Abstract
The Swedish national guidelines for treatment of acute myeloid leukemia (AML) recommend analysis of measurable residual disease (MRD) by multiparameter flow cytometry (MFC) in bone marrow in the routine clinical setting. The Swedish AML registry contains such MRD data in AML patients diagnosed 2011–2019. Of 327 patients with AML (non-APL) with MRD-results reported in complete remission after two courses of intensive chemotherapy 229 were MRD-negative (70%), as defined by <0.1% cells with leukemia-associated immunophenotype in the bone marrow. MRD-results were reported to clinicians in real time. Multivariate statistical analysis adjusted for known established risk factors did not indicate an association between MFC-MRD and overall survival (HR: 1.00 [95% CI 0.61, 1.63]) with a median follow-up of 2.7 years. Knowledge of the importance of MRD status by clinicians and individualized decisions could have ameliorated the effects of MRD as an independent prognostic factor of overall survival.
Introduction
The survival of patients with acute myeloid leukemia (AML) has increased over time [Citation1]. This is partly due to improvements in supportive care and the increased use of allogeneic hematopoietic stem cell transplantation (allo-SCT) [Citation2,Citation3]. Approximately 80–90% of younger (<65 years) and 50% of older (>70 years) AML patients eligible to receive intensive chemotherapy attain complete remission [Citation1,Citation2]. However, the relapse rate is high, and survival after relapse is limited [Citation3].
In AML, acquired cytogenetic abnormalities and gene mutations defined at diagnosis influence treatment strategies and outcomes. Genetic risk classification was established more than twenty years ago as a strong prognostic marker and has guided the use of allo-SCT as part of the primary treatment [Citation4]. Recently, several genetic markers have been added to the MRC risk classification by the European LeukemiaNet (ELN) to improve the assessment of risk of relapse and death [Citation5].
Assessment of measurable residual disease (MRD) has the potential to improve the prediction of relapse-free survival (RFS) and overall survival (OS) in AML [Citation6]. A recent meta-analysis provided further evidence of an association between pre-allo-SCT MRD and post-transplant relapse and survival [Citation6]. MRD can be detected using multiparameter flow cytometry (MFC) and molecular techniques, including real-time quantitative PCR (RT-qPCR), digital PCR, and next-generation sequencing–based technologies [Citation5]. These methods differ in sensitivity and applicability depending on the specific immunophenotypical and genetic features of the targeted leukemia [Citation5]. MFC-MRD has lower sensitivity than RT-qPCR but has the advantage to be applicable in the majority of all AML patients in contrast to RT-qPCR [Citation5]. MRD is increasingly analyzed in clinical practice although firm guidelines regarding its implementation in treatment decisions for AML patients are yet to be established [Citation3,Citation7].
Since 2012, the Swedish national guidelines for AML [Citation8] recommend the assessment of MRD by MFC following two courses of intensive chemotherapy treatment and after completed consolidation. The MRD analysis is performed at five experienced university hospital laboratories across Sweden and the MRD results have been reported to the Swedish AML Registry regularly since 2013. We here report the first analysis on the relationship between MRD and OS in a population-based setting using an extended Cox regression model adjusted for well-established risk factors.
Material and methods
Study population
This analysis of registry data was performed according to the Ethics Review Committee decision in Lund (Dnr 2015/260). Data was extracted on 20th Feb 2020 from the national Swedish AML Registry, with an overall coverage of 98% of adult patients with AML and complete survival follow-up [Citation1,Citation9]. In this study, we included records from patients who were in CR1 after two courses of intensive chemotherapy, and then had a valid MRD assessment, as recommended in the Swedish guidelines. In total, MRD results were available for 455 patients diagnosed with AML between August 2011 and August 2019 (), among which 327 (72%) within the time frame recommended by the guidelines. The measurements of MRD were performed within the routine clinical practice, with a median time of 68 days from diagnosis to MRD sampling (range 47–114 days). The median follow-up was 2.7 years.
Figure 1. Study disposition including statistically analyzed MRD groups (MRD: measurable residual disease).
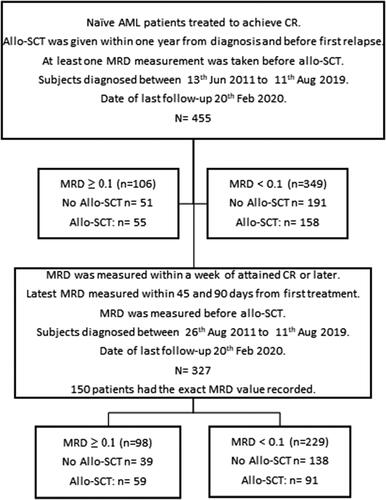
Patients received treatment according to the Swedish national AML guidelines [Citation8], which were established in 2005 and updated regularly, but hitherto without major changes in treatment recommendations. Intensive chemotherapy for AML (excluding acute promyelocytic leukemia [APL]) is recommended for most patients up to age 75–80 years [Citation10]. This treatment consists of cytarabine 1 g/sqm/2h b.i.d. for five days and daunorubicin 60 mg/sqm/8h for three days. Most patients are eligible to receive consolidation chemotherapy after recovery with identical drugs and dosages. Early intensification with a second identical induction course given before recovery from course #1 was an option for some patients judged to be at high risk. In some cases, patients were switched to alternate intensive treatment, mostly FA-Ida (fludarabine, cytarabine and idarubicin) or ACE (amsacrine, cytarabine, etoposide) [Citation8]. Most patients had one or two further consolidations including intermediate dose cytarabine. Allo-SCT was considered as consolidation in first complete remission (CR1) if feasible for patients with intermediate or adverse risk, but patients without FLT3-ITD and sustained MRD-negative complete remission could be followed without allo-SCT, considering age and comorbidities.
The Swedish national guidelines since 2012 recommend collecting MRD data after the second course of intensive chemotherapy for all patients similar to other international guidelines [Citation3,Citation7]. MRD results were always available to clinicians in real-time. The guidelines state that MRD-positivity is a risk factor for relapse, but in high-risk patients the decision to perform allo-SCT should not be influenced by MRD. Furthermore, there is a general recommendation to proceed to allo-SCT also in MRD-negative intermediate-risk patients if the patients do not have other risk factors of treatment-related mortality and have a suitable HLA-matched donor [Citation5]. The Swedish national guidelines were recently updated (April 2019) recommending routine collection of molecular markers for MRD when feasible, such as with CBF and NPM1-mutated AML but this did not influence the outcome of this specific study.
Analysis of MRD
Analysis of MRD was performed on bone marrow aspirates using MFC according to the methodological guidelines implemented in the pediatric NOPHO-DBH AML-2012 study (EudraCT 2012-002934-35). Analyses were performed in a harmonized fashion in five university hospital laboratories using either the 8-color panel for FACSCantoII (Becton Dickinson) as previously described [Citation11], or a modified 10 color panel containing the same antigens for use on Navios (Beckman Coulter). All involved laboratories participated in digital quality control ring trials performed nationally and/or within NOPHO, and in regular educational meetings for quality assurance. MRD positivity was in this analysis defined as at least 0.1% cells with leukemia-associated immunophenotype (LAIP) described at diagnosis, which was specifically reported into the AML registry.
Statistical analyses
The association of MRD with OS was investigated using an extended Cox regression model. A directed acyclic graph was defined to determine the minimum set of variables to be included to reduce confounding. For classification of patients based on allo-SCT, we only considered allo-SCT performed in CR1. Allo-SCT was modeled as a time dependent covariate. Interactions between allo-SCT and MRD, MRD and type of AML, and MRD and genetic risk classification were also included in the model. To minimize overfitting, variable selection was performed using a priori defined priority order of variables. The highest priority was given to AML type, age, sex, allo-SCT, MRD, genetic risk, and the interaction terms. Surviving patients were censored at the date of last follow-up regardless of treatment received. Relapse-free survival (RFS), defined as the time from diagnosis to reported first relapse or death due to any cause, whichever occurs first, was analyzed in the same manner as OS. The fulfillment of the proportional hazard assumption was assessed visually by log-log plots. The goodness-of-fit was investigated using the Cox-Snell residuals. Formal test for goodness-of-fit was not applied since they are not expected to have enough power to be informative in our study [Citation12]. Several sensitivity analyses for OS were performed to assess the robustness of the model estimates and are results are shown in Supplementary Appendix. A subset of 150 patients whose MRD measurements were collected as recommended in the national Swedish guidelines also had a report on the exact MRD level. Therefore, we performed a supplementary analysis using the exact MRD value as a covariate in the model. The analysis was performed in a similar manner as the primary analysis. To investigate the effect of the timeframe selected for finding MRD measurements fulfilling the guidelines request, we also analyzed change of sampling time frame from 45–90 days to 45–60 days (n = 138) and to 45–180 days (n = 408). We also investigated the effect of transplant-related mortality by censoring patients who received allo-SCT at the transplantation date. The confidence intervals were calculated using bootstrapping. For the calculation of Kaplan–Meier curves, allo-SCT was modeled as a time-dependent covariate [Citation13]. Cumulative incidence curves calculated using the Cox models are shown in Supplementary Appendix (Figures A2 and A3).
Sample size calculations were not performed for this study. The width of the confidence intervals is used as an estimate of precision. The statistical significance level was set to 0.05. The objectives of the study were exploratory and therefore correction for multiple comparisons was not implemented. The statistical analyses were performed using Stata IC 14.2 software (StataCorp LLC, College Station, TX, USA).
Results
Demographics and baseline characteristics for the 327 patients with MRD data collected according to the Swedish guidelines are listed in . The median age at allo-SCT in CR1 was 57 years (range 18–72 years). Overall, MRD-positive patients had similar clinical characteristics as compared to MRD-negative patients. Patients diagnosed during the same time period but with MRD-results sampled outside our studied time interval had similar characteristics as those with MRD-results within the time period. A higher proportion of patients in the MRD-positive group received allo-SCT than in the MRD-negative group.
Table 1. Demographics and baseline disease characteristics at diagnosis for all 455 AML patients with available MRD data.
Treatment outcomes are shown in . In Table A6 in Supplementary Appendix, treatment outcomes for intermediate risk patients are shown. The proportion of patients who died was around 30% for both MRD-positive and negative patients. Kaplan–Meier survival curves for OS were calculated for MRD-positive and negative patients who received vs did not receive allo-SCT (). Patients who received allo-SCT had the best survival. The Kaplan–Meier curves for MRD-positive and negative patients who received allo-SCT overlapped, as did the curves among patients who did not receive allo-SCT in CR1.
Figure 2. Kaplan–Meier curve for overall survival for patients with measurable residual disease as determined by MFC collected according to the Swedish AML guidelines (Allo-SCT: allogeneic stem cell transplantation; MRD: measurable residual disease; OS: overall survival).
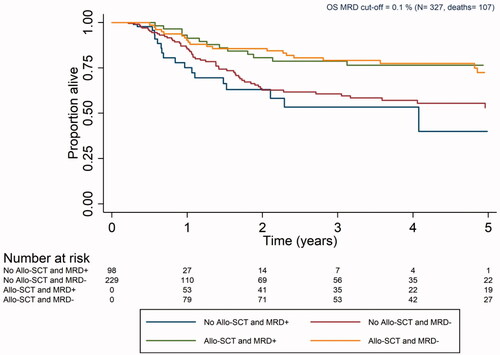
Table 2. Treatment outcomes.
Cox model results for OS are listed in and showed expected findings. Higher age and diabetes were associated with poor survival. Patients with intermediate and adverse genetic risk had approximately two- and fourfold higher hazard than patients with favorable risk, respectively. Patients with de novo AML had better survival than patients with AML secondary to antecedent hematologic disease, mainly myelodysplastic syndrome or myeloproliferative neoplasia (s-AML) and therapy-related AML (t-AML). However, MRD was not associated with overall survival (HR: 1.00, 95% CI [0.61, 1.63], p value 0.982). The results from the sensitivity analyses were concordant with the results obtained for OS and are shown in Supplementary Appendix (Table A3). A Cox model for RFS was also calculated ( and ) and the results were comparable to those obtained for OS.
Figure 3. Kaplan–Meier curve for relapse-free survival for patients with measurable residual disease as determined by MFC collected according to the Swedish AML guidelines (Allo-SCT: allogeneic stem cell transplantation; MRD: measurable residual disease; RFS: relapse-free survival).
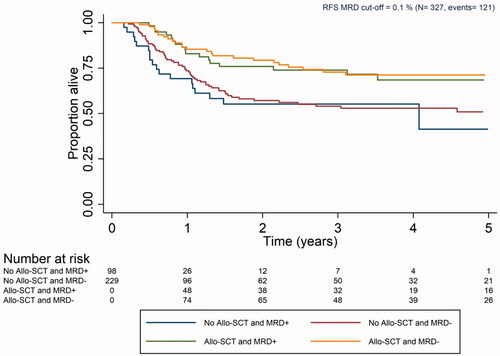
Table 3. Cox Model for overall survival (OS) and relapse-free survival (RFS) with bootstrap 95% CI for patients with MFC MRD collected according to the Swedish AML guidelines.
Discussion
Measurable residual disease has the potential to provide information about the risk of relapse and death in AML [Citation2,Citation3,Citation14,Citation15]. However, it remains unclear how MRD should be analyzed and interpreted in order to guide therapeutic decisions [Citation3]. Uncertainties include which patient category should be analyzed, the time point for sampling, if single or multiple samplings are required, use of blood versus bone marrow, which technique is optimal, which sensitivity is required, if there is added value in relation to other prognostic markers, and most importantly how we should use the MRD results to guide clinical decisions.
We here investigated the impact of MFC-MRD from the bone marrow collected at a predefined clinical timepoint, as recommended in the Swedish national AML guidelines. In this study, approximately two-thirds of the patients in CR1 were MRD-negative after the second cycle of intense chemotherapy. This is in line with what has been observed in recently reported studies [Citation16,Citation17] To assess the association of MRD with survival, we implemented an extended Cox regression model adjusted for well-established risk factors. Some of the results are concordant with those reported earlier in the literature [Citation6,Citation14,Citation16]. Hence, we found that age, AML type, genetic risk and diabetes all were associated with survival [Citation2,Citation18,Citation19]. However, the main finding was that our model failed to show that MRD is associated with survival when MRD is assessed in bone marrow aspirates by MFC after two cycles of intense chemotherapy in a ‘real life’ clinical setting. The results obtained for RFS was similar to the findings of the OS analyses.
A recent meta-analysis indicates that MRD-negativity is associated with superior survival [Citation15]. The studies included in the meta-analysis did not prospectively adjust consolidation therapy based on MRD-status [Citation15]. We hypothesize that the lack of observed association between MRD and OS in our study is both related to patient and leukemia characteristics, and to the implementation of MRD-information during the planning of subsequent treatment, which was generally based on the national guidelines. We noted that MRD-positive patients received allo-SCT more often than MRD-negative patients during the whole study period. We observed, as expected, that there was a larger proportion of intermediate and high genetic risk among MRD-positive patients (87% vs 72%), which partially explain the larger proportion of allo-SCT patients in this group. Other factors than genetic risk were also expected to be considered during the evaluation of the feasibility for allo-SCT. Patients eligible for allo-SCT are in general younger, are sufficiently fit to tolerate the procedure and have an acceptable control of the disease. These latter factors are positively associated with survival. Although the use of MFC-MRD results were defined in the guidelines as an indication for allo-SCT, it is likely that the treating physician and local transplantation team considered MRD-results during treatment planning and made an individual decision for every patient. MRD-positivity after induction and/or consolidation could support intensified treatment, such as adding one or more cycles of chemotherapy; however, the number of courses did not differ between the groups. They may also have decided to perform allo-SCT more often in MRD-positive patients to overcome the putative negative effects of an MRD-positive specimen. MRD-positivity could also be an indication for fully ablative conditioning before allo-SCT rather than reduced intensity conditioning [Citation20], although the choice between myeloablative versus reduced conditioning mostly has been based on age and comorbidities. There is also a possibility that in some cases with MFC-MRD-negativity the decision to proceed to allo-SCT was abandoned because of acquired toxicities of intensive chemotherapy, patient´s refusal or lack of suitable stem cell donor. Whether MRD was used to support clinical decisions was not recorded in the registry. This effect created an imbalance between the MRD positive and negative groups that cannot be modeled in the analysis and may have caused the lack of statistical significance of the association between MRD and OS.
The European Society for Medical Oncology (ESMO) recently updated their AML treatment guidelines [Citation3], which now recommend that AML patients in CR with ELN favorable-risk should be consolidated with chemotherapy without allo-SCT, while patients in CR with ELN intermediate- or adverse-risk should undergo allo-SCT, if feasible [Citation3]. We observed that patients who received allo-SCT and were MRD-positive benefited from allo-SCT regardless of their genetic risk. Randomized trials would be needed to investigate whether allo-SCT could prolong survival in eligible patients regardless of their MRD status or risk classification, but such studies are difficult to perform. Venditti et al [Citation21] presented the results from the GIMEMA AML1310 trial where intermediate risk AML patients were to receive autologous stem cell transplantation (auto-SCT) or allo-SCT depending on the MRD level. The authors found no significant difference in 2-year OS between MRD negative (79% [95% CI 66–94]) and MRD positive (70% [95% CI 57–86], p value 0.713) patients [Citation21]. It also remains unproven whether additional therapy, e.g. hypomethylating agents with or without venetoclax, with the aim to transform MRD-positivity to negativity before allo-SCT would be beneficial.
Our study has strengths and limitations. We have a representation of AML patients treated in a routine clinical setting. The patients were treated uniformly according to national guidelines. Therefore, our results reflect the outcomes of typical AML patients who attain CR1 within two cycles of induction in real-life clinical practice. We analyzed overall survival, which is a well-defined endpoint. Survival follow-up was complete due to the direct connection with the national population database, and the median follow-up of surviving patients was 2.7 years, which is likely to cover most events [Citation1].
We acknowledge that the interpretation of our results remains difficult, partly due to the unavoidable heterogeneity in a population-based study, and the lack of valid control groups. We recently published data on improved outcomes in the recent decade [Citation1], with the assumption that increased allo-SCT rates and better supportive care would have contributed to the improvement, as well as MRD assessments. Therefore, a comparison of the observed survival time to that of historical controls would be difficult to interpret. Despite national sampling of data for eight years we did not achieve sufficient sample size to assess impact of MRD in specific disease subsets. MRD was measured at slightly different time points, but sensitivity analyses yielded overall similar results irrespective of time points between our limits. Mechanisms behind the collection of MRD values outside the timeframe recommended in the guidelines remain uncertain. We are concerned that patients with poorer prognostic characteristics may be over-represented in those without MRD results, which hampers the comparison of survival between patients with or without MRD measurements. However, the clinical characteristics of patients without reported MRD data according to the guidelines seemed comparable to the study group. The data was collected during several years, and changes in the definition of genetic risk could cause misclassification in some patients, since a reclassification according to the ELN 2017 definition was not doable. We incorporated information about available main clinical and demographical characteristics, but there might well be significant differences in medical and health-related issues between MRD-positive and negative patients not possible to consider in our model. Finally, several laboratories did perform the analyses, but the method across laboratories was harmonized. From April 2019, monitoring of NPM1-mutations and core binding factor-leukemia was initiated, but these changes are not likely to have had significant impact on the currently studied patient population.
In summary, we investigated the association between MRD and survival in Swedish AML patients treated in clinical practice. MRD analyses were performed with MFC after two courses of intensive chemotherapy and results were available to clinicians in real time. We observed that MRD-negative patients received allo-SCT in CR1 to a lesser extent than MRD-positive patients. Our analyses do not indicate that MRD was associated with survival. However, the access to MRD-results in real-time is likely to support medical decisions on further AML treatment, and thus may have influence on the subsequent overall survival.
Author contributions
AR, VL, and GJ designed the study and wrote the manuscript. AR performed the statistical analyses. All hematology departments in Sweden provided clinical data. All authors critically reviewed and approved the manuscript.
GLAL-2020-1593-File008.docx
Download MS Word (43.7 KB)Acknowledgments
The Swedish AML Registry is supported by the Swedish Association of Local Authorities and Regions, Region Skåne and Regional Cancer Center South.
Disclosure statement
The authors declare no competing financial interests.
References
- Juliusson G, Hagberg O, Lazarevic VL, et al. Improved survival of men 50 to 75 years old with acute myeloid leukemia over a 20-year period. Blood. 2019;134:1558–1561.
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136–1152.
- Heuser M, Ofran Y, Boissel N, et al. Acute myeloid leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:697–712.
- Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 Trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92:2322–2333.
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447.
- Buckley SA, Wood BL, Othus M, et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: a meta-analysis. Haematologica. 2017 May;102:865–873.
- Schuurhuis GJ, Heuser M, Freeman S, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018;131:1275–1291.
- Knowledge bank for cancer treatment - Regionala cancercentrum i samverkan. 2020. [cited 2020 Sep 23]. Available from: https://kunskapsbanken.cancercentrum.se/diagnoser/aml/
- Juliusson G, Antunovic P, Derolf Å, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187.
- Juliusson G, Billström R, Gruber A, et al., for the Swedish Adult Acute Leukemia Registry Group. Attitude towards remission induction for elderly patients with acute myeloid leukemia influences survival. Leukemia. 2006;20:42–47.
- Delsing Malmberg E, Rehammar A, Pereira MB, et al. Accurate and sensitive analysis of minimal residual disease in acute myeloid leukemia using deep sequencing of single nucleotide variations. J Mol Diagn. 2019;21:149–162.
- Grant S, Chen YQ, May S. Performance of goodness-of-fit tests for the Cox proportional hazards model with time-varying covariates. Lifetime Data Anal. 2014;20:355–368.
- Benichou J. A review of adjusted estimators of attributable risk. Stat Methods Med Res. 2001;10:195–216.
- Jongen-Lavrencic M, Grob T, Hanekamp D, et al. Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med. 2018;378:1189–1199.
- Short NJ, Zhou S, Fu C, et al. Association of measurable residual disease with survival outcomes in patients with acute myeloid leukemia: a systematic review and meta-analysis. JAMA Oncol. 2020;6:1890.
- Terwijn M, van Putten WL, Kelder A, et al. High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: data from the HOVON/SAKK AML 42A study. J Clin Oncol. 2013 Nov 1;31:3889–3897.
- Freeman SD, Hills RK, Virgo P, et al. Measurable residual disease at induction redefines partial response in acute myeloid leukemia and stratifies outcomes in patients at standard risk without NPM1 mutations. J Clin Oncol. 2018;36:1486–1497.
- Deschler B, Lubbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107:2099–2107.
- Ali NA, O'Brien JM, Jr Blum W, et al. Hyperglycemia in patients with acute myeloid leukemia is associated with increased hospital mortality. Cancer. 2007;110:96–102.
- Hourigan CS, Dillon LW, Gui G, et al. Impact of conditioning intensity of allogeneic transplantation for acute myeloid leukemia with genomic evidence of residual disease. J Clin Oncol. 2020;38:1273–1283. 20
- Venditti A, Piciocchi A, Candoni A, et al. GIMEMA AML1310 trial of risk-adapted, MRD-directed therapy for young adults with newly diagnosed acute myeloid leukemia. Blood. 2019;134:935–945.
