 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The objective of this experiment was to evaluate the influence of nanoselenium (NANO-Se) addition on milk production, milk fatty acid synthesis, the development and metabolism regulation of mammary gland in dairy cows. Forty-eight Holstein dairy cows averaging 720 ± 16.8 kg of body weight, 66.9 ± 3.84 d in milk (dry matter intake [DIM]) and 35.2 ± 1.66 kg/d of milk production were divided into four treatments blocked by DIM and milk yields. Treatments were control group, low-Se (LSe), medium-Se (MSe) and high-Se (HSe) with 0, 0.1, 0.2 and 0.3 mg Se, respectively, from NANO-Se per kg dietary dry matter (DM). Production of energy- and fat-corrected milk (FCM) and milk fat quadratically increased (p < 0.05), while milk lactose yields linearly increased (p < 0.05) with increasing NANO-Se addition. The proportion of saturated fatty acids (SFAs) linearly decreased (p < 0.05), while proportions of monounsaturated fatty acids (MUFAs) linearly increased and polyunsaturated fatty acids (PUFAs) quadratically increased. The digestibility of dietary DM, organic matter (OM), crude protein (CP), neutral detergent fiber (NDF) and acid detergent fiber (ADF) quadratically increased (p < 0.05). Ruminal pH quadratically decreased (p < 0.01), while total VFA linearly increased (p < 0.05) with increasing NANO-Se addition. The acetic to propionic ratio decreased (p < 0.05) linearly due to the unaltered acetic molar percentage and a quadratical increase in propionic molar percentage. The activity of CMCase, xylanase, cellobiase and pectinase increased linearly (p < 0.05) following NANO-Se addition. The activity of α-amylase increased linearly (p < 0.01) with an increase in NANO-Se dosage. Blood glucose, total protein, estradiol, prolactin, IGF-1 and Se linearly increased (p < 0.05), while urea nitrogen concentration quadratically decreased (p = 0.04). Moreover, the addition of Se at 0.3 mg/kg from NANO-Se promoted (p < 0.05) mRNA and protein expression of PPARγ, SREBP1, ACACA, FASN, SCD, CCNA2, CCND1, PCNA, Bcl-2 and the ratios of p-ACACA/ACACA and BCL2/BAX4, but decreased (p < 0.05) mRNA and protein expressions of Bax, Caspase-3 and Caspase-9. The results suggest that milk production and milk fat synthesis increased by NANO-Se addition by stimulating rumen fermentation, nutrients digestion, gene and protein expressions concerned with milk fat synthesis and mammary gland development.
Introduction
The primary goal in dairy industry development is the production of healthy heifers with normal mammary glands that can synthesize and secrete large quantities of high-quality milk.Citation1 However, the proliferation of mammary epithelial cells is impacted by many factors, including nutrition,Citation2 hormonesCitation3 and environment.Citation4
Selenium (Se) is an essential element for the normal growth, development and health of dairy cows and plays a crucial role in many metabolic functions of tissues and organs because of its antioxidant capability.Citation5,Citation6 Se is required for the synthesis of many Se-dependent enzymes, containing glutathione peroxidase (GSH-Px), selenoprotein-P, thioredoxin reductase and thioredoxin. These enzymes regulate the intracellular inflammatory and apoptotic signal cascades to preserve the intracellular environment.Citation7,Citation8 Numerous studies have emphasized the link established between Se and the performance of dairy cows. Supplementation with Se has been shown to increase the production of milkCitation9–11 and milk fat and protein,Citation12 increase nutrient digestibility,Citation11,Citation12 ruminal total volatile fatty acid (VFA) concentration and microbial enzymatic activity.Citation9,Citation11 Supplementation with Se decrease mastitis and embryonic mortality.Citation5,Citation13 Se plays a role in adjusting the cell proliferation and apoptosis in relation to several protein kinases and intracellular signaling pathways.Citation14 Recently, the addition of Se has been shown to promote the proliferation of lens epithelial cells, following ultra-violet B-induced damageCitation6, and of spermatogonial stem cells of sheep.Citation15 Furthermore, Zhang et al. demonstrated that supplementation of 50 nmol/L of Se in cell culture fluid increased cell viability and relative growth rate of bovine mammary epithelial cells (BMECs).Citation16
As a new way of supplementing Se, nanoselenium (NANO-Se, typically 20–60 nm) that uses a protein as a dispersant and the element Se as a membrane, has more active centers, higher biological activity and less toxicity than those of other Se supplements (selenite and selenate) and can increase the expressions of Se-containing proteins more effectively.Citation17 Dietary NANO-Se addition is more effective than sodium selenite in increasing the Se concentration and GSH-Px activities in milk and blood and upregulating the mammary mRNA expression levels of GSH-Px.Citation18 Accordingly, we hypothesized that supplementation with NANO-Se could enhance the lactation performance and milk fatty acid synthesis by stimulating mammary gland development. Furthermore, we sought to clarify the regulatory mechanism underlying the effect of NANO-Se addition on mammary gland development by quantifying the expressions of the genes and proteins involved in fatty acid synthesis and mammary gland cell proliferation. Our study sought to investigate the impacts of NANO-Se addition on the lactation performance, nutrient digestion, expressions of genes and proteins involved in milk fatty acid synthesis, and cell proliferation of the mammary gland in dairy cows.
Materials and methods
The research proposal of the current investigation was evaluated and authorized by the Shanxi Agriculture University Animal Care and Use Committee (Taigu, China) before the study commencement (IACUC Issue No. SXAU-EAW-2021C.FU.00301401).
Holstein cows, experimental design and diets
A randomized block design experiment was performed with 48 multiparous Holstein dairy cows (720 ± 16.8 kg body weight, 66.9 ± 3.84 d in milk and 35.2 ± 1.66 kg/d milk yield) at the beginning of the study. The feeding experiment was conducted for 120 days, comprising a 15-d covariate period followed by a 15-d adaptation period and a subsequent 90-d sampling period. The production data collected during the covariate period were used to formulate a basal diet (). The diet was formulated based on the NRC recommendations.Citation19 Cows were blocked by days in milk and milk yield during the covariate period and divided into one of four groups in a randomized block design. The groups included a control group (without NANO-Se) and low-Se (LSe), medium-Se (MSe) and high-Se (HSe) treatment groups with 0, 0.1, 0.3 and 0.5 mg Se, respectively, from NANO-Se per kg dietary dry matter (DM). The amount of Se added was based on a previous study,Citation18 in which it was found that 0.3 mg of Se from NANO-Se per kg dietary DM has an influence on dry matter intake (DMI). The NANO-Se additive (feed grade, 995 g/kg Se; Sichuan Jilongda Biotechnology Group Co. LTD., Guanghan, China) was blended into the basal diet (). The basal diet and NANO-Se additive were mixed as a total mixed ratio (TMR) twice daily. Cows were housed in a naturally ventilated, two-row, head-to-head, free-stall barn equipped with a Calan gate Feeding System for monitoring individual intake and were milked three times (at 05:30, 13:30 and 20:30 h) per day, fed the same standard diet ad libitum, and had free access to water.
Table 1. Ingredients and chemical composition of the basal diet (DM basis).
Data and sample collection
Cows were weighed on two consecutive days at 16:00 h on day 1 of the covariate period and on days 1 and 90 of the sampling period. The provided TMR and the refused TMR were determined daily for each dairy cow during the entire study to estimate the DMI. Samples were collected every 5 d of the covariate period and every 10 d during the sampling period and were stored at −20 °C. Milk production was measured daily for each cow during the entire experiment. Milk samples were collected from each cow every 5 d of the covariate period and every 10 d during the sampling period from each milking on three consecutive milkings within a day. Concurrently, samples were collected and stored at 4 °C with 2-bromo-2-nitropropane-1, 3-diol. At 06:30 and 18:30 h during days 1–15 of the covariate period and days 70–87 of the sampling period, all cows were dosed with 5 g of chromic oxide powder placed in a gelatin capsule to serve as a digestion marker. Approximately, 250 g of fecal samples were collected from each cow’s rectum at 07:00, 13:00, 19:00 and 01:00 h during days 8–15 of the covariate period and days 78–87 of the sampling period and stored at −20 °C. During days 88–89, samples of TMR, refusals, and feces of each cow were composited, dried at 55 °C for 72 h, and ground to pass through a 1-mm screen with a cutter mill (Beishengwei Experimental Instrument, Changzhou, China).
Ruminal fluid was collected from each animal via an oral stomach tube at before the morning feeding, and 3, 6 and 9 hours after feeding on day 5 of the covariate period and days 44 and 89 of the sampling period. The initial 150 mL of ruminal fluid was discarded to minimize saliva contamination, and the next 200 mL was reserved and filtered through four layers of medical gauze. The ruminal pH of each cow was determined once using a portable pH meter (ST3100/F; Zhejiang Scientific Equipment, Zhejiang, China). Thereafter, 5 mL of the filtrate was mixed with 1 mL of 250 g/L of meta-phosphoric acid before being stored at −20 °C for VFA analysis. Furthermore, 5 mL of the filtrate was mixed with 1 mL of 20 g/L sulfuric acid before being stored at −20 °C for the analysis of ammoniacal nitrogen. For microbial enzymatic activity analyses, 50 mL of the filtrate was frozen in liquid nitrogen and subsequently stored at −80 °C.
At 10:30 h on day 15 of the covariate period and day 90 of the sampling period, blood samples from each dairy cow were collected into 10-mL evacuated tubes (serum separation gel coagulant tube, Hunan Liuyang Medical Instrument, Liuyang, China) via the coccygeal vessel. Blood samples were promptly placed on ice and transported to the laboratory to separate the serum by centrifugation at 2000 × g and 4 °C for 12 min, whereafter the serum samples were stored at −20 °C.
Mammary tissue biopsies were performed from 16:00 to 20:00 h on day 15 of the covariate period and day 90 of the sampling period. Approximately, 1 g of mammary gland secretory tissue was collected by surgical biopsy from the midpoint section of the rear quarterCitation20 and was rapidly frozen in liquid nitrogen and kept at −80 °C for total RNA extraction.
Chemical analyses
The contents of DM, nitrogen, ether extract, and crude ash in TMR, refusal and feces samples were measured as described in AOAC.Citation21 The organic matter (OM) content was estimated as the difference between the DM and crude ash contents. The neutral detergent fiber (NDF) content was determined as previously described using heat-stable alpha-amylase and sodium sulfite and expressed including residual ash.Citation22 The acid detergent fiber (ADF) content was analysed as described in AOAC.Citation21 The fat, true protein, and lactose contents of the milk were analysed as described in AOAC (method 972.16).Citation21 Milk fat extraction and trans-methylation of the esterified FA were performed as described in a previous report.Citation23 Fatty acid methyl esters (FAMEs) were used for gas chromatographic measurement of the fatty acid composition in milk (Agilent 7890 A, Agilent Technologies, Santa Clara, CA). FAMEs were identified by comparisons with retention times of the standards: FAME Mix C4-C24 Unsaturates (Sigma, Roedermark, Germany), methyl trans-11 C18:1 (Sigma), and methyl cis-9 and trans-11 CLA (Matreya1255, Pleasant Gap). According to the method of Rico and Harvatine,Citation24 the mean proportion of FA in total milk lipids was calculated for each sample. The chromium content of the feces was measured using atomic absorption spectrophotometry (AA-1800H; Shanghai Meisei Instrument Co., LTD., Shanghai, China) as described by Williams et al.Citation25 Ruminal VFA concentrations were analysed using gas chromatography (GC-7890; Agilent Technology, Beijing, China). The ammoniacal nitrogen content was analysed according to the procedure of AOAC.Citation21 The microbial enzyme activities of ruminal fluid (cellobiose, carboxymethyl cellulase [CMCase], α-amylase, xylanase, pectinase and protease) were determined as described by Agarwal et al.Citation26 Biochemical kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) for glucose (no. A154-2-1), total protein (A045-3-1), albumin (no. A028-1-1), urea nitrogen (no. C013-2-1) and triglyceride (no. A110-2-1) were used to analyse the serum concentrations of glucose, total protein, albumin, urea nitrogen and triglycerides using an Automatic Biochemical Analyzer (BS-400, Nanjing Badeng Co., LTD, Nanjing, China). ELISA kits (Beijing Biolide Biotechnology Co., LTD, Beijing, China) for bovine estradiol (bovine, no. BL-E28820M), prolactin (bovine, no. BL-E28845M) and insulin-like growth factor 1 (IGF-1; bovine, no. BL-E21687M) were used to analyse E2, prolactin and IGF-1 with a Konelab autoanalyzer (Thermo Fisher Scientific Oy, Vantaa, Finland). Levels of Se in feed, milk and serum were determined as described by Webb et al.Citation27
Extraction of mammary gland RNA and real-time PCR
Total RNA was isolated from 100 mg of mammary gland tissue using a total RNA extraction kit (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. The concentration and quality of the extracted RNA were measured on a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE). For all preparations, the ratio of absorbance at 260 and 280 nm was close to 2.0. Denaturing agarose gel electrophoresis and ethidium bromide staining were used to evaluate RNA integrity. cDNA was synthesized using 500 ng of total RNA from each sample per 10 µL of the sample reaction mixture using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories GmbH, Munich, Germany) according to the manufacturer’s specifications. Reactions were conducted at 37 °C for 15 min and 85 °C for 5 min. To identify possible genomic or environmental DNA contamination, a negative control reaction without reverse transcriptase was performed for each sample.Citation28
The mRNAs of fatty acid synthase (FASN), acetyl-coenzyme A carboxylase-α (ACACA), fatty acid-binding protein 3 (FABP3), peroxisome proliferator-activated receptor gamma (PPARG or PPARγ), stearoyl-CoA desaturase (SCD), sterol regulatory element-binding factor 1 (SREBPF1), proliferating cell nuclear antigen (PCNA), cyclin A2 (CCNA2), cyclin D1 (CCND1), B-cell lymphoma 2 (BCL2), BCL2-associated X 4 (BAX4), BCL2/BAX4, Caspase 3 (CASP3) and Caspase 9 (CASP9) were quantified via quantitative reverse transcription-PCR using an iCycler and iQ-SYBR Green Supermix (Bio-Rad). Both glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin (ACTB) were selected as potential internal control genes with reference to the study of Busato et al.Citation29 The relative expressions of GAPDH and ACTB were compared, and no significant differences were found among the treatments; therefore, GAPDH was used as the housekeeping gene. Real-time PCR primers for the target genes are listed in . Real-time quantitative PCR was performed with an ABI QuantStudio5 instrument (Thermo Fisher Scientific, Fremont, NY). A 20-µL reaction mixture comprised 2 µL of cDNA, 10 µL of SYBR Premix Taq II (TaKaRa, San Jose, CA), 0.8 µL of forward primer (10 µM), 0.8 µL of reverse primer (10 µM), 0.4 µL of ROX Reference Dye II (TaKaRa) and 6.0 µL of nuclease-free water. The PCR cycling conditions were as follows: 1 cycle at 95 °C for 20 s for the original denaturation step, 40 cycles at 95 °C for 15 s, annealing at the annealing temperature of each target gene for 30 s and extension at 55 °C for 20 s.
Table 2. Real-time PCR primers for amplifying the target genes.
Western blot analysis
The protein content was analysed using a Thermo Scientific Pierce BCA Protein Assay Kit (23225; Thermo Fisher Scientific) according to the manufacturer’s instructions; β-actin was used as the loading control. Equal quantities of protein (20 µg) were separated on a 12% SDS-PAGE, and the separated proteins were transferred onto nitrocellulose membranes (1704271; Bio-Rad). Membranes were blocked with 6% (wt/vol) BSA in tris-buffered saline plus Tween (TBST) for 2 h at 25 °C and were then incubated overnight at 4 °C with the following primary antibodies diluted in TBST: mouse anti-cyclin A1 (1:2000; cat. no. NB100-2660; Novusbio Biologicals, Centennial, CO), mouse anti-PCNA (1:2000; cat. no. 2586; Cell Signaling Technology, Danvers, MA), rabbit anti-Akt (1:2000; cat. no. 9272; Cell Signaling Technology), rabbit anti-phosphorylated-AktSer473 (1:2000; cat. no. bs-0876R; Bios Antibodies, Boston, MA), rabbit anti-mTOR (1:2000; cat. no. bs-1992R; Bios Antibodies), rabbit anti-phosphorylated-mTORSer2448 (1:2000; cat. no. bs-3495R; Bios Antibodies), rabbit anti-PPARγ (1:2000; cat. no. bs-4590R; Bios Antibodies), rabbit anti-phosphorylated-ACACA (1:2000; cat. no. bs-12954R; Bios Antibodies), rabbit anti-ACACA (1:2000; cat. no. bs-11912R; Bios Antibodies), rabbit anti-FASN (1:2000; cat. no. bs-60347R; Bios Antibodies), rabbit anti-SCD1 (1:2000; cat. no. bs-3787R; Bios Antibodies), rabbit anti-BCL2 (1:2000; cat. no. bs-20351R; BIOSS, Beijing, China), mouse anti-BAX (1:2000, cat. no. bs-0127M; BIOSS, Beijing, China), rabbit anti-Caspase 3 (1:2000, cat. no. bs-0081R; BIOSS, Beijing, China), rabbit anti-Caspase 9 (1:2000, cat. no. bs-0049R; BIOSS, Beijing, China) and rabbit anti-β-actin (1:10,000; cat. no. 4970; Cell Signaling Technology).
Membranes were washed five times with 1× TBST for 5 min to remove excess antibodies. Membranes were then incubated at 25 °C for 2 h with either of the following secondary antibodies diluted in TBST: goat anti-rabbit (1:5000; cat. no. E-AB-1003; Elabscience, Wuhan, China) or goat anti-mouse (1:5000; cat. no. RS0001; Immunoway, Plano, TX). A SuperSignal West Pico Chemiluminescence Substrate (Thermo Fisher Scientific) was used to visualize the western blots before being semi-quantified using ImageJ software version 1.4.3.67 (National Institutes of Health, Bethesda, MD).
Calculations and statistical analysis
Dietary net energy for lactation (NEL) was estimated by multiplying the NEL-3X density of the feed ingredient with the dietary content.Citation30 Energy-corrected milk (ECM) and fat-corrected milk (FCM) were calculated according to the NRC.Citation19 The feed efficiency was calculated for each animal as milk/DMI and ECM/DMI. Data were analysed as a randomized complete-block design via the mixed procedure of SAS (PROC MIXED; SAS, 2002). Data for DMI, milk production, feed efficiency, milk fatty acid composition, nutrient digestibility and blood parameters were analysed using the following statistical model:
where Yiklm represents the dependent variable; µ represents the overall mean; β represents the regression coefficient; Vk represents the covariate measurement; Bi represents the effect of block i; Dj represents the effect of day j; Ck(i) represents the effect of cow k within block i; Ll represents the effect of NANO-Se treatment l; DLjl represents the interaction between day and NANO-Se treatment; and Eijkl represents the residual error.
Data for ruminal fermentation and microbial enzymatic activity were analysed using the following statistical model for which there were repeated measurements over time (pH, VFA and enzymatic activity):
where Yiklm represents the dependent variable; µ represents the overall mean; β represents the regression coefficient; Vk represents the covariate measurement; Bi represents the effect of block i; Dj represents the effect of day j; Ck(i) represents the effect of cow k within block i; Ll represents the effect of NANO-Se treatment l; DLjl represents the interaction between day and NANO-Se treatment; Eijkl represents the whole plot error; Tm represents the effect of time m; LTml represents the interaction between NANO-Se treatment and time; and Sijklm represents the subplot error.
The spatial covariance structure SP was used for estimating the covariances, and the subject of the repeated measurements was defined as cow (block × day × treatment). Linear and quadratic orthogonal contrasts were performed using the CONTRAST statement of SAS with coefficients estimated based on the dose of added NANO-Se. Western blot data were analysed using the SigmaPlot version 12.5 (Systat Software, San Jose, CA) statistical analysis package. Statistical differences in the means of treatments were evaluated using the Student’s t-test. Data in graphs are presented as mean ± SEM. The effects of the factors were considered significant at p < 0.05 unless other trends were declared at p < 0.10.
Results
Feed intake, milk production, feed efficiency and milk fatty acid composition
Although the DMI was not affected by the increase in NANO-Se addition (), the production of milk tended to increase linearly (p = 0.064), whereas the FCM (4%, p = 0.049), ECM (p = 0.048), and milk fat production (p = 0.028) increased quadratically. The milk fat content increased quadratically (p = 0.039) and the milk protein content tended to increase quadratically (p = 0.071), whereas the milk lactose content did not change following NANO-Se addition. The feed efficiency, described as either milk yields/DMI or ECM yields/DMI, also quadratically increased (p < 0.05) following NANO-Se addition. As expected, the milk Se content increased linearly (p = 0.003) following NANO-Se addition.
Table 3. Effects of nanoselenium (NANO-Se) supplementation on the dry matter intake (DMI), lactation performance and feed efficiency in dairy cows.
Although the proportion of C16:0 fatty acids was quadratically increased (p = 0.009), the proportion of saturated fatty acids (SFAs) decreased linearly (p = 0.021) due to the quadratically decreased levels of C6:0 and C12:0, and the linearly decreased levels of C8:0 and C10:0 with increasing NANO-Se addition (). For monounsaturated fatty acids (MUFA), the linear (p = 0.036) increase in the C18:1 fatty acid content caused by the linear (p = 0.024) increase in the C18:1 cis-9 fatty acid content resulted in a linear (p = 0.033) increase in the MUFA content, regardless of the linear (p = 0.016) increase in the C12:1 cis-9 fatty acid content. Furthermore, the proportion of polyunsaturated fatty acids (PUFAs) increased quadratically (p = 0.024) because of the quadratically (p = 0.012) increased content of nonconjugated C18:2 fatty acids and the linearly (p = 0.031) increased content of C20:0:3n-6 fatty acids with increasing NANO-Se addition. Thus, the proportion of short-chain fatty acids (SCFAs) decreased quadratically (p = 0.042), whereas the proportion of mixed sourced fatty acids (MSFAs) increased quadratically (p = 0.007) following NANO-Se addition. The proportion of preformed fatty acids (PFA) increased linearly (p = 0.008), whereas odd- and branched-chained fatty acids were not influenced by NANO-Se addition. For the desaturation ratios, only the ratio of rumenic acid (RA; cis-9, trans-11 C18:2) to the sum of RA and vaccenic acid (VA; trans-11 C18:1) was increased quadratically (p = 0.044) with increasing NANO-Se addition.
Table 4. Effects of nanoselenium (NANO-Se) supplementation on milk fatty acid composition of milk fat in lactating dairy cows (g/100 g of fatty acids).
Digestibility, ruminal fermentation and microbial enzymatic activities
The digestibility of dietary DM, OM, crude protein (CP), NDF and ADF was increased quadratically (p < 0.05) with NANO-Se addition, although the digestibility of ether extract was unaffected (). The ruminal pH was quadratically decreased (p = 0.006), whereas the ruminal total VFA concentration increased linearly (p = 0.033) following NANO-Se addition. The molar percentage of acetate was unaffected by NANO-Se addition, whereas that of propionate was increased quadratically (p = 0.004) and that of butyrate was decreased quadratically (p = 0.047) following NANO-Se addition. Moreover, the acetate-to-propionate ratio was decreased quadratically (p = 0.034) with increasing NANO-Se addition. Conversely, the molar percentages of valerate, isobutyrate, and isovalerate were unaffected by NANO-Se addition. The ruminal ammonia N concentration decreased quadratically (p = 0.021) following NANO-Se addition. Although the enzymatic activity of protease was unaffected by NANO-Se addition, the activity of CMCase, xylanase, cellobiase, and pectinase increased linearly (p < 0.05) following NANO-Se addition. The activity of α-amylase increased linearly (p = 0.005) with an increase in NANO-Se dosage.
Table 5. Effects of nanoselenium (NANO-Se) supplementation on nutrient digestibility and ruminal fermentation in lactating dairy cows.
Blood metabolites
The blood concentrations of glucose, albumin, and total protein were increased quadratically (p < 0.05) following NANO-Se addition (). Furthermore, the concentration of estradiol, prolactin, IGF-1 and Se increased linearly (p < 0.05) with increasing NANO-Se addition. Conversely, the blood urea nitrogen (BUN) content decreased quadratically (p = 0.044) following NANO-Se addition.
Table 6. Effects of nanoselenium (NANO-Se) addition on blood metabolites in lactating dairy cows.
Expressions of genes and proteins involved in fatty acid synthesis
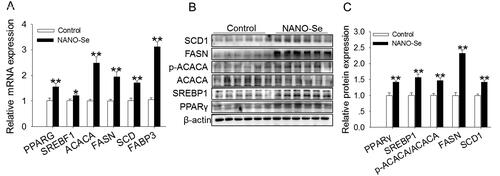
Regarding fatty acid synthesis, 0.3 mg/kg of Se from NANO-Se significantly increased the mRNA levels of PPARG (p < 0.01), SREBPF1 (p < 0.05), ACACA (p < 0.01), FASN (p < 0.01), SCD (p < 0.01) and FABP3 (p < 0.01) in comparison with those of the control (). The protein levels of PPARγ, SREBP1, p-ACACA/ACACA, FASN (p < 0.01) and SCD (p < 0.01) were uniformly increased upon addition of 0.3 mg/kg of Se from NANO-Se compared with those in the control ().
Figure 1. Effects of dietary nanoselenium (NANO-Se) addition on the expressions of genes and proteins related to fatty acid synthesis in the bovine mammary gland. (A) mRNA levels of peroxisome proliferator-activated receptor gamma (PPARG or PPARγ), sterol regulatory element-binding factor 1 (SREBF1), acetyl-coenzyme a carboxylase-α (ACACA), fatty acid synthase (FASN), stearoyl-CoA desaturase (SCD) and fatty acid-binding protein 3 (FABP3) in bovine mammary glands treated with NANO-Se (0 and 0.3 mg/kg of Se). GAPDH was used as the reference gene. The relative mRNA expression level of each target gene was standardized to that in the control group. (B) Western blot analysis of PPARγ, sterol regulatory element-binding protein 1 (SREBP1), ACACA, FASN, SCD1 and FABP3 in bovine mammary gland tissues; β-actin was used as the loading control. (C) Mean ± SEM of immunopositive bands of PPARγ, SREBP1, ACACA, FASN, SCD1 and FABP3. The relative protein expression level of each target protein was standardized to that in the control group. *p < 0.05 and **p < 0.01 versus the control group.
Expressions of genes and proteins involved in cell proliferation
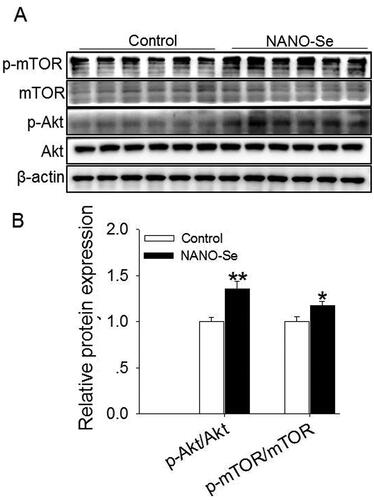
The CCNA2 and CCND1 genes encode CCNA2 and D1, respectively. Supplementation of 0.3 mg/kg of Se from NANO-Se increased the mRNA levels of PCNA (p < 0.05), CCNA2 (p < 0.01) CCND1 (p < 0.05), BCL2 (p < 0.05) and BCL2/BAX4 (p < 0.01) and decreased those of BAX4 (p < 0.01), CASP3 (p < 0.01) and CASP9 (p < 0.01) () compared with those in the control. The protein levels of PCNA (p < 0.01), CCNA1 (p < 0.01), BCL2 (p < 0.05) and BCL2/BAX (p < 0.01) were increased, whereas those of BAX (p < 0.01), Caspase-3 (p < 0.01) and Caspase-9 (p < 0.01) were decreased upon addition of 0.3 mg/kg of Se from NANO-Se compared with those in the control (). Furthermore, the probable involvement of the Akt/mTOR signaling pathway was assessed (). The protein expression ratios of p-Akt:Akt (p < 0.01) and p-mTOR:mTOR (p < 0.05) were also significantly increased following NANO-Se addition ().
Figure 2. Effects of dietary nanoselenium (NANO-Se) addition on proliferation-related mRNA and protein expressions in the bovine mammary gland. (A) mRNA levels of proliferating cell nuclear antigen (PCNA), cyclin A2 (CCNA2), cyclin D1 (CCND1), B-cell lymphoma 2 (BCL2), BCL2-associated X 4 (BAX4), BCL2/BAX4, Caspase 3 (CASP3) and Caspase 9 (CASP9) in bovine mammary glands treated with NANO-Se (0 and 0.3 mg/kg of Se). GAPDH was used as the reference gene. The relative mRNA expression level of each target gene was standardized to that in the control group. (B) Western blot analysis of PCNA, cyclin A1 (CCNA1), BCL2, BAX, BCL2/BAX, Caspase 3 and Caspase 9 in bovine mammary gland tissues; β-actin was used as the loading control. (C) Mean ± SEM of the immunopositive bands of PCNA, CCNA1, BCL2, BAX, BCL2/BAX, Caspase 3 and Caspase 9. The relative protein expression level of each target protein was standardized to that in the control group. *p < 0.05 and **p < 0.01 versus the control group.
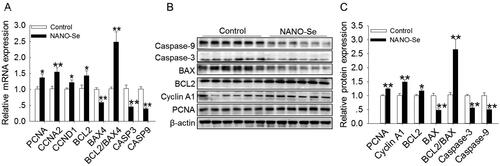
Figure 3. Effects of dietary nanoselenium (NANO-Se) addition on the Akt-mTOR signaling pathway in the bovine mammary gland. (A) Western blot analysis of Akt, p-Akt, mTOR and p-mTOR in bovine mammary glands treated with NANO-Se (0 and 0.3 mg/kg of Se from NANO-Se); β-actin was used as the loading control. (B) Mean ± SEM of the immunopositive bands of p-Akt/Akt and p-mTOR/mTOR. The relative protein expression level of each target protein was standardized to that in the control group. *p < 0.05 and **p < 0.01 versus the control group.
Discussion
Feed intake, milk yields and feed efficiency
The linear increase in the DMI of dairy cows agrees with the increased dietary NDF and ADF digestibility following the NANO-Se addition in this study and a previous positive correlation between dietary DMI and NDF digestibility in dairy cows.Citation31 Similarly, Zhang et al. demonstrated that the increased DMI was due to the increased NDF digestibility with coated sodium selenite addition.Citation11 The increase in the production of FCM, ECM and milk fat was attributed to the positive effects of NANO-Se supplementation on DMI, total VFA concentration, digestibility and the proliferation of mammary gland cells. Moreover, the increase in feed efficiency indicated that dietary NANO-Se addition enhanced the utilization of nutrients in cows. Acetate and butyrate produced by rumen fermentation are precursor substances of milk fatty acid synthesisCitation32 and approximately 60% of glucose used for the synthesis of milk lactose is derived from hepatic gluconeogenesis via propionate.Citation33 Therefore, the increased ruminal acetate and propionate contents resulted in a higher production of milk fat and FCM with NANO-Se supplementation. Similarly, previous studies demonstrated that yields of milk and milk fat were increased following 0.3 mg/kg of Se addition from Se-yeastCitation9,Citation10 or from coated sodium seleniteCitation11 in comparison with those in control diets. The linearly increased concentration of Se in dairy cow milk from 20.4 µg/kg (control) to 47.9 µg/kg (0.5 mg of Se from NANO-Se per kg dietary DM) revealed a dose effect of the NANO-Se addition and confirmed the findings observed by previous studies where 2–40 mg/d of Se-yeast was provided to cows.Citation34–36
Milk fat composition
The reduction of SFA proportions and the elevation of MUFA and PUFA proportions in milk fat following NANO-Se addition implied that supplementation with NANO-Se stimulated the dehydrogenation of SFA in the mammary gland and then improved the synthesis of MUFA and PUFA. These findings were confirmed by the increased mRNA expression of SCD and the increased ratio of RA to the sum of RA and VA with increasing NANO-Se addition. The proportions of SCFA were decreased and those of MSFA and PFA, and in particular C16:0, cis-C18:1, nonconjugated C18:2 and C20:3n-6, were increased following NANO-Se addition. These findings agree with the result of Pulido et al. who demonstrated a reduction in the content of several SCFAs and an increase in that of the C18:1 fatty acids.Citation37 De novo synthesis of SCFAs depends on the plasma concentration of acetate and β-hydroxybutyric acid and is downregulated by increasing the plasma concentration of long-chain fatty acids through the activity of acetyl CoA carboxylase.Citation38,Citation39 Although the ruminal acetate concentration increased, the proportions of SCFAs in milk fat decreased quadratically, whereas the proportions of MSFA and PFA increased following NANO-Se addition. Nevertheless, the unchanged DMI and fat digestibility cannot support the increase in the proportions of long-chain fatty acids,Citation37 and thus other mechanisms could be involved. Vázquez-Añón et al. suggested oxidative protection of cis-9 C18:1 in feed to explain the elevation in the proportion of this FA in milk in response to Se supplementation.Citation40
Nutrient digestion and ruminal fermentation
The increased response in nutrient digestibility following NANO-Se addition was caused by enhanced ruminal fermentation and microbial enzymatic activity, supporting the improved FCM and ECM production. Furthermore, the quadratic influence of NANO-Se on dietary DM, OM, CP, NDF and ADF digestibility suggested that greater doses of NANO-Se would decrease dietary DM, OM, CP, NDF and ADF digestibility and support the quadratic production of FCM and ECM following the increased NANO-Se doses. Zhang et al. also found that the positive effect of a high concentration of Se on the antioxidant function was gradually diminished in healthy BMECs.Citation16 Based on these results, a moderate dose of NANO-Se (0.3 mg/kg of Se) is beneficial for nutrient digestion.
The quadratical decreased ruminal pH with increasing NANO-Se addition as in previous observations was due to the the increased total VFA concentration.Citation11 The increased total VFA concentration and acetate concentrations (84.7, 86.0, 87.8 and 86.6 mM for 0, 0, 0.1, 0.3 and 0.5 mg of Se from NANO-Se per kg dietary DM, respectively) were in agreement with the increased dietary DM digestibility and were attributed to ruminal enzymatic activities (CMCase, cellobiase, xylanase and pectinase) following NANO-Se addition. Furthermore, the quadratically decreased acetate-to-propionate ratios were due to the unaltered acetate molar percentage and linearly increased propionate molar percentage. This implied that the ruminal fermentation pattern tended toward propionate formation under increased NANO-Se addition conditions.
In cows, dietary fiber is degraded to acetate by the ruminal bacteria, protozoa and fungi that secrete cellulolytic enzymes.Citation41 Feed lignocellulosic tissues can be degraded by ruminal fungi, and approximately 30% of fiber digestion and 10% of VFA production could be due to ruminal protozoa.Citation42 Therefore, the linear increase in ruminal CMCase, cellobiase and xylanase activities with an increasing NANO-Se dosage was a consequence of the increased populations of total bacteria, including Ruminococcus albus and Ruminococcus flavefaciens and total anaerobic fungi.Citation11 Moreover, this result supported the enhanced total ruminal VFA and increased dietary NDF and ADF digestibility. Dietary Se can be used by ruminal microbes for protein and cell wall synthesizesCitation43 and subsequently the increased antioxidant status of the ruminal microbes is beneficial for microbial growth.Citation44 Furthermore, populations of total ruminal bacteria and protozoa have been shown to increase by supplementation with additional Se in sheep dietsCitation44 and in dairy cow diets.Citation11 The quadratic increase in α-amylase activity and a linear increase in pectinase activity were consistent with the increase in the R. amylophilus population,Citation11,Citation45 indicating that NANO-Se addition promoted ruminal starch degradation and supporting the increase in propionate molar percentage. The unaltered ruminal protease activity was mainly associated with the unchanged Prevotella ruminicola population.Citation11 Furthermore, this finding confirmed that microbial protein synthesis was promoted, as demonstrated by the linear decline in the ammonia N concentration with increased NANO-Se supplementation because ruminal microbes utilize carbon skeletons and ammonia N to synthesize microbial proteins.Citation46 The results were consistent with the addition of Se at 0.3 mg/kg from NANO-Se in sheep dietsCitation15 and from Se-yeast in dairy cow diets.Citation9
Blood metabolites
Levels of blood glucose quadratically increased following NANO-Se addition, which agrees with the results of previous studies with Se-yeastCitation9 and coated sodium seleniteCitation11 addition in dairy cows, and may be ascribed to an increase in the ruminal propionate output,Citation47 since propionate produced by ruminal fermentation is transported to the liver and subsequently converted to glucose.Citation48 The total protein, albumin and BUN contents are indicators of the efficiency of protein utilization.Citation49 We observed increased albumin and total protein levels and decreased BUN content following NANO-Se supplementation. This may suggest that the feed either promoted the efficiency of protein utilization for milk protein secretion or lowered the mobilization of body proteins.Citation50 Estradiol, prolactin and IGF-1 are recognized as being vital for mammary duct development,Citation51 and ovary-derived estradiol, prolactin and IGF-1 stimulate epithelial cell proliferation.Citation52 In addition, IGF-1 activates the PI3K/Akt signaling pathway through its receptor and promotes the proliferation of epithelial cells.Citation53 Therefore, the NANO-Se-induced elevation of serum estradiol, prolactin and IGF-1 levels seen in this study supports the notion that NANO-Se stimulates mammary gland development. The linearly increased level of blood Se with increasing NANO-Se addition indicated that NANO-Se was effectively absorbed. Moreover, Han et al. demonstrated that the blood Se content and GSH-Px activity were increased by NANO-Se addition in comparison with that of sodium selenite addition.Citation18
Expressions of genes and proteins implicated in fatty acid synthesis
In the mammary gland, both PPARG and SREBPF1 regulate the expressions of ACACA, FASN, SCD and FABP3 mRNA.Citation54,Citation55 Moreover, both FASN and ACACA are involved in the synthesis of milk fatty acids from acetic acid and β-hydroxybutyric acidCitation54 and are positively correlated with the secretion of C4–16 fatty acids.Citation56 SCD catalyses the synthesis of MUFA from SFA.Citation57,Citation58 Furthermore, FABP3 is related to long-chain fatty acid absorption and transport in BMECs.Citation58 The increased levels of mRNA and proteins of FASN, ACACA, PPARG, SCD and SREBPF1 following NANO-Se supplementation indicated that the expressions of genes and proteins involved in the synthesis of SCFAs and medium-chain fatty acids were upregulated, supporting the elevation in the milk fat yield and milk MUFA. The increased mRNA expression of FABP3 indicated that NANO-Se addition stimulated the genes involved in long-chain fatty acid synthesis, absorption and transport in the bovine mammary gland.
Expressions of genes and proteins implicated in cell proliferation and apoptosis
Stimulating cell proliferation and suppressing cell apoptosis in the mammary gland should be the critical control point that promotes lactation persistence in dairy cows.Citation59 Cyclin D modulates the transition from the G1 phase to the S phase of the cell cycle, while cyclin A is considered essential for initiating and completing DNA replication in the S phase.Citation60 In addition, PCNA is an indispensable component of both DNA replication and repair.Citation61 Moreover, previous studies demonstrated the involvement of cyclin D3 and PCNA in modulating mammary epithelial cell proliferation.Citation62 We demonstrated that NANO-Se addition increased the expressions of mRNA cyclin (CCNA2 and CCND1) and PCNA mRNA and those of CCNA1 and PCNA proteins, indicating that NANO-Se stimulates the proliferation of mammary epithelial cells. Apoptosis is executed in cells via a highly complex signaling cascade by two paths, the mitochondrial and death receptor pathways, and is induced by the BCL2 family proteins. Furthermore, the BCL2 family proteins participate in the modulation of apoptotic cell death, including antiapoptotic (BCL2, etc.) and proapoptotic (BAX, etc.) members.Citation63 Since BCL2 prevents apoptosis by inhibiting the activity of BAX, a high BCL2/BAX ratio is an indication of suppressed apoptosis. Therefore, the ratio of the levels of BCL2/BAX proteins could indicate the status of cellular apoptosis. Both the mitochondrial and death receptor pathways converge onto the common pathway of executioner caspases (Caspase-3 and Caspase-9),Citation64 which play a crucial part in the execution phase of cell apoptosis, and reducing caspase expression suppresses the initiation of apoptosis. Herein, the increased expressions of BCL2 mRNA and protein and the BCL2/BAX ratio and the decreased expressions of BAX mRNA and protein and Caspase-3 and Caspase-9 suggested that the addition of NANO-Se promoted the expressions of apoptotic markers. Therefore, NANO-Se likely modulated proliferation by stimulating the expressions of proliferative markers and inhibiting the expressions of apoptotic markers. Similarly, Zhang et al. demonstrated that the addition of 50–200 nM Se significantly increased the cell viability and relative growth rate of BMECs, with 50 nM Se being the optimum dose.Citation16
Other studies have implicated the Akt signaling pathway in the modulation of the proliferation and apoptosis of various cells, such as breast cancer cellsCitation65 and BMECs.Citation66 Furthermore, the mTOR signaling pathway is implicated in regulating the proliferation of various cells.Citation67 In this study, the addition of NANO-Se stimulated Akt and mTOR phosphorylation. These findings suggest that activation of the Akt-mTOR signaling pathway may stimulate the expressions of proliferative markers and consequently increase milk production and milk fat synthesis with NANO-Se addition.
Conclusion
In conclusion, we demonstrated that supplementation of NANO-Se in dairy cow diets improved milk yields and the feed efficiency by stimulating total tract nutrient digestion, ruminal fermentation and mammary gland fatty acid synthesis. Moreover, the addition of NANO-Se activated the Akt/mTOR pathway and promoted the expressions of proliferative markers. It is advisable to study the effect of nano-Se compared to common mineral sources of selenium.
Authors contributions
Yapeng Liu: Formal analysis, writing (original draft) and visualization. Jing Zhang: Visualization, writing (original draft) and project administration. Lijun Bu: Data curation. Caixia Pei: Methodology and validation. Wenjie Huo: Methodology. Chengqiang Xia: Software and validation. Qiang Liu: Conceptualization, funding acquisition, software, supervision and editing.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Akers RM. A 100-year review: mammary development and lactation. J Dairy Sci. 2017;100(12):10332–10352.
- Molenaar AJ, Maclean PH, Gilmour ML, et al. Effect of whole-milk allowance on liveweight gain and growth of parenchyma and fat pads in the mammary glands of dairy heifers at weaning. J Dairy Sci. 2020;103(6):5061–5069.
- Macrina AL, Kauf ACW, Kensinger RS. Effect of bovine somatotropin administration during induction of lactation in 15-month-old heifers on production and health. J Dairy Sci. 2011;94(9):4566–4573.
- McCabe CJ, Suarez-Trujillo A, Teeple KA, Casey TM, Boerman JP. Chronic prepartum light-dark phase shifts in cattle disrupt circadian clocks, decrease insulin sensitivity and mammary development, and are associated with lower milk yield through 60 days postpartum. J Dairy Sci. 2021;104(2):2422–2437.
- Xiao JX, Khan MZ, Ma YL, et al. The antioxidant properties of selenium and vitamin E; their role in periparturient dairy cattle health regulation. Antioxidants. 2021;10(10):1555.
- Zhong JX, Jin SS, Wu KS, Yu GC, Tu LL, Liu L. Effect of nano-selenium loaded with Lycium barbarum polysaccharide on the proliferation of lens epithelial cells after UVB damage in vitro. Int J Ophthalmol. 2022;15(1):9–14.
- Huang Z, Rose AH, Hoffmann PR. The role of selenium in infammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2012;16(7):705–743.
- Li F, Lutz PB, Pepelyayeva Y, Arnér ES, Bayse CA, Rozovsky S. Redox active motifs in selenoproteins. Proc Natl Acad Sci USA. 2014;111(19):6976–6981.
- Wang C, Liu Q, Yang WZ, et al. Effects of selenium yeast on rumen fermentation, lactation performance and feed digestibilities in lactating dairy cows. Livest Sci. 2009;126(1–3):239–244.
- Ullah H, Khan RU, Mobashar M, et al. Effect of yeast-based selenium on blood progesterone, metabolites and milk yield in Achai dairy cows. Ital J Anim Sci. 2019;18(1):1445–1450.
- Zhang ZD, Wang C, Du HS, et al. Effects of sodium selenite and coated sodium selenite on lactation performance, total tract nutrient digestion and rumen fermentation in Holstein dairy cows. Animal. 2020;14(10):2091–2099.
- Najafnejad B, Aliarabi H, Tabatabaei MM, Alimohamady R. Effects of different forms of selenium supplementation on production performance and nutrient digestibility in lactating dairy cattle. Paper presented at: Proceedings of the Second International Conference on Agriculture and Natural Resources; December 2013; Kermanshah, Iran, p. 25–26.
- Zhang Y, Xu Y, Chen B, Zhao B, Gao XJ. Selenium defciency promotes oxidative stress-induced mastitis via activating the NF-κB and MAPK pathways in dairy cow. Biol Trace Elem Res. 2021;200(6):2716–2726.
- He SL, Guo X, Tan WH, et al. Effect of selenium deficiency on phosphorylation of the AMPK pathway in rats. Biol Trace Elem Res. 2016;169(2):254–260.
- Shi L, Duan YL, Yao XL, Song RG, Ren YS. Effects of selenium on the proliferation and apoptosis of sheep spermatogonial stem cells in vitro. Anim Reprod Sci. 2020;215:106330.
- Zhang BQ, Guo YM, Yan SM, Guo XY, Zhao YL, Shi BL. The protective effect of selenium on the lipopolysaccharide-induced oxidative stress and depressed gene expression related to milk protein synthesis in bovine mammary epithelial cells. Biol Trace Elem Res. 2020;197(1):141–148.
- Zhang JS, Wang XF, Xu TW. Elemental selenium at nano size (Nano-se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with se-methylselenocysteine in mice. Toxicol Sci. 2008;101(1):22–31.
- Han LQ, Pang K, Fu T, Phillips CJC, Gao TY. Nano-selenium supplementation increases selenoprotein (Sel) gene expression profiles and milk selenium concentration in lactating dairy cows. Biol Trace Elem Res. 2021;199(1):113–119.
- NRC (National Research Council). Nutrient Requirements of Dairy Cattle. 7th ed. Washington, DC: National Academies Press; 2001.
- Farr VC, Stelwagen K, Cate LR, Molenaar AJ, McFadden TB, Davis SR. An improved method for the routine biopsy of bovine mammary tissue. J Dairy Sci. 1996;79(4):543–549.
- AOAC. Official Methods of Analysis. 17th ed. Arlington, VA: Association of Official Analytical Chemists; 2000.
- Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74(10):3583–3597.
- Chouinard PY, Corneau L, Barbano DM, Metzger LE, Bauman DE. Conjugated linoleic acids alter milk fatty acid composition and inhibit milk fat secretion in dairy cows. J Nutr. 1999;129(8):1579–1584.
- Rico DE, Harvatine KJ. Induction of and recovery from milk fat depression occurs progressively in dairy cows switched between diets that differ in fiber and oil concentration. J Dairy Sci. 2013;96(10):6621–6630.
- Williams CH, David DJ, Iismaa O. The determination of chromic oxide in faeces samples by atomic absorption spectrophotometry. J Agric Sci. 1962;59(3):381–385.
- Agarwal N, Kamra DN, Chaudhary LC, Agarwal I, Sahoo A, Pathak NN. Microbial status and rumen enzyme profile of crossbred calves fed on different microbial feed additives. Lett Appl Microbiol. 2002;34(5):329–336.
- Webb S, Bartos J, Boles R, Hasty E, Thuotte E, Thiex NJ. Simultaneous determination of arsenic, cadmium, calcium, chromium, cobalt, copper, iron, lead, magnesium, manganese, molybdenum, nickel, selenium, and zinc in fertilizers by microwave acid digestion and inductively coupled plasma-optical emission spectrometry detection: single-laboratory validation of a modification and extension of AOAC 2006.03. J AOAC Int. 2014;97(3):700–711.
- Kuzinski J, Zitnan R, Viergutz T, Legath J, Schweige M. Altered Na+/K+-ATPase expression plays a role in rumen epithelium adaptation in sheep fed hay ad libitum or a mixed hay/concentrate diet. Vet Med. 2011;56(1):36–48.
- Busato S, Mezzetti M, Logan P, Aguilera N, Bionaz M. What’s the norm in normalization? A frightening note on the use of RT-qPCR in the livestock science. Gene. 2019;721S:100003.
- NASEM (National Academies of Sciences, Engineering, and Medicine). Nutrient Requirements of Dairy Cattle. 8th ed. Washington, DC: National Academies Press; 2021.
- Allen MS. Effects of diet on short-term regulation of feed intake by lactating dairy cattle. J Dairy Sci. 2000;83(7):1598–1624.
- Mansson HL. Fatty acids in bovine milk fat. Food Nutr Res. 2008;52:1–3.
- Reynolds CK. Production and metabolic effects of site of starch digestion in dairy cattle. Anim Feed Sci Technol. 2006;130(1–2):78–94.
- Givens DI, Allison R, Cottrill B, Blake JS. Enhancing the selenium content of bovine milk through alteration of the form and concentration of selenium in the diet of the dairy cow. J Sci Food Agric. 2004;84(8):811–817.
- Heard JW, Stockdale CR, Walker GP, et al. Increasing selenium concentration in milk: effects of amount of selenium from yeast and cereal grain supplements. J Dairy Sci. 2007;90(9):4117–4127.
- Doyle PT, Stockdale CR, Jenkin ML, et al. Producing milk with uniform high selenium concentrations on commercial dairy farms. Anim Prod Sci. 2011;51(2):87–94.
- Pulido E, Fernández M, Prieto N, et al. Effect of milking frequency and α-tocopherol plus selenium supplementation on sheep milk lipid composition and oxidative stability. J Dairy Sci. 2019;102(4):3097–3109.
- Chilliard YD, Ferlay A, Mansbridge RM, Doreau M. Ruminant milk fat plasticity: Nutritional control of saturated, polyunsaturated, trans and conjugated fatty acids. Ann Zootech. 2000;49(3):181–205.
- Loften JR, Linn JG, Drackley JK, Jenkins TC, Soderholm CG, Kertz AF. Invited review: Palmitic and stearic acid metabolism in lactating dairy cows. J Dairy Sci. 2014;97(8):4661–4674.
- Vázquez-Añón M, Nocek J, Bowman G, et al. Effects of feeding dietary antioxidant in diets with oxidized fat on lactation performance and antioxidant status of the cow. J Dairy Sci. 2008;91(8):3165–3172.
- Orpin CG. The role of ciliate protozoa and fungi in the rumen digestion of plant cell walls. Anim Feed Sci Technol. 1984;10(2–3):121–143.
- Wang Y, McAllister TA. Rumen microbes, enzymes, and feed digestion-a review. Asian Australas J Anim Sci. 2002;15(11):1659–1676.
- Hidiroglou M, Heaney DP, Jenkins KJ. Metabolism of inorganic selenium in rumen bacteria. Can J Physiol Pharmacol. 1968;46(2):229–232.
- Mihaliková K, Gresáková L, Boldizárová K, Faix S, Leng L, Kisidayová S. The effects of organic selenium supplementation on the rumen ciliate population in sheep. Folia Microbiol (Praha). 2005;50(4):353–356.
- Cheng KF, Wang C, Zhang GW, et al. Effects of betaine and rumen-protected folic acid supplementation on lactation performance, nutrient digestion, rumen fermentation and blood metabolites in dairy cows. Anim Feed Sci Technol. 2020;262:114445.
- Reynolds CK, Kristensen NB. Nitrogen recycling through the gut and the nitrogen economy of ruminants: an asynchronous symbiosis. J Anim Sci. 2008;86(14):E293–E305.
- Foley PA, Kenny DA, Lovett DK, Callan JJ, Boland TMO, Mara FP. Effect of dl-malic acid supplementation on feed intake, methane emissions, and performance of lactating dairy cows at pasture. J Dairy Sci. 2009;92(7):3258–3264.
- Chan TM, Freedland RA. The effect of propionate on the metabolism of pyruvate and lactate in the perfused rat liver. Biochem J. 1972;127(3):539–543.
- Nousiainen J, Shingfield KJ, Huhtanen P. Evaluation of milk urea nitrogen as a diagnostic of protein feeding. J Dairy Sci. 2004;87(2):386–398.
- Lee C, Hristov AN, Heyler KS, et al. Effects of dietary protein concentration and coconut oil supplementation on nitrogen utilization and production in dairy cows. J Dairy Sci. 2011;94(11):5544–5557.
- Rosen JM. On hormone action in the mammary gland. CSH Perspect Biol. 2012;4(2):a013086–a013086.
- Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor α is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA. 2006;103(7):2196–2201.
- Zhou Y, Li S, Li J, Wang D, Li Q. Effect of microRNA-135a on cell proliferation, migration, invasion, apoptosis and tumor angiogenesis through the IGF-1/PI3K/Akt signaling pathway in non-small cell lung cancer. Cell Physiol Biochem. 2017;42(4):1431–1446.
- Bionaz M, Loor J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genomics. 2008;9(1):366.
- Ma L, Corl BA. Transcriptional regulation of lipid synthesis in bovine mammary epithelial cells by sterol regulatory element binding protein-1. J Dairy Sci. 2012;95(7):3743–3755.
- Bernard L, Leroux C, Chilliard Y. Expression and nutritional regulation of lipogenic genes in the ruminant lactating mammary gland. Adv Exp Med Biol. 2008;606:67–108.
- Lehner R, Kuksis A. Biosynthesis of triacylglycerols. Prog Lipid Res. 1996;35(2):169–201.
- Sheng R, Yan SM, Qi LZ, Zhao YL, Jin L, Guo XY. Effect of the ratios of acetate and β-hydroxybutyrate on the expression of milk fat- and protein-related genes in bovine mammary epithelial cells. Czech J Anim Sci. 2015;60(12):531–541.
- Bae D, Chon JW, Kim DH, Kim H, Seo KH. Effect of folic acid supplementation on proliferation and apoptosis in bovine mammary epithelial (MAC-T) cells. Anim Biotechnol. 2020;33(1):13–21.
- Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140(15):3079–3093.
- Park SY, Mi SJ, Chang WH, Yu HS, Jang SB. Structural and functional insight into proliferating cell nuclear antigen. J Microbiol Biotechnol. 2016;26(4):637–647.
- Zhang J, Ye J, Yuan C, et al. Exogenous H2S exerts biphasic effects on porcine mammary epithelial cells proliferation through PI3K/Akt-mTOR signaling pathway. J Cell Physiol. 2018;233(10):7071–7081.
- Shamas-Din A, Kale J, Leber B, Andrews DW. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb Perspect Biol. 2013;5(4):a008714.
- Pisani C, Ramella M, Boldorini R, et al. Apoptotic and predictive factors by Bax, Caspases 3/9, Bcl-2, p53 and Ki-67 in prostate cancer after 12 Gy single-dose. Sci Rep. 2020;10(1):7050.
- Huang J, Qin YY, Lin CF, Huang XG, Zhang FR. MTHFD2 facilitates breast cancer cell proliferation via the AKT signaling pathway. Exp Ther Med. 2021;22(1):703.
- Liu LH, Sun B, Zhang F, et al. lncRNA MPFAST promotes proliferation and fatty acid synthesis of bovine mammary epithelial cell by sponging miR-103 regulating PI3K-AKT pathway. J Agric Food Chem. 2022;70(38):12004–12013.
- Li L, Liu L, Qu B, Li X, Gao X, Zhang M. Twinfilin 1 enhances milk bio-synthesis and proliferation of bovine mammary epithelial cells via the mTOR signaling pathway. Biochem Biophys Res Commun. 2017;492(3):289–294.
