patients which is important to adjust for unmeasured confounders.
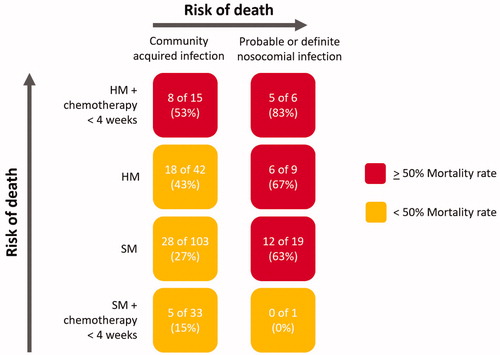
We have observed that appreciable mortality exists in this cohort of UK cancer patients hospitalized with COVID-19. Patients with haematological malignancies are at the highest risk of death within this group, either because of the immunosuppressive effects of cytotoxic chemotherapy, their underlying disease or both. Nosocomial infection carries a high mortality, especially in those who have HM and have received chemotherapy recently () not because of where infection takes place, but most likely because the patients who acquire infection in hospital represent a particularly vulnerable group. Preventing in-hospital transmission to highly vulnerable patients should be a priority for care providers going forward into the first full winter season since the emergence of COVID-19 in the UK. Potential measures include ‘clean’ sites where cancer patients can be admitted away from patients being cared for with COVID-19, the use of dedicated teams for the most vulnerable patients, regular testing of staff, and strict adherence to hospital infection control measures. Further work is now underway to understand the impact of COVID-19 in cancer patients in the UK when controlled for key factors including age and ethnicity.
Figure 3. Risk of death relating to nosocomial infection, type of malignancy and whether chemotherapy was received within 4 weeks of COVID-19. The color codes signify the risk of death. HM: haematological malignancy; SM: solid malignancy. HM and SM groups refer to all patients who did not receive chemotherapy within 28 days of COVID-19.
Acknowledgments
All authors approved the manuscript.
GLAL-2020-1384-File004.docx
Download MS Word (17.2 KB)GLAL-2020-1384-File003.docx
Download MS Word (2.6 MB)Disclosure statement
None to be declared related to the study. RL: Speaker fees BMS and Astrazeneca; AA: spouse shares in Astra Zeneca, Merck paid conference fee; CP: grant funding from Pfizer and Daiichi Sankyo and honoraria from Pfizer, Roche, Daiichi Sankyo, Exact sciences and Eli Lilly.
Additional information
Funding
References
- Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269.
- Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. bioRxiv. 2020:2020.02.07.937862.
- Chemaly RF, Vigil KJ, Saad M, et al. A multicenter study of pandemic influenza A (H1N1) infection in patients with solid tumors in 3 countries: early therapy improves outcomes. Cancer. 2012;118:4627–4633.
- Dignani MC, Costantini P, Salgueira C, et al. Pandemic 2009 Influenza A (H1N1) virus infection in cancer and hematopoietic stem cell transplant recipients; a multicenter observational study. F1000Res. 2014;3:221.
- Campbell AP, Guthrie KA, Englund JA, et al. Clinical outcomes associated with respiratory virus detection before allogeneic hematopoietic stem cell transplant. Clin Infect Dis. 2015;61:192–202.
- Docherty AB, Harrison EM, Green CA, et al., ISARIC4C investigators. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985.
- Gov.uk. Guidance on shielding and protecting people who are clinically extremely vulnerable from COVID-19. 2020. Available from: https://www.gov.uk/government/publications/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19
- Palmieri C, Palmer D, Openshaw PJ, et al. Cancer datasets and the SARS-CoV-2 pandemic: establishing principles for collaboration. ESMO Open. 2020;5:e000825.
- Organization WH. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf?sfvrsn=bc7da517_2
- NHS England. Letter: Interim data collection – hospital-onset COVID-19. London (UK): NHS England and NHS Improvement; 2020. Publications approval reference: 001559.
- Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791.
- He W, Chen L, Chen L, et al. COVID-19 in persons with haematological cancers. Leukemia. 2020;34:1637–1645.
- Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901.
- Yu J, Ouyang W, Chua MLK, et al. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6:1108–1110.
- El-Sharif A, Elkhatib WF, Ashour HM. Nosocomial infections in leukemic and solid-tumor cancer patients: distribution, outcome and microbial spectrum of anaerobes. Future Microbiol. 2012;7:1423–1429.
- Kamboj M, Sepkowitz KA. Nosocomial infections in patients with cancer. Lancet Oncol. 2009;10:589–597.
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337.
- Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069.
- Lee LYW, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926.
- Malard F, Genthon A, Brissot E, et al. COVID-19 outcomes in patients with hematologic disease. Bone Marrow Transplant. 2020;55:2180–2184.
- Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918.
- Pinato DJ, Zambelli A, Aguilar-Company J, et al. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov. 2020;10:1465–1474.
- Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904–913.
- Mato AR, Roeker LE, Lamanna N, et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136:1134–1143.
- Sanchez-Pina JM, Rodríguez Rodriguez M, Castro Quismondo N, et al. Clinical course and risk factors for mortality from COVID-19 in patients with haematological malignancies. Eur J Haematol. 105:597–607.
- Miyashita H, Mikami T, Chopra N, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;31:1088–1089.
- Joharatnam-Hogan N, Hochhauser D, Shiu KK, Rush H, Crolley V, Butcher E, et al. Outcomes of the 2019 Novel Coronavirus in patients with or without a history of cancer – a multi-centre North London experience. medRxiv. 2020:2020.04.16.20061127.
- Lee LYW, Cazier JB, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309–1316.
- Montopoli M, Zumerle S, Vettor R, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N= 4532). Ann Oncol. 2020;31:1040–1045.
- Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436.
- Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. 2020:2020.06.22.20137273.
