Abstract
The liver plays crucial roles in material metabolism and immune response. Bacterial endotoxin can cause various liver diseases, thereby causing significant economic losses to pig industry. Tryptophan is an essential amino acid in piglets. However, whether tryptophan can alleviate liver injury and inflammation by regulating necroptosis and pyroptosis has not been clarified. This study aimed to investigate whether dietary tryptophan can alleviate lipopolysaccharide (LPS)-induced liver injury in weaned piglets. 18 weaned piglets were randomly distributed to three treatments, each with 6 replicates: (1) control; (2) LPS-challenged control; (3) LPS + 0.2% tryptophan. After feeding with control or 0.2% tryptophan-supplemented diets for 35 d, pigs were intraperitoneally injected with saline or LPS (100 mg/kg body weight). At 4 h post-injection, blood samples and liver were collected. Results indicated that tryptophan reduced alanine aminotransferase, aspartate aminotransferase, decreased the mRNA expression and protein expression of 70-kDa heat shock proteins. Moreover, tryptophan increased the mRNA expression and protein expression of claudin-1, occludin and zonula occludens and decreased hydrogen peroxide and malondialdehyde contents, and increased catalase, glutathione peroxidase and total superoxide dismutase activities and proinflammatory cytokine levels in the liver. Meanwhile, tryptophan inhibited pyroptosis-related and necroptosis-related protein expression in liver. Collectively, tryptophan could relieve liver damage, increased the antioxidant capacity and reduced inflammation by inhibiting pyroptosis and necroptosis signaling pathways.
Introduction
The liver plays a crucial role in material metabolism and immune response.Citation1 Inflammatory responses and oxidative stress can act on the liver and lead to liver damage.Citation2 Lipopolysaccharides (LPS), potent endotoxins produced by Gram-negative bacteria, can induce inflammatory response and oxidative stress by stimulating Kupffer cells in the liver and damage the parenchymal structure of the liver, ultimately leading to liver damage.Citation3 Therefore, inhibiting inflammatory response and oxidative stress in the liver is an effective measure to treat liver injury.Citation4
Tryptophan ensures normal growth and development in pigs.Citation5 Tryptophan and its metabolites can regulate animal reproduction, immunity, feed intake, neurological function and stress tolerance. Inflammatory response is harmful to animal health.Citation6 Tryptophan has exerted inflammatory effects in the body.Citation7,Citation8 Therefore, it may have a protective effect on liver and alleviate liver damage. Intraperitoneal injection of tryptophan alleviates chronic liver injury in rats.Citation9 Ohta et al. reported that melatonin, a metabolite of tryptophan, alleviates acute liver injury through antioxidative effects.Citation10 Another study reported that oral tryptophan alleviates liver toxicity caused by carbon tetrachloride and protects the liver.Citation11 However, the exact molecular mechanisms by which tryptophan relieves oxidative stress and inflammatory responses, and protects the liver are unclear.
Necroptosis is a newly discovered form of cell death that depends on kinase receptor-interacting protein (RIP) 1 and the kinase receptor-interacting protein 3 (RIP3) and has necrotic and apoptotic characteristics. Necroptosis can cause a variety of liver diseases and necroptotic cells strictly follow the regulation of intracellular signals,Citation12 morphologically manifested as cell swelling, mitochondrial dysfunction, burst-like rupture of cell membranes, release of cellular contents, and a peripheral inflammation.Citation13 The binding of RIP1 to FAS-associated death domain (FADD) enhances caspase-8 activation.Citation14 When the activity of caspase-8 is reduced, necroptosis is activated and RIP1 binds to RIP3 to produce necrosomes and then induces the autophosphorylation of RIP3. Activated RIP3 recruits and phosphorylates mixed lineage kinase domain-like (MLKL) to trigger necroptosis.Citation15 RIP3 can interact with phosphoglycerate mutase 5 (PGAM5) to promote MLKL phosphorylation.Citation16 Necroptosis ultimately causes the release of intracellular inflammatory damage-associated molecular patterns (DAMPs), which promote the occurrence of inflammatory responses and lead to tissue damage.Citation17 However, whether tryptophan supplementation can alleviate liver injury and inflammation by regulating necroptosis has not been clarified.
Pyroptosis is a caspase-dependent form of proinflammatory programmed cell death that increases cell permeability and promotes the swelling and rupture of cell membranes and release of intracellular proinflammatory factors.Citation18 During pyroptosis, intracellular proinflammatory cytokines including interleukin-1β (IL-1β) and IL-18, are released, and thus pyroptosis can promote inflammation and lead to a variety of liver diseases.Citation19 Caspase-1 can mediate pyroptosis and can be activated by the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome.Citation20 When exposed to pathogen-associated molecular patterns (PAMPs) or DAMPs, NLRP3 binds to apoptosis-associated speck-like protein containing a CARD (ASC) to form a complex that cleaves pro-caspase-1 into mature caspase-1.Citation18 Caspase-1 cleaves and activates gasdermin-D (GSDMD), which eventually promotes cell membrane perforation, releases many proinflammatory cytokines, aggravates inflammatory response, and promotes the development of a liver disease.Citation19 Although the inhibition of hepatic pyroptosis may be helpful in the treatment of liver injury, whether tryptophan can alleviate liver injury and inflammation by inhibiting pyroptosis is unclear and needs to be elucidated. In the current study, we tested the hypothesis that tryptophan supplementation can alleviate LPS challenge-induced piglet liver injury and inflammation by regulating necroptosis and pyroptosis signaling pathway.
Materials and methods
Animal and experimental design
All animal experiments and procedures were approved by Sichuan Agricultural University Animal Care and Use Committee with permission number YYS202108. Eighteen castrated barrows (Duroc × Landrace × Yorkshire; weaned at 24 ± 1 d of age; body weight of 8.64 ± 1.00 kg) were randomly assigned to three treatment groups with six replicates, each of which was based on initial body weight. The room temperature and relative humidity were 30 °C and 50%-60%. Throughout the experiment, the piglets had free access to drinking water and feed. All nutrients in the basal diet () were determined based on the standard of the National Research Council (2012). A total of eighteen weaned piglets were randomly distributed to three treatments with 6 replicate pens per treatment and 1 piglet per pen: (1) non-challenged control (CONTR; the piglets were fed with a basal diet and injected with 0.9% sterile saline); (2) LPS-challenged control (LPS; the piglets were fed with the same basal diet and challenged by injection with E. coli LPS [E. coli serotype 055: B5; Sigma Chemical Inc. St. Louis MO USA]); (3) LPS + 0.2% L-tryptophan (Trp + LPS group; the piglets were fed with a 0.2% Trp diet [CJ International Trading Co., Ltd., South Korea] and injected with E. coli LPS). After feeding with control or 0.2% tryptophan-supplemented diets for 35 d, pigs were intraperitoneally injected with saline or LPS (100 mg/kg body weight). The dose of tryptophan and LPS were selected based on previous studies.Citation1,Citation21,Citation22 In order to get isonitrogenous diets, 0.17% and 0% alanine (purity > 99%) were supplemented to the control and 0.2% Trp diets, respectively. After administration with LPS or saline, all piglets were fasted for 4 h until slaughter in order to avoid the potential influence on the liver tissue caused by feed intake change.
Table 1. Composition and nutrient levels of basal diet (DM basis).
Sample collection
4 h after the injection of saline or LPS, all piglets were performed with electric shock and slaughtered. Livers were quickly collected.
Liver histology
Liver tissue fixed for 24 h was embedded in paraffin, and the cross-sections of the fragments were cut at 5 μm and stained with hematoxylin and eosin. Hepatocyte pathological changes were assessed using light microscopy based on the method of previous study.Citation23
Serum biochemical parameters
Briefly, at 4h after LPS or saline injection, the serum was separated and the content of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were detected.
Detection of antioxidant status in liver
The activities of antioxidant enzymes and non-enzyme antioxidants were evaluated by employing commercially available kits. In brief, the content of liver catalase (CAT), superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), hydrogen peroxide (H2O2) and malondialdehyde (MDA) were detected by the kit from Nanjing Jiancheng Institute of Biological Engineering.
Real-time PCR analysis
Quantitative real-time PCR detection in liver tissue were based on our previous studies.Citation22,Citation24 lists the gene primers used. The 2-ΔΔCt technique was used to calculate relative mRNA expression.
Table 2. Primer sequences used for Real-time PCR.
Western blot
Western blot procedure was carried out as described by previous study.Citation25 Briefly, after separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the proteins were subsequently electrophoretically transferred onto polyvinylidene fluoride membranes (Millipore, Eschborn Germany). Block the membrane with skim milk for 2 h at room temperature. Membranes were incubated with primary antibodies overnight at 4 °C. The primary antibodies including β-actin (1:1000; #4970S), zonula occludens-1 (ZO1) (1:1000, #66452-1-lg), occludin (1:1000, #27260-1-AP), claudin-1 (1:1000, #13050-1-AP), caspase-1 (1:1000, #22915-1-AP), 70-kDa heat shock proteins (HSP70) (1:5000, #10995-1-AP) and NLRP3 (1:1000, #19771-1-AP) were supplied by Proteintech Group, Inc. (Wuhan, China). The Phospho-MLKL (1:1000, #91689) rabbit antibody was purchased from Cell Signaling Technology (Chicago, USA). The GSDMD (1:1000, #DF12275) rabbit antibody was obtained from Affinity Biosciences (Beijing, China). The HRP conjugated anti-rabbit (1:1000, #abs20040) and HRP conjugated anti-mouse (1:1000, # abs20039ss) were purchased from Absin Biotechnology (Shanghai, China). Then, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody for 1.5 h at 37 °C. Bands on the membrane were visualized by extreme hypersensitivity ECL chemiluminrscence kit (Beyotime, Shanghai, China). Band density was determined using Image lab analysis software (version 6.1 Bio-Rad).
Statistical analysis
All the data were analyzed using T-test by SPSS 27.0 (SPSS, Inc., Chicago, IL, USA). To discover the effect of LPS challenge, the LPS group was compared to the control group, and the LPS group was compared to the Trp + LPS group to determine the effect of tryptophan treatment within the LPS groups. All data were presented as means ± standard error. p < 0.05 was considered statistically significant.
Results
Liver histology
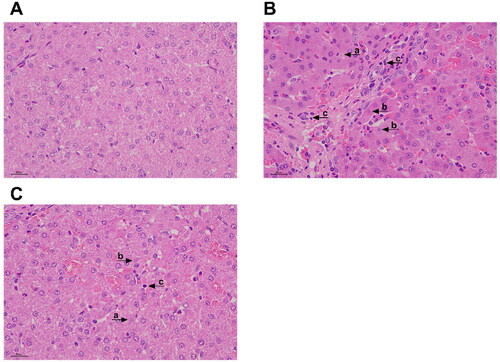
No obvious pathological changes were found in the piglets of the control group (). However, pathological changes of liver injury, such as hepatocyte lysis, pyknosis, inflammatory cell infiltration, hepatic sinusoidal congestion, and disordered arrangement of hepatocyte cords were found in piglets in the LPS group (). Relative to the piglets in the LPS group, the liver damage of the piglets in the Trp + LPS group was significantly reduced ().
Figure 1. Effects of tryptophan on liver Morphology in piglets after 4h lipopolysaccharide (LPS) challenge in piglets. (A) CONTR group, piglets were fed basal diet and injected with normal saline. Normal liver histology. (B) LPS group, piglets were fed a basal diet and injected intraperitoneally with LPS. Piglet liver showed significant pathological changes, including (a) heptatocyte caryolysis, (b) karyopycnosis, (c) inflammatory cell infiltration, hyperemia in hepatic sinusoids and hepatic cell cords arrangement in disorder were found. (C) Trp + LPS group, piglets were fed a basal diet supplemented with 0.2% tryptophan and injected intraperitoneally with LPS. n = 6 (1 pig/pen). original magnifications 400×. scale bars = 22.4 µm.
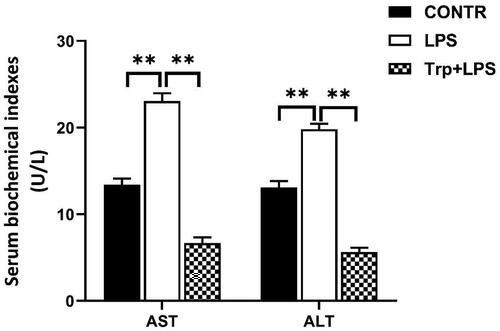
Analysis of ALT and AST in the piglets
Relative to the control group, LPS group had higher serum ALT and AST activities (p < 0.05) (). Piglets in the Trp + LPS group had lower serum ALT and AST activities relative to piglets in the LPS group (p < 0.05) ().
Figure 2. Tryptophan decreases serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities after 4h lipopolysaccharide (LPS) challenge in piglets. P values < 0.05 were considered significant. P values between 0.05 and 0.10 were regarded as a tendency. Values are presented as mean ± standard error, n = 6. CONTR: control group; LPS: piglets challenged with LPS; Trp + LPS: piglets fed with 0.2% tryptophan and challenged with LPS. * denotes P < 0.05, ** means P < 0.01.
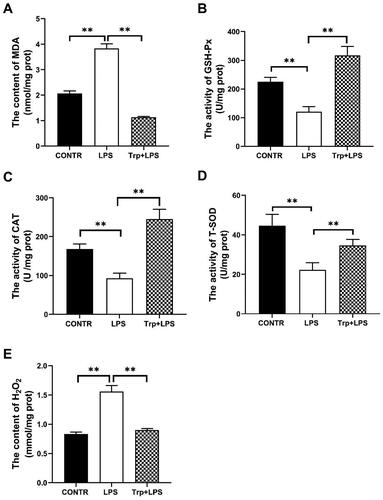
Antioxidant indicators in the liver
The antioxidant indicators of liver of piglets are summarized in . Relative to the control group, the levels of MDA and H2O2 in the liver of the LPS group were significantly enhanced, and the activities of CAT, GSH-Px and T-SOD were significantly reduced (p < 0.05). Relative to the LPS group, the Trp + LPS group significantly decreased MDA and H2O2 contents, and significantly increased CAT, GSH-Px and T-SOD activities (p < 0.05).
Figure 3. Effects of tryptophan on antioxidant indicators in the liver in piglets after 4h lipopolysaccharide (LPS) challenge in piglets. (A) the content of malondialdehyde (MDA). (B) the activity of glutathione peroxidase (GSH-Px). (C) the activity of catalase (CAT). (D) the activity of superoxide dismutase (T-SOD). (E) the content of hydrogen peroxide (H2O2). P values < 0.05 were considered significant. P values between 0.05 and 0.10 were regarded as a tendency. Values are showed as mean ± standard error, n = 6. CONTR: control group; LPS: piglets challenged with LPS; Trp + LPS: piglets fed with 0.2% tryptophan and challenged with LPS. *denotes P < 0.05, **means P < 0.01.
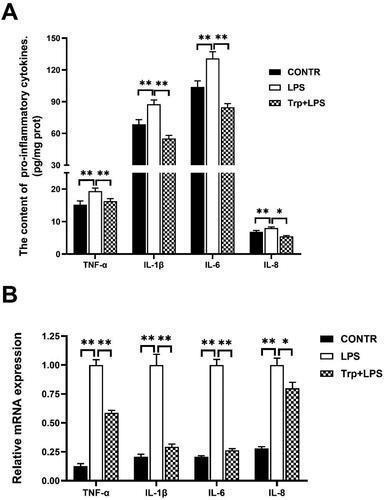
mRNA expression and levels of pro-inflammatory cytokines in the liver
The levels and mRNA expression abundance of IL-1β, IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) in liver of LPS group were significantly higher than those of the control group (p < 0.05) (). Relative to LPS group, Trp + LPS group significantly decreased pro-inflammatory cytokines levels and mRNA expression (p < 0.05) ().
Figure 4. Effects of tryptophan on content and mRNA expression of liver pro-inflammatory cytokines in piglets after 4h lipopolysaccharide (LPS) challenge in piglets. (A) the content of liver pro-inflammatory cytokines (n = 5). (B) mRNA expression of liver pro-inflammatory cytokines (n = 6). P values < 0.05 were considered significant. P values between 0.05 and 0.10 were regarded as a tendency. Values are showed as mean ± standard error. CONTR: control group; LPS: piglets challenged with LPS; Trp + LPS: piglets fed with 0.2% tryptophan and challenged with LPS. TNF-α: tumor necrosis factor-α. IL-1β: interleukin-1 β. * denotes P < 0.05, ** means P < 0.01.
Tight junction-related mRNA and protein expression
The mRNA and protein expression of claudin-1 and occludin were significantly decreased in the LPS group and the mRNA expression of ZO-1 were significantly reduced in the LPS group relative to the control group (p < 0.05) ( and 6). Relative to LPS group, Trp + LPS group had higher mRNA abundance and protein expression of claudin-1, occludin and ZO-1 in liver (p < 0.05) ( and Citation6)
Figure 5. Effects of tryptophan on mRNA expressions of tight junction (A), HSP70 gene (B), necroptosis (C), and pyroptosis (D) in liver after 4h lipopolysaccharide (LPS) challenge in piglets. P values < 0.05 were considered significant. P values between 0.05 and 0.10 were regarded as a tendency. Values are showed as mean ± standard error, n = 6. CONTR: control group; LPS: piglets challenged with LPS; Trp + LPS: piglets fed with 0.2% tryptophan and challenged with LPS. NLRP3: NOD-like receptor family pyrin domain containing 3. ASC: apoptosis-associated speck-like protein containing a CARD. GSDMD: gasdermin-D. ZO-1: zonula occludens-1. RIP1: the kinases receptor-interacting protein 1. RIP3: the kinases receptor-interacting 3. MLKL: the mixed lineage kinase domain-like protein. FADD: fas-associated death domain. PGAM5: phosphoglycerate mutase 5. HSP70: the 70-kDa heat shock proteins. * denotes P < 0.05, ** means P < 0.01.
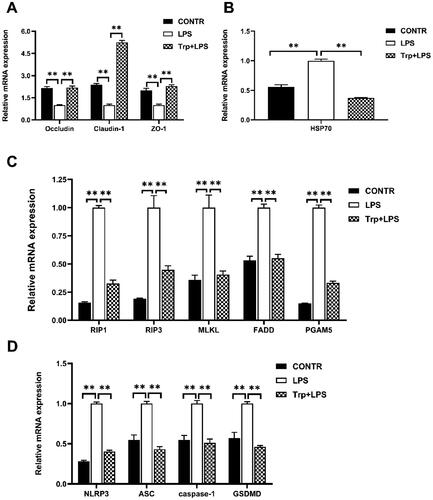
Figure 6. Effects of tryptophan on protein expressions of tight junction in liver after 4h lipopolysaccharide (LPS) challenge in piglets. P values < 0.05 were regarded as significant. P values between 0.05 and 0.10 were regarded as a tendency. Values are presented as mean ± standard error, n = 4. CONTR control group; LPS piglets challenged with LPS; Trp + LPS piglets fed with 0.2% tryptophan and challenged with LPS. ZO-1: zonula occludens-1. ns denotes not significant, * means P < 0.05, ** denotes P < 0.01.
HSP70 mRNA and protein expression in liver
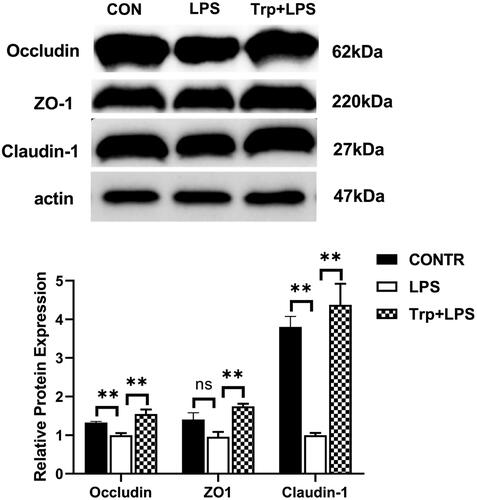
The mRNA expression of the HSP70 were significantly enhanced in the LPS group relative to the control group (). Relative to LPS group, Trp + LPS group had lower mRNA abundance of HSP70 in liver (p < 0.05) (). Relative to the LPS group, the Trp + LPS group significantly reduced the relative protein expression of HSP70 in the liver (p < 0.05) ().
Figure 7. Effects of tryptophan on expression pyroptosis-related proteins in liver after 4h lipopolysaccharide (LPS) challenge in piglets. P values < 0.05 were considered significant. P values between 0.05 and 0.10 were regarded as a tendency. Values are presented as mean ± standard error, n = 4. CONTR: control group; LPS: piglets challenged with LPS; Trp + LPS: piglets fed with 0.2% tryptophan and challenged with LPS. NLRP3: NOD-like receptor family pyrin domain containing 3. GSDMD: gasdermin-D. MLKL: the mixed lineage kinase domain-like protein. HSP70: the 70-kDa heat shock proteins. * denotes P < 0.05, ** means P < 0.01.
Necroptosis-related mRNA and protein expression in liver
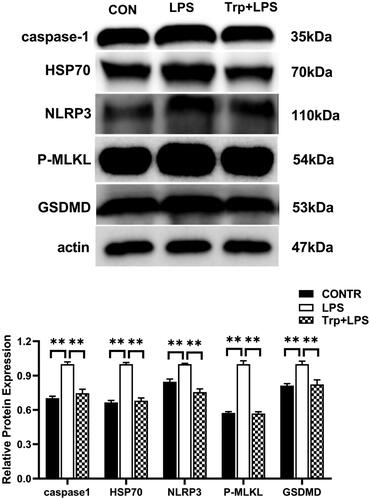
The mRNA expression of RIP1, RIP3, MLKL, FADD and PGAM5 were significantly enhanced in the LPS group relative to the control group (). Relative to LPS group, Trp + LPS group had lower mRNA abundance of RIP1, RIP3, MLKL, FADD and PGAM5 in liver (p < 0.05) (). Relative to the control group, the LPS group significantly increased the relative protein expression of MLKL in the liver (). Relative to the LPS group, the Trp + LPS group significantly decreased the relative protein expression of MLKL in the liver (p < 0.05) ().
Pyroptosis-related mRNA and protein expression in liver
The mRNA expression of NLRP3, ASC, caspase-1 and GSDMD and the relative protein expression of NLRP3, caspase-1 and GSDMD in the liver were significantly increased in the LPS group relative to the control group (p < 0.05) ( and 7). Relative to LPS group, Trp + LPS group had lower mRNA abundance of NLRP3, ASC, caspase-1 and GSDMD, and the relative protein expression of NLRP3, caspase-1 and GSDMD in liver (p < 0.05) ( and 7).
Discussion
The liver plays a crucial role in material metabolism and immune response. In animal husbandry, many factors (such as bacterial infection, stress, and feed toxin residue) can lead to liver structural damage and dysfunction, and resulting in decreased animal health, thereby causing significant economic losses to pig industry. As a result, alleviating piglets’ liver injury and inflammation, and understanding its regulative factors are critical. Our previous studies had found that tryptophan can modulate the composition of intestinal microflora and alleviate lipopolysaccharide-induced muscle fiber type transformation.Citation22,Citation26 Therefore, we extended our current study into the liver to investigate the role of tryptophan on injury and inflammation using the same animals. Tryptophan plays a crucial regulatory role in animal growth, development, and physiological metabolism and has antioxidative and anti-inflammatory effects.Citation6 Tryptophan improves growth performance mainly by affecting animal appetite and feed intake. In this study, 0.2% tryptophan supplementation improved the growth performance of weaned piglets before LPS challenge [increased average daily feed intake and average daily gain, but did not exert an effect on feed-to-meat ratio (data not shown)].Citation27 This study aimed to examine the effects and molecular mechanisms of dietary tryptophan supplementation on LPS-induced liver injury. LPS induced changes in the liver histology of piglets, particularly by promoting hepatocyte nuclear lysis, karyopyknosis, and fibroblast proliferation. This result was agreement with the result of Li et al.Citation28 However, supplementation with tryptophan in the diet reversed piglet liver damage after LPS challenge. ALT and AST activities are sensitive indicators reflecting liver injury.Citation4 Our findings suggested that LPS challenge resulted in increased ALT and AST activities in the sera, inducing liver injury. Dietary tryptophan can reduce the activities of ALT and AST in sera after intraperitoneal injection with LPS. Similar to our findings, the findings of Dremza et al showed that melatonin, a tryptophan metabolite, reduces ALT and AST activities in the sera of acetaminophen-gavaged rats.Citation29 Another study reported that tryptophan can reduce the activities of ALT and AST in the sera of mice with liver injury.Citation30 These results suggested that tryptophan can alleviate LPS-induced liver damage in piglets.
Tight junctions play an important role in cellular homeostasis. The permeability of paracellular pathways is closely related to tight junctions.Citation1 In this experiment, tryptophan upregulated the gene and protein expression of ZO-1, claudin-1, and occludin in piglet liver after LPS challenge. Similarly, previous studies reported that the protein expression of tight junctions in porcine epithelial cells treated with tryptophan increased in vitro.Citation31,Citation32 This finding suggested that tryptophan maintains the barrier function of the liver by upregulating the expression of tight junction-related proteins.
The liver is an important organ because of its role in immunological homeostasis and metabolism.Citation1 These vital roles are usually disrupted by LPS, inflammatory factors and pathogens. The LPS directly binds to TLR4 and leads to the production of proinflammatory cytokines and nitric oxide (NO) in the liver. The increased release of proinflammatory factors including IL-1β, IL-6 and other cytokines are closely associated with liver injury.Citation33,Citation34 In this experiment, challenge with LPS increased the levels and mRNA expression of proinflammatory cytokines in the piglet liver, caused hepatic inflammatory response. Tryptophan can alleviate LPS-induced liver inflammation in piglets. This result was consistent with the result of a previous study, which demonstrated that dietary tryptophan supplementation could treat colitis in pigs by reducing the gene expression of proinflammatory cytokines.Citation23,Citation35 Similar findings have been found in humans; treatment with tryptophan and its metabolite melatonin reduces proinflammatory cytokines levels in patients. In addition, HSP70 is considered a marker of cellular damage and inflammatory response.Citation36 Dietary tryptophan decreased HSP70 mRNA and protein expression in piglet livers after LPS challenge. This was agreement with the result of previous study.Citation37 These results presented evidence demonstrating that 0.2%tryptophan supplementation alleviates LPS-induced liver inflammatory response in animal husbandry.
Oxidative stress is associated with inflammatory responses.Citation38 T-SOD, CAT, and GSH-Px act as antioxidant enzymes to scavenge reactive oxygen species in the liver and alleviate liver damage caused by oxidative stress.Citation28 MDA and H2O2 are important indicators in response to oxidative stress.Citation39 In this study, tryptophan enhanced the activities of hepatic T-SOD, CAT, and GSH-Px and decreased liver MDA and H2O2 content after LPS challenge. Similarly, in the study of Mao et al. tryptophan alleviated hepatic oxidative stress caused by the intraperitoneal injection with diquat, enhanced the activities of T-SOD and GSH-Px, and decreased MDA content.Citation40 Tryptophan and its derivatives can scavenge reactive oxygen species and increase the body’s antioxidant capacity.Citation41 This result suggested that tryptophan is an effective measure to treat liver injury by increasing the activity of antioxidant enzymes, relieving oxidative stress, and then alleviating liver damage.
To explore the possible molecular mechanisms by which tryptophan alleviates hepatic oxidative stress and inflammatory responses, we evaluated necroptosis signaling. Necroptosis is a novel form of cell death with the features of necrosis and apoptosis and associated with tissue damage.Citation42 Necroptosis leads to the release of cellular DAMPs, which activate signaling pathways, such as TLRs and NODs, to initiate and maintain inflammatory response.Citation43 Necroptosis can be activated by extracellular stimuli such as TNF-α and TLRs agonists.Citation1 Therefore, to explore the underlying mechanisms by which tryptophan reduces hepatic inflammatory response and tissue loss our experiments assessed the expression of necroptosis-related signaling pathways. In our study, LPS challenge upregulated the mRNA expression of RIP1, RIP3, MLKL, FADD, and PGAM5 and enhanced the phosphorylation of MLKL in piglet liver, suggesting that LPS induced the necroptosis of hepatocytes. Tryptophan supplementation inhibited the LPS-induced necroptosis of hepatocytes, decreased the mRNA expressions of RIP1, RIP3, MLKL, FADD, and PGAM5 and alleviated inflammatory response. Further protein analysis showed that tryptophan decreased the phosphorylation of MLKL. This results suggested that tryptophan alleviates liver injury and inflammatory response by inhibiting necroptosis signaling pathway.
NLRP3 inflammasome-mediated hepatocyte pyroptosis is a novel mechanism of liver injury.Citation44 GSDMD is cleaved by activated caspase-1, promoting the synthesis of proinflammatory cytokines.Citation45 Pyroptosis releases numerous proinflammatory cytokines, which damages the liver.Citation46 In this experiment, LPS challenge increased the mRNA expression of NLRP3, ASC, caspase-1, and GSDMD and the protein expression of NLRP3 and GSDMD in piglet liver, indicating that LPS caused the pyroptosis of hepatocytes. However, tryptophan reversed LPS-induced pyroptosis and downregulated the mRNA expression of NLRP3, ASC, caspase-1, and GSDMD. Protein analysis showed that tryptophan reduced the protein expression of NLRP3 and GSDMD. These results suggested that tryptophan can alleviate liver injury and inflammatory response by inhibiting the pyroptosis signaling pathway.
Conclusion
In conclusion, tryptophan supplementation can alleviate LPS-induced histology injury, decrease the serum ALT and AST activities and mRNA and protein expression of HSP70. Furthermore, tryptophan supplementation can decrease pro-inflammatory cytokines levels and mRNA expression, increase the mRNA expression and protein expression of claudin-1, occludin and ZO-1. Evidences in present study suggested that tryptophan supplementation can decrease the necroptosis-related indicators, such as RIP1, RIP3, MLKL, FADD and PGAM5 mRNA expression and MLKL protein expression and decrease pyroptosis-related indicators, such as NLRP3, ASC, caspase-1 and GSDMD mRNA expression and protein expression of NLRP3, caspase-1 and GSDMD in liver. Taken together, tryptophan can alleviate liver injury and inflammatory response by inhibiting pyroptosis and necroptosis signaling pathways. This study not only offers new insights into the function of tryptophan, but also indicates the necessity for further investigating the effect of tryptophan on liver health in vivo.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Zhang L, Wang X, Chen S, et al. Medium-Chain triglycerides attenuate liver injury in lipopolysaccharide-challenged pigs by inhibiting necroptotic and inflammatory signaling pathways. Int J Mol Sci. 2018;19(11):3697.
- Reyes-Gordillo K, Shah R, Muriel P. Oxidative stress and inflammation in hepatic diseases: current and future therapy. Oxid Med Cell Longev. 2017;2017:3140673–3140672.
- Li Q, Liu Y, Che Z, et al. Dietary L-arginine supplementation alleviates liver injury caused by Escherichia coli LPS in weaned pigs. Innate Immun. 2012;18(6):804–814.
- Huang FF, Yang Y, Wang LM, et al. Holly polyphenols attenuate liver injury, suppression inflammation and oxidative stress in lipopolysaccharide-challenged weaned pigs. Food Agric Immunol. 2022;33(1):35–46.
- Rao Z, Li J, Shi B, et al. Dietary tryptophan levels impact growth performance and intestinal microbial ecology in weaned piglets via tryptophan metabolites and intestinal antimicrobial peptides. Animals. 2021;11(3):817.
- Xie C, Zhang Y, Niu K, et al. Enteromorpha polysaccharide-zinc replacing prophylactic antibiotics contributes to improving gut health of weaned piglets. Anim Nutr. 2021;7(3):641–649.
- Liu J, Zhang Y, Li Y, et al. L-tryptophan enhances intestinal integrity in diquat-challenged piglets associated with improvement of redox status and mitochondrial function. Animals. 2019;9(5):266.
- Liu G, Tao J, Lu J, et al. Dietary tryptophan supplementation improves antioxidant status and alleviates inflammation, endoplasmic reticulum stress, apoptosis, and pyroptosis in the intestine of piglets after lipopolysaccharide challenge. Antioxidants. 2022;11(5):872.
- Ohta Y, Sahashi D, Sasaki E, et al. Alleviation of carbon tetrachloride-induced chronic liver injury and related dysfunction by L-tryptophan in rats. Ann Clin Biochem. 1999;36(4):504–510.
- Ohta Y, Kongo M, Sasaki E, et al. Therapeutic effect of melatonin on carbon tetrachloride-induced acute liver injury in rats. J Pineal Res. 2000;28(2):119–126.
- Ayinde TO, Olayaki LA, Ojulari LS, et al. Hepatoprotective effect of tryptophan in carbontetrachloride-induced hepatotoxicity in male wistar rats. Int J Basic Appl Physiol. 2021;10:25.
- Schwabe RF, Luedde T. Apoptosis and necroptosis in the liver: a matter of life and death. Nat Rev Gastroenterol Hepatol. 2018;15(12):738–752.
- Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517(7534):311–320.
- Dillon CP, Oberst A, Weinlich R, et al. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep. 2012;1(5):401–407.
- Dovey CM, Diep J, Clarke BP, et al. MLKL requires the inositol phosphate code to execute necroptosis. Mol Cell. 2018;70(5):936–948.e7.
- Fu G, Wang B, He B, Feng M, Yu Y. LPS induces cardiomyocyte necroptosis through the Ripk3/Pgam5 signaling pathway. J Recept Signal Transduct Res. 2021;41(1):32–37.
- Dondelinger Y, Declercq W, Montessuit S, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7(4):971–981.
- Broz P, Pelegrín P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20(3):143–157.
- Guo H, Xie M, Zhou C, et al. The relevance of pyroptosis in the pathogenesis of liver diseases. Life Sci. 2019;223:69–73.
- Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):3328.
- Liang H, Dai Z, Kou J, et al. Dietary l-tryptophan supplementation enhances the intestinal mucosal barrier function in weaned piglets: implication of tryptophan-metabolizing microbiota. Int J Mol Sci. 2018;20(1):20.
- Wang F, Sun W, Liu G, et al. Tryptophan alleviates lipopolysaccharide-induced muscle fiber type transformation from type I to II and modulates Sirt1/AMPK/PGC-1α signaling pathway in pigs. Anim Biotechnol. 2023. doi:10.1080/10495398.2022.2136679.
- Wang L, Hou Y, Yi D, et al. Dietary supplementation with glutamate precursor α-ketoglutarate attenuates lipopolysaccharide-induced liver injury in young pigs. Amino Acids. 2015;47(7):1309–1318.
- Mo W, Wu X, Jia G, et al. Roles of dietary supplementation with arginine or N-carbamylglutamate in modulating the inflammation, antioxidant property, and mRNA expression of antioxidant-relative signaling molecules in the spleen of rats under oxidative stress. Anim Nutr. 2018;4(3):322–328.
- Chen X, Jia G, Liu G, et al. Effects of apple polyphenols on myofiber-type transformation inlongissimus dorsi muscle of finishing pigs. Anim Biotechnol. 2021;32(2):246–253.
- Liu G, Lu J, Sun W, et al. Tryptophan supplementation enhances intestinal health by improving gut barrier function, alleviating inflammation, and modulating intestinal microbiome in lipopolysaccharide-challenged piglets. Front Microbiol. 2022;13:919431.
- Liu G, Sun W, Wang F, et al. Dietary tryptophan supplementation enhances mitochondrial function and reduces pyroptosis in the spleen and thymus of piglets after lipopolysaccharide challenge. Animal. 2023;17(3):100714.
- Li Y, Zhao X, Jiang X, et al. Effects of dietary supplementation with exogenous catalase on growth performance, oxidative stress, and hepatic apoptosis in weaned piglets challenged with lipopolysaccharide. J Anim Sci. 2020;98(3):skaa067.
- Dremza IK, Cheshchevik VT, Zabrodskaia SV, et al. Hepatotoxic effects of acetaminophen. Protective properties of tryptophan-derivatives. Biomed Khim. 2010;56(6):710–718.
- Nagamura Y, Uesugi K, Naito J, et al. Macrophages modulate liver cell function via tryptophan metabolites. Adv Exp Med Biol. 1996;398:381–386.
- Wang Q, Liu D, Song P, et al. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front Biosci. 2015;20(7):1116–1143.
- Liu G, Gu K, Wang F, et al. Tryptophan ameliorates barrier integrity and alleviates the inflammatory response to enterotoxigenic Escherichia coli K88 through the CaSR/Rac1/PLC-γ1 signaling pathway in porcine intestinal epithelial cells. Front Immunol. 2021;12:748497.
- Tacke F, Luedde T, Trautwein C. Inflammatory pathways in liver homeostasis and liver injury. Clin Rev Allergy Immunol. 2009;36(1):4–12.
- Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–7218.
- Kim CJ, Kovacs-Nolan JA, Yang C, et al. L-tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J Nutr Biochem. 2010;21(6):468–475.
- Zhong X, Li W, Huang X, et al. Impairment of cellular immunity is associated with overexpression of heat shock protein 70 in neonatal pigs with intrauterine growth retardation. Cell Stress Chaperones. 2012;17(4):495–505.
- Celinski K, Konturek PC, Slomka M, et al. Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with non-alcoholic fatty liver disease–14 months follow up. J Physiol Pharmacol. 2014;65(1):75–82.
- Zhu R, Wang Y, Zhang L, et al. Oxidative stress and liver disease. Hepatol Res. 2012;42(8):741–749.
- Li S, Tan HY, Wang N, et al. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. 2015;16(11):26087–26124.
- Mao X, Lv M, Yu B, et al. The effect of dietary tryptophan levels on oxidative stress of liver induced by diquat in weaned piglets. J Anim Sci Biotechnol. 2014;5(1):49.
- Reiter RJ, Tan DX, Cabrera J, et al. Melatonin and tryptophan derivatives as free radical scavengers and antioxidants. Adv Exp Med Biol. 1999;467:379–387.
- Xu Q, Guo J, Li X, et al. Necroptosis underlies hepatic damage in a piglet model of lipopolysaccharide-induced sepsis. Front Immunol. 2021;12:633830.
- Wang L, Tu Z, Wang H, et al. Flaxseed oil improves liver injury and inhibits necroptotic and inflammatory signaling pathways following lipopolysaccharide challenge in a piglet model. J Funct Foods. 2018;46:482–489.
- Wree A, Eguchi A, McGeough MD, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59(3):898–910.
- Liu X, Zhang Z, Ruan J, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153–158.
- Pan Y, Yu S, Wang J, et al. N-acetyl-L-tryptophan attenuates hepatic ischemia-reperfusion injury via regulating TLR4/NLRP3 signaling pathway in rats. PeerJ. 2021;9:e11909.
