Abstract
This study explored the effects of different vitamin B5 (VB5) levels on intestinal growth and function of weaned piglets. Twenty-one piglets (7.20 ± 1.11 kg) were included in a 28-day feeding trial with three treatments, including 0 mg/kg (L-VB5), 10 mg/kg (Control) and 50 mg/kg (H-VB5) of VB5 supplement. The results showed that: Large intestine weight/body weight was the highest in H-VB5 group, Control and H-VB5 groups had significantly higher villus height and villus height/crypt depth than the L-VB5 in the ileum (p < .05). Goblet cells (ileal crypt) and endocrine cells (ileal villus) significantly increased in Control and H-VB5 (p < .05). The H-VB5 group exhibited significantly higher levels of ki67 and crypt depth in the cecum and colon, colonic goblet cells and endocrine cells were both rising considerably (p < .05). Isobutyric acid and isovaleric acid were significantly reduced in the H-VB5 group (p < .05), and there was a decreasing trend in butyric acid (p = .073). At the genus level, the relative abundance of harmful bacteria such as Clostridium_Sensu_Structo_1 Strecto_1, Terrisporbacter and Streptococcus decreased significantly and the relative abundance of beneficial bacteria Turicibacter increased significantly in H-VB5 group (p < .05). Overall, the addition of 50 mg/kg VB5 primarily enhanced the morphological structure, cell proliferation and differentiation of the ileum, cecum and colon. It also had a significant impact on the gut microbiota and short-chain fatty acids.
Introduction
Pigs in all phases of growth and development need a healthy digestive system to maintain their general metabolism, physiology, health condition and performance.Citation1 Previous research has shown that even in the absence of disease, the pig’s gastrointestinal tract can be compromised.Citation2 Low feed intake after weaning, for example, can result in piglets’ intestinal lumens lacking adequate nutrients.Citation3 Furthermore, numerous studies have demonstrated that the presence of nutrients in the lumen after weaning has a direct impact on the maintenance of intestinal structure and function.Citation4,Citation5 Piglet weaning also results in significant changes in the structure and function of the gastrointestinal tract, as well as an inflammatory state, which damages the villous-crypt structure, the gastrointestinal barrier function and the microbiota.Citation6,Citation7 It has been shown that dietary supplementation with appropriate doses of vitamins, can adjust the intestinal function of weaned piglets and reduce the risk of inflammation and other diseases.Citation8–11
Pantothenic acid, a form of water-soluble vitamin B5 (VB5), serves as a crucial raw material for the synthesis of coenzyme A (CoA) in cells. It plays a vital role in various metabolic pathways, such as glucose and fat metabolism, ensuring the maintenance of body homeostasis.Citation12 While plants and microorganisms are capable of synthesizing VB5, animal cells lack this ability. Additionally, acetyl CoA can be converted into CoA.Citation13 By increasing CoA levels and promoting glutathione synthesis, VB5 can enhance the inflammatory process and reduce oxidative stress.Citation14,Citation15 Moreover, VB5 significantly contributes to strengthening animals’ immunity and resistance against bacterial infections. It regulates the maturation of macrophages, promotes macrophage phagocytosis and restricts the growth of mycobacteria within macrophage cells.Citation16 Activation of pro-inflammatory pathways, such as NF-κB and PI3K-AKT, leads to the production of cytokines in innate immune cells like macrophages, neutrophils and dendritic cells.Citation17 Previous study found that VB5 is capable of activating different inflammatory signaling molecules (NF-κB, AKT, ERK and P38) and enhancing the expression of tumor necrosis factor-alpha (TNFα) and interleukin-6 (IL-6). These molecules play a crucial role as immune factors against tuberculosis.Citation18 Furthermore, VB5 reduces triglyceride synthesis in the liver, promotes protein synthesis and conversion and facilitates the complete oxidation of fatty acids while decreasing ketone production.Citation19 Inadequate intake of VB5 can lead to liver fat deposition and metabolic disorders.Citation20 Deficiency in VB5 has been associated with growth failure, weight loss, dermatitis, dyslipidemia, neuropathy and adrenal disease.Citation21 Therefore, supplementing animals with sufficient VB5 can improve their physiological condition.
The National Research Council (NRC) recommends adding 10 mg/kg of VB5 to the diet of weaned piglets weighing 7–11 kg. However, due to severe intestinal damage after weaning and reduced feed intake, piglets struggle to obtain enough feed to meet their VB5 needs. Therefore, it is necessary to increase the dose of VB5 in the diet of weaned piglets to meet their requirements, promote immune function and improve disease resistance. In this study, we hypothesized that the VB5 levels recommended by the NRC would lead to VB5 deficiency after weaning, which would worsen weaning stress. To address this, we added VB5 in higher amounts than theCitation22 recommended levels (1 and 5 times) to the basal diet. This ensured a relatively high level of VB5 in the diet, which is crucial for normal growth and integrity of the piglet.Citation23–25 A large number of studies have shown that supplementing the diet with VB5 can improve the growth performance of poultry, ruminants and growing pigs.Citation12,Citation24,Citation26,Citation27 The intestines of piglets grow faster than other body parts after weaning. Currently, there is limited data available on the effects of VB5 on the physicochemical aspects of weaned piglets’ intestines. Therefore, this study aimed to investigate the effects of different levels of VB5 on growth performance, intestinal development, short-chain fatty acid (SCFA) concentration and intestinal microbiota of weaned piglets. This may provide valuable insights into determining the optimal dosage of VB5 supplementation for weaned piglets.
Materials and methods
Animals and the experimental design
Twenty-one 21-day-old weaned piglets ([Large White × Landrace] × Duroc) were randomly assigned to three dietary treatments with seven replicate pens (one piglet per replicate pen) according to body weight (BW, 7.20 ± 1.11 kg) for 28-day period experiment, respectively. The L-VB5, control and H-VB5 groups were supplemented with an additional 0, 10 and 50 mg/kg of VB5 in the basal diet. The basal diet composition shown in was designed to meet the nutrient requirements of weaned piglets.Citation22 Phase I (day 0–14) basal diet contained 9.59 mg/kg VB5. On days 15, 16 and 17, the phase I feed was gradually replaced with the phase II ration at 25%, 50% and 75%, respectively, until all the feed of phase I was replaced. The phase II basal diet (days 18–28) contained VB5 of 9.18 mg/kg. During the trial, the piglets were kept in individual cages and were free to feed and drink. The trough was kept clean and the feed fresh to prevent the feed from getting moldy. Pig’s house was regularly cleaned and disinfected, and air circulation was regularly ventilated.
Table 1. Ingredient and chemical composition of piglet diets, as-fed basis.
Sample collection
During the first week of the trial, one piglet in the control group died and the remaining piglets were sampled. The piglets were euthanized after fasting overnight. Following the measurement of the weight and total length of the small and large intestines from each piglet, the organ indexes (the ratio of the intestine’s total weight or total length to BW) were computed using the piglets’ 28-day BW. Approximately 2 cm samples of duodenum, jejunum, ileum, cecum and colon were taken and stored in a 4% formalin solution. The rest of duodenum, jejunum, ileum, cecum and colon were wrapped in tin foil, snap frozen in liquid nitrogen and then stored at −80 °C.Citation28
Growth performance measurement
Piglets were weighed at 0,14 and 28d of the trial to determine weight gain. Each pig’s feed consumption was also weighed daily to determine feed intake. The average daily gain (ADG), average daily feed intake (ADFI) and feed conversion ratio (FCR) for each pen were calculated at the end of the experiment.Citation29 Record diarrhea per piglet per day to calculate diarrhea rate.Citation30
Intestinal histology and morphology analysis
Intestinal tissues were soaked in 4% formalin solution, sectioned using standard paraffin embedding methods and stained with hematoxylin and eosin (H&E). The height of villi and the depth of crypts were observed using a light microscope (Leica Imaging Systems Ltd, Cambridge, UK). Twenty intact villi and corresponding crypts were selected from each piglet and measured using Image-Pro Plus 6.0 software (Media Cybernetics, San Diego, CA). Goblet cells were mounted for Alcian blue-periodic Acid-shiff staining (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), and they were counted in 20 intact villi and crypts.Citation28,Citation30
Immunohistochemistry
Paraffin sections of jejunum with a thickness of 4 μm were dewaxed in xylene, then rehydrated with gradient alcohol and washed three times in phosphate buffer (pH =7.4). Block the endogenous peroxidase with 3% hydrogen peroxide. Then it was washed with PBS 3 times, then boiled twice in 0.01 mol/L and pH 6.0 citrate buffer for antigen repair. A total of 5% bovine serum albumin (Boster Biological Technology Co. Ltd, Wuhan, China) was reacted at 37 °C for 30 min. The sections were then incubated overnight with Ki67 antibody and Chga antibody (Cambridge, UK, Abcam;1:700 dilution) at 4 °C, and then washed with PBS 3 times. Then incubated with secondary antibodies (ZSGB-BIO, Beijing, China) at 37 °C for 1 h, washed with PBS 3 times, and positive cells were detected with diaminobenzidine kit (ZSGB-BIO). After anti-staining with hematoxylin, it was dehydrated by gradient alcohol. The samples were photographed at a magnification of 10 using a Leica DM 3000 microscope. For each piglet, 20 complete villi and crypts were collected.Citation31
Determination of SCFAs
Using a 10 mL centrifuge tube, weigh 1.000 g of fresh colon contents. Add 5 mL of distilled water, and centrifuge at 10000 × g for 15 min. In a 9:1 ratio, combine supernatant fluid with a 25% metaphosphoric acid solution. After centrifuging overnight, the solution was centrifuged in a 4 °C centrifuge (10000 × g) for 10 min and filtered through a membrane filter with 0.45 μm pore diameter and transferred to an injection vial. The SCFAs concentrations of samples were determined by using Agilent Technologies 7890B GC System (AGILENT, USA) with flame ionization detector on a DB-FFAP column (30 μm long × 250 μm diameter × 0.25 μm film thickness). Nitrogen was used as the carrier gas with a flow rate of 0.8 mL/min.Citation9
16S rRNA high-throughput sequencing
Genomic DNA was extracted from colon stool samples, and then the V3–V4 region of 16s rRNA gene was amplified by PCR (universal bacterial primers 515F (5′-ACTCCTACGGGAGGCAGCAG-3′) and the reverse primer 806R (5′-GGACTACHVGGGTWTCTAAT-3′)). Samples were sequenced on the Illumina HiSeq2500 platform provided by Majorbio Bio-pharm Technology (Shanghai, China). Using a single-end sequencing method, a small fragment library was constructed for single-end sequencing. After distinguishing the samples, clustering and species classification analysis were conducted using the operational taxonomic units (similarity level of 97%) of the clean data. Then, the alpha and beta diversity measurements were done using QIIME (Version 1.9.1) software.Citation11,Citation32 Raw 16s rRNA data sequences have been uploaded to the SRA database (PRJNA915325).
Statistical analysis
SPSS 22.0 statistical software (version 17.0; IBM Corp., Chicago, IL) was used for all statistical analyses. The data were examined for normal distribution and variance homogeneity. Use one-way analysis of variance if the means have a normal distribution; otherwise, use the chi-square test. Duncan’s multiple comparisons were used to estimate treatment differences, and values were presented as means ± SEM. p < .05 meant a significant difference, and.05 < p < .10 meant there is a trend of significant difference.
Results
Growth performance
During the 28-day trial period, there was no significant difference in initial BW, final weight, ADG, ADFI, FCR and diarrhea scores of piglets (). The results in show that there were no differences in the length and weight of the small intestine and the weight of the large intestine in piglets; however, the length of the large intestine (P = 0.083) increased when the diet was supplemented with 50 mg/kg VB5. There were no differences in organ indices for small intestine weight/BW, small intestine length/BW, large intestine length/BW, small intestine weight/small intestine length and large intestine weight/large intestine length, whereas, increasing the dietary VB5 concentration significantly increased large intestine weight/BW (p = 0.016).
Table 2. The growth performance in piglet.Table Footnotea
Table 3. Effect of vitamin B5 on intestinal organ indices.Table Footnotea
Intestinal morphology and immunohistochemistry
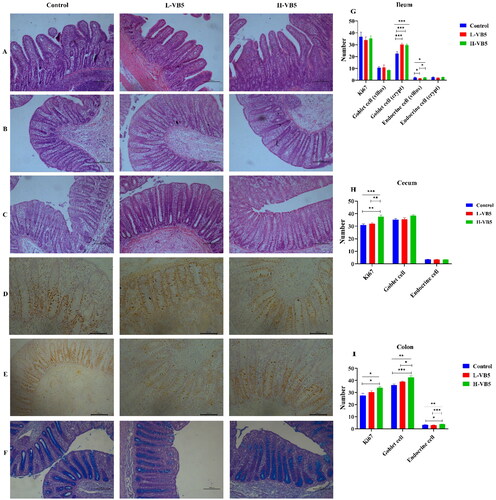
There were significant differences in the ileal villus height (p = .016) and villus height/crypt depth (p = .011) between the treatments with the addition of VB5 and L-VB5 groups. The depth of the crypt in the cecum and colon was significantly increased in the H-VB5 group compared to the Control and the L-VB5 group ().
Table 4. Effects of vitamin B5 on intestinal morphology.Table Footnotea
The number of goblet cells in the ileal crypt site was significantly increased in the L-VB5 and H-VB5 groups (p < .001), while the number of endocrine cells in the ileal villus site was significantly increased in the Control and H-VB5 groups (p = .029) as shown in . The crypt depth (p = .017), the number of Ki67 positive cells (p = .001) and goblet cells (P = .08) of cecum were significantly increased in H-VB5 group, whereas VB5 did not affect cecal endocrine cells. The number of Ki67 positive cells (p = .023), goblet cells (p = .001) and endocrine cells (p = .001) of colon were significantly increased in H-VB5 group.
Figure 1. Intestinal morphological structure and immunohistochemical analysis of weaned piglets fed with different dietary concentrations of VB5 (magnification 100×). (A) ileum morphology. (B) Colon morphology. (C) Cecum morphology. (D) Immunohistochemistry staining with a Ki67 antibody in the colon. (E) Immunohistochemistry staining with a Ki67 antibody in the cecum. (F) Alcian blue-periodic acid-shiff staining in the colon. (G)Immunohistochemistry of the ileum. (H) Immunohistochemistry of the cecum. (I) Immunohistochemistry of the colon. n = 7. *p < .05, **p < .01, ***p < .001.
Microbial assessment in colon contents
Colonic microbial alpha diversity indices showed no differences in Sobs, ACE, Shannon, Simpson and Chao1 indices for the three groups, as shown in . The principal co-ordinates analysis (PcoA) and non-metric multidimensional scaling (NMDS) analysis () showed no difference in the composition of the three groups at the OUT level.
Figure 2. Effect of vitamin B5 on the composition of colonic bacterial. (A) NMDS plot of functional profiles among groups. (B) PCoA plot of functional profiles among groups. (C) Colon bacterial communities at the genus level. (D) Colonic differential bacteria at the genus level. n = 7. *p < .05, **p < .01, ***p < .001.
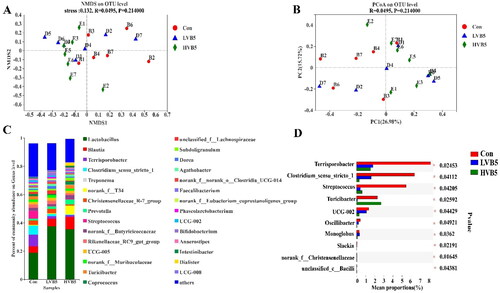
Table 5. Alpha diversity index.Table Footnotea
At the genus level, the top five dominant species were Lactobacillus, Blautia, Terrisporobacter, Clostridium_sensu_stricto_1 and Treponema (). The top 10 statistically different species at the genus level are shown in . Terrisporobacter, Clostridium_sensu_stricto_1, Streptococcus and UCG-002 in the H-VB5 group had significantly lower abundance (p < .05) compared to the Control group. Turicibacter in the Control and H-VB5 groups showed a significant increase in abundance compared to the L-VB5 group (p = .025).
Effect of VB5 on metabolic function of microbiota
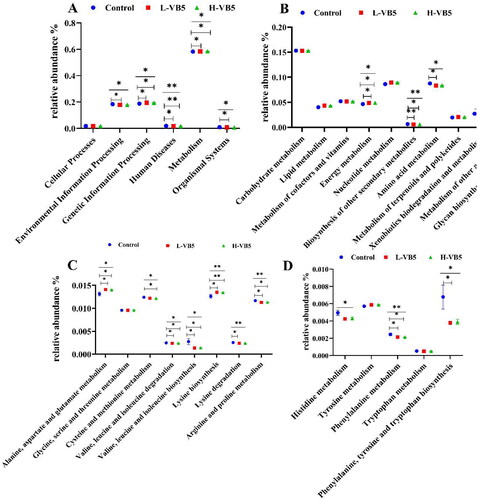
The effects of additional VB5 addition on colonic bacteria were focused on six aspects, and the most significant effect on metabolism, which include energy metabolism, biosynthesis of other secondary metabolites, amino acid metabolism, xenobiotics biodegradation and metabolism, metabolism of other amino acids and glycan biosynthesis and metabolism (). The addition of VB5 enhanced the alanine, aspartic acid and glutamic acid metabolism; cysteine and methionine metabolism; valine, leucine and isoleucine degradation; valine, leucine and isoleucine biosynthesis; lysine biosynthesis; lysine degradation; arginine and proline metabolism; histidine metabolism; phenylalanine metabolism and phenylalanine, tyrosine and tryptophan biosynthesis ().
Correlation analysis of gut morphology and microbes
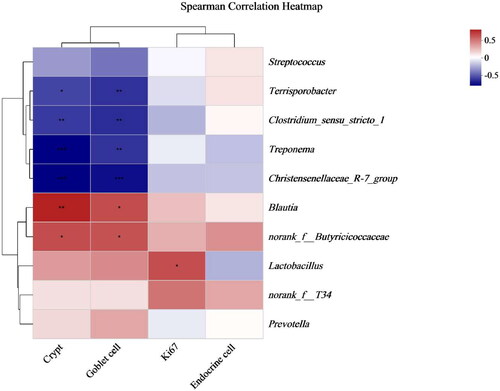
Spearman correlation analysis was conducted between the top 10 bacterial genera, and the depth of the colonic crypts, ki67-positive cells, endocrine cells and goblet cells are directly reflected by a heatmap (). The results indicated that Blautiu and norank_f__Butyricicoccaceae were positively correlated with crypt depth and goblet cells. Terrisporobacter, Clostridium_sensu_stricto_1, Treponema and Christensenellaceae_R-7_group were negatively correlated with crypt depth and goblet. Lactobacillus was positively correlated with ki67.
Effect of VB5 supplementation on the SCFAs concentration
There were no differences in the levels of acetic acid, propionic acid and valeric acid in the three groups; however, the concentration of isobutyric acid (p = .007) and isovaleric acid (p = .034) significantly decreased with increasing VB5 levels, and the content of butyric acid was decreased with increasing VB5 levels (p = .073) as shown in .
Table 6. Effect of VB5 on colonic SCFAs.Table Footnotea
Discussion
Previous report had shown that there was no significant effect of six different levels of pantothenic acid on weight gain rate, feed intake and blood biochemical properties of piglets,Citation33 In addition, Groesbeck et al. have reported no effect of increasing the amount of pantothenic acid on ADG, ADFI and FCR of test pigs.Citation24 In the present study, the addition of 0, 10 and 50 mg/kg VB5 did not affect the growth performance of piglets, which is consistent with Groesbeck’s and Krehl’s results. Weaning stress has been linked to changes in intestinal morphology and structure, including shortening of intestinal length, villus atrophy and crypt hyperplasia, which in turn reduces the absorption rate of piglets.Citation30,Citation34 The magnitude of villus height/crypt depth can represent the level of intestinal digestive and absorptive capacity.Citation35 Piglets absorb nutrients through the villus site, and an increase in villus height indicates that more nutrients are in contact with the villus, allowing more nutrients to be absorbed.Citation36 The villus height was higher in the Control, H-VB5 group than in the L-VB5 group in this experiment, indicating that VB5 effectively protected the intestinal villus and reduced the damage to the intestine caused by weaning stress. Furthermore, VB5 increases the ratio of villus height/crypt depth, most likely due to the high concentration of VB5 increased the rate of migration of crypt stem cells from the bottom to the top villus, which has a positive effect on intestinal repair.Citation37 Organ index can indicate the effectiveness of organ function,Citation9 and the impact of nutrition on intestine development can be determined by intestinal weight and length.Citation38 The addition of 50 mg/kg VB5 significantly increased the proportion of large intestine weight/BW ratio in piglets, and the large intestine length showed an increasing trend. The rapid proliferation and differentiation of intestinal epithelial cells is a prerequisite for normal intestinal growth.Citation39 In this study, the crypt depth of the cecum and colon was significantly increased in the H-VB5 group. Ki67-positive proliferating cells were significantly increased in the colon and cecum. The goblet and endocrine cells were significantly increased in the colon. In addition, the goblet cells in the cecum showed an increasing trend, which may explain the increased weight of intestinal tissues in the H-VB5 group. This shows that VB5 supplementation has a positive effect on the development of large intestine in weaned piglets.
The villus mediates nutrient absorption and acts as a barrier in the intestinal lumen against antigens, pathogens and toxins.Citation40 To ensure the function of the small intestine and to maintain a continuous and effective barrier function in a harsh intestinal environment, intestinal villus epithelial cells renew rapidly through cell proliferation and specific differentiation.Citation41 The majority of ki67 cells are found in the intestinal crypt, and counting ki67 cells is a common way to measure the proliferation of intestinal epithelial cells.Citation42 This study showed that VB5 did not affect the number of ileal ki67-positive cells. Previous studies have shown that the decrease in VH is either the result of increased cell loss or decreased crypt cell proliferation.Citation37,Citation43 The height of the ileal villus was significantly higher in the Control and H-VB5 groups than in the L-VB5 group, likely because VB5 protects the intestinal villus and reduces the rate of epithelial cell loss from the villus.Citation37 The H-VB5 group showed a significant increase in ki67-positive proliferating cells in the colon and cecum, as well as an increase in crypt depth and an increase in epithelial cells. These results indicate that high-dose VB5 alters intestinal morphology primarily by promoting continuous proliferation and differentiation of crypt in the colon and cecum to produce new enterocytes.
By the creation of energy by immune cells, VB5 contributes to the maintenance of host immunity.Citation44 Goblet cells, which are responsible for secreting and synthesizing mucus, are found in the mucosal epithelium. Goblet cells are required for the intestinal environment to be stable.Citation45 The L-VB5 group had significantly more goblet cells in the ileal crypts than the control group in this study, likely to reduce the intestinal damage caused by microbial diseases from early weaning by secreting large amounts of mucus.Citation37 Pantothenic acid is predominantly present in cells in the active form of CoACitation46 and acetyl CoA synthesizes acetylcholine,Citation47,Citation48 which is an effective inducer of intestinal mucus secretion and increases the rate of mucus secretion.Citation49 Acetylcholine mainly targets goblet cells in the small intestinal crypts.Citation49 The significant increase in the number of goblet cells in the crypt of the H-VB5 group could be attributed to the increase in CoA in the body caused by the addition of 50 mg/kg of VB5, which promotes the differentiation of goblet cells and the secretion of mucus, thereby protecting the intestine. The addition of 50 mg/kg of VB5 significantly increased the number of both colonic and the cecum goblet cells also showed an elevated trend, indicating that VB5 improved the chemical barrier of the intestine, boosted the immunity of the body, and enhanced the disease resistance. Many gut hormones are produced by enteroendocrine cells, and they play critical roles in the control of hunger, insulin secretion, and food digestion and absorption.Citation50 In this investigation, endocrine cells increased significantly in the H-VB5 and control group. This indicates that VB5 promotes the differentiation of intestinal enteroendocrine cells.
It is generally recognized that SCFAs play a crucial role in maintaining the balance of the intestinal microbiota and the function of the intestinal epithelium.Citation51 The concentration of isobutyric acid and isovaleric acid is proportional to the amount of undigested protein in the intestine, and their primary source is the fermentation of undigested protein flowing from the ileum into the hindgut by hindgut microorganisms.Citation51 In this study, the VB5-containing diet may improve ileum intestinal morphology and nutrient digestion, thereby improving the feed utilization of piglets.Citation52 This diet may cause more branched-chain amino acid digestion, resulting in a significant decrease in isobutyric acid and isovaleric acid concentrations in the H-VB5 group. SCFAs play a crucial role in maintaining the structural integrity and function of colon epithelium.Citation53 SCFA can effectively stimulate the proliferation of intestinal epithelial cells by serving as an energy substrate, generally, colon epithelial cells primarily utilize butyrate.Citation9 In this experiment, the concentrations of butyric acid, isobutyric acid and isovaleric acid were found to be the lowest in the colon contents of the 50 mg/kg VB5 group. Additionally, the 50 mg/kg VB5 group exhibited improved intestinal morphology, cell proliferation and differentiation. These findings suggest that VB5 may enhance the utilization of SCFAs by colon epithelial cells, thereby promoting colon development, which may explain why the highest number of proliferating and differentiated cells in the H-VB5 group. The Spearman correlation analysis of gut morphology and microbes showed that the abundance of Terrisporobacter and Clostridium_sensu_stricto_1 was negatively correlated with the crypt depth and goblet cells. The content of Terrisporobacter and Clostridium_sensu_stricto_1 was significantly decreased in the H-VB5 group, indicating that VB5 may influence the colon’s morphology and structure by modifying the number of microorganisms present there.
The intestinal microbiota, as a critical component in metabolic control, plays an important role in catabolism and nutrient absorption.Citation54 In our study, the effects of VB5 supplementation on intestinal microflora are variable. At the genus level, the addition of 50 mg/kg VB5 significantly increased the abundance of beneficial bacteria (Turicibacter)Citation55 and reduced the abundance of harmful bacteriaCitation56 (Terrisporobacter, Clostridium_sensu_stricto_1, Streptococcus) and UCG-002 compared with the Control group abundance. Turicibacter plays a few beneficial roles in immune interactions within the porcine microbiome, thereby promoting growth performance.Citation57 Clostridium_sensu_stricto_1, a butyrate producer, is generally considered pathogenic and is associated with inflammation and neonatal diarrhea, as well as causing colonic mucosal damage that induces inflammation.Citation58 In contrast, Terrisporobacter, on the other hand, is an emerging anaerobic pathogen that produces toxins such as trimethylamine-N-oxide, which is associated with oxidative stress and inflammation in the intestine of weaned pigs.Citation56,Citation59 Streptococcus is a pathogenic microorganism responsible for various diseases in pigs.Citation60 UCG-002 are beneficial bacteria that promote fiber breakdown and digestion and are involved in the regulation of enteritis and reducing intestinal inflammation.Citation61 In addition, they can ferment lacto-oligosaccharides to produce SCFA for microbial regulation and epithelial development. The reduced abundance of UCG-002 in the H-VB5 group may be due to the addition of 50 mg/kg VB5, which increased villus height of the ileum and endocrine cells of the colon. Therefore, increasing in intestinal absorptive capacity, a decrease in carbohydrate reaching the posterior end of the colon, and a positive correlation between UCG-002 abundance and the concentration of isobutyric acid. This may also account for the lowest isobutyric acid concentration in the colonic contents of the H-VB5 group. In conclusion, the altered intestinal microbiota induced by the additional 50 mg/kg VB5 treatment in the basal diet could result in improved intestinal health and stabilization of the immune status and intestinal environment. Further Tax4Fun analysis of microbiota function predictions revealed that the addition of VB5 significantly altered amino acid metabolism-related enrichment pathways. Many amino acids are believed to be required for the maintenance of intestinal health and to regulate intestinal immunity.Citation62 All of these findings suggest that the addition of 50 mg/kg VB5 improves the health of the piglet gut.
Conclusions
The results of this study indicate that the inclusion of 50 mg/kg VB5 in the diet of weaned piglets can enhance the length and weight of the large intestine, as well as improve the intestinal morphology of the ileum, cecum and colon. Additionally, it was observed that VB5 influences colon development by impacting the abundance of colon microbial populations and the content of SCFAs. Furthermore, the supplementation of 50 mg/kg VB5 led to an increase in the abundance of beneficial bacteria (Turicibacter) in the pig colon. These findings provide a scientific basis for the use of VB5 to improve the health of weaned piglets by regulating organ index, intestinal morphology, intestinal flora and SCFA concentration.
Animal welfare statement
All animal experimental procedures were performed according to protocols approved by the Animal Care Advisory Committee of Hunan Normal University, Changsha, Hunan, China.
Authors’ contribution
Xin Wang: Investigation, Writing – original draft. Yan Qin: Data curation, Visualization, Investigation. Jianzhong Li: Investigation. Pengfei Huang: Investigation. Yali Li: Investigation. Jing Huang: Investigation. Qiye Wang: Visualization. Huansheng Yang: Supervision, Funding acquisition, Writing – review and editing.
Disclosure statement
Authors declare no conflict of interests for this article.
Additional information
Funding
References
- Pluske JR, Turpin DL, Kim J-C. Gastrointestinal tract (gut) health in the young pig. Anim Nutr. 2018;4(2):1–14.
- Bischoff SC. ‘Gut health’: a new objective in medicine? BMC Med. 2011;9(1):24.
- Dong GZ, Pluske JR. The low feed intake in newly-weaned pigs: problems and possible solutions. Asian Australas J Anim Sci. 2007;20(3):440–452.
- Goldstein R, Hebiguchi T, Luk G, et al. The effects of total parenteral nutrition on gastrointestinal growth and development. J Pediatr Surg. 1985;20(6):785–791.
- Shulman R, Fiorotto M, Sheng H, Garza C. Effect of different total parenteral nutrition fuel mixes on the body composition of infant miniature pigs. Pediatr Res. 1984;18(3):261–265.
- Jayaraman B, Nyachoti CM. Husbandry practices and gut health outcomes in weaned piglets: a review. Anim Nutr. 2017;3(3):205–211.
- Moeser A, Pohl CS, Rajput M. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Anim Nutr. 2017;3(4):313–321.
- Chen C, Wang Z, Li J, et al. Dietary vitamin E affects small intestinal histomorphology, digestive enzyme activity, and the expression of nutrient transporters by inhibiting proliferation of intestinal epithelial cells within jejunum in weaned piglets. J Anim Sci. 2019;97(3):1212–1221.
- Wang L, Zou L, Li J, Yang H, Yin Y. Effect of dietary folate level on organ weight, digesta pH, short-chain fatty acid concentration, and intestinal microbiota of weaned piglets. J Anim Sci. 2021;99(1):skab015.
- Wang Z, Li J, Wang Y, et al. Dietary vitamin A affects growth performance, intestinal development, and functions in weaned piglets by affecting intestinal stem cells. J Anim Sci. 2020;98(2):skaa020.
- Wang Z, Yi Z, Wang Q, et al. Effect of different levels of niacin on serum biochemical parameters, antioxidant status, cytokine levels, inflammatory gene expression and colonic microbial composition in weaned piglets. Animals (Basel). 2022;12(21):3018.
- Ragaller V, Lebzien P, Südekum K, Hüther L, Flachowsky G. Pantothenic acid in ruminant nutrition: a review. J Anim Physiol Anim Nutr (Berl). 2011;95(1):6–16.
- Nitto T, Onodera K. Linkage between coenzyme a metabolism and inflammation: roles of pantetheinase. J Pharmacol Sci. 2013;123(1):1–8.
- Slyshenkov V, Dymkowska D, Wojtczak L. Pantothenic acid and pantothenol increase biosynthesis of glutathione by boosting cell energetics. FEBS Lett. 2004;569(1–3):169–172.
- Wojtczak L, Slyshenkov V. Protection by pantothenic acid against apoptosis and cell damage by oxygen free radicals—the role of glutathione. Biofactors. 2003;17(1–4):61–73.
- He W, Hu S, Du X, et al. Vitamin B5 reduces bacterial growth via regulating innate immunity and adaptive immunity in mice infected with mycobacterium tuberculosis. Front Immunol. 2018;9:365.
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–995.
- Lerner T, Borel S, Gutierrez M. The innate immune response in human tuberculosis. Cell Microbiol. 2015;17(9):1277–1285.
- Abiko Y. Metabolism of coenzyme A. In: Metabolism of Sulfur Compounds. New York: Academic Press; 1975:1–25.
- Lin Y-H, Lin H-Y, Shiau S-Y. Estimation of dietary pantothenic acid requirement of grouper, Epinephelus malabaricus according to physiological and biochemical parameters. Aquaculture. 2012;324–325:92–96.
- Kobayashi D, Kusama M, Onda M, Nakahata N. The effect of pantothenic acid deficiency on keratinocyte proliferation and the synthesis of keratinocyte growth factor and collagen in fibroblasts. J Pharmacol Sci. 2011;115(2):230–234.
- NRC. Nutrient Requirements of Swine. 11th rev. ed. Washington, DC: The National Academies Press; 2012.
- Böhmer B, Roth-Maier D. Effects of high-level dietary B-vitamins on performance, body composition and tissue vitamin contents of growing/finishing pigs. J Anim Physiol Anim Nutr (Berl). 2007;91(1–2):6–10.
- Groesbeck C, Goodband R, Tokach M, Dritz S, Nelssen J, DeRouchey J. Effects of pantothenic acid on growth performance and carcass characteristics of growing-finishing pigs fed diets with or without ractopamine hydrochloride. J Anim Sci. 2007;85(10):2492–2497.
- Lorenzett M, Armién A, Henker L, et al. Motor and somatosensory degenerative myelopathy responsive to pantothenic acid in piglets. Vet Pathol. 2023;60(1):101–114.
- Tang J, Zhang B, Liang S, et al. Effects of pantothenic acid on growth performance and antioxidant status of growing male white Pekin ducks. Poult Sci. 2020;99(9):4436–4441.
- Wang B, Zhang X, Yue B, et al. Effects of pantothenic acid on growth performance, slaughter performance, lipid metabolism, and antioxidant function of Wulong geese aged one to four weeks. Anim Nutr. 2016;2(4):312–317.
- Deng Q, Wang Y, Wang X, et al. Effects of dietary iron level on growth performance, hematological status, and intestinal function in growing-finishing pigs. J Anim Sci. 2021;99(1):skab002.
- Zhou J, Qin Y, Xiong X, et al. Effects of iron, vitamin A, and the interaction between the two nutrients on intestinal development and cell differentiation in piglets. J Anim Sci. 2021;99(10):skab258.
- Deng Q, Shao Y, Wang Q, et al. Effects and interaction of dietary electrolyte balance and citric acid on the intestinal function of weaned piglets. J Anim Sci. 2020;98(5):skaa106.
- Wang L, Yan S, Li J, et al. Rapid communication: The relationship of enterocyte proliferation with intestinal morphology and nutrient digestibility in weaning piglets. J Anim Sci. 2019;97(1):353–358.
- Yang C, Wang M, Tang X, et al. Effect of dietary amylose/amylopectin ratio on intestinal health and cecal microbes’ profiles of weaned pigs undergoing feed transition or challenged with lipopolysaccharide. Front Microbiol. 2021;12:693839.
- Krehl WA. Pantothenic acid in swine nutrition. Nutr. Rev. 1957;15(9):275–277.
- Yang H, Xiong X, Yin Y. Development and renewal of intestinal villi in pigs. In: Nutritional and Physiological Functions of Amino Acids in Pigs. Vienna; New York: Springer; 2013: 29–47.
- Montagne L, Pluske J, Hampson D. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim Feed Sci Technol. 2003;108(1–4):95–117.
- Wang M, Huang H, Hu Y, et al. Effects of dietary microencapsulated tannic acid supplementation on the growth performance, intestinal morphology, and intestinal microbiota in weaning piglets. J Anim Sci. 2020;98(5):skaa112.
- Wang M, Wang L, Tan X, et al. The developmental changes in intestinal epithelial cell proliferation, differentiation, and shedding in weaning piglets. Anim Nutr. 2022;9:214–222.
- Mansbach C, Gorelick F. Development and physiological regulation of intestinal lipid absorption. II. Dietary lipid absorption, complex lipid synthesis, and the intracellular packaging and secretion of chylomicrons. Am J Physiol Gastrointest Liver Physiol. 2007;293(4):G645–650.
- Yang H, Wang X, Xiong X, Yin Y. Energy metabolism in intestinal epithelial cells during maturation along the crypt-villus axis. Sci Rep. 2016;6(1):31917.
- Gehart H, Clevers HJ. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol. 2019;16(1):19–34.
- Yang H, Xiong X, Wang X, Yin Y. Mammalian target of rapamycin signaling pathway changes with intestinal epithelial cells renewal along crypt-villus axis. Cell Physiol Biochem. 2016;39(2):751–759.
- Yang H, Li F, Kong X, et al. Chemerin regulates proliferation and differentiation of myoblast cells via ERK1/2 and mTOR signaling pathways. Cytokine. 2012;60(3):646–652.
- Pluske JR, Hampson DJ, Williams IH. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest Prod Sci. 1997;51(1–3):215–236.
- Yoshii K, Hosomi K, Sawane K, Kunisawa J. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front Nutr. 2019;6:48.
- McCauley HA, Guasch G. Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends Mol Med. 2015;21(8):492–503.
- Lanska DJ. The discovery of niacin, biotin, and pantothenic acid. Ann Nutr Metab. 2012;61(3):246–253.
- Moiseenok A, Kanunnikova N. Brain CoA and acetyl CoA metabolism in mechanisms of neurodegeneration. Biochemistry (Mosc). 2023;88(4):466–480.
- Wang Y, Yang H, Geerts C, et al. The multiple facets of acetyl-CoA metabolism: Energetics, biosynthesis, regulation, acylation and inborn errors. Mol Genet Metab. 2023;138(1):106966.
- Birchenough GM, Johansson ME, Gustafsson JK, Bergström JH, Hansson GC. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015;8(4):712–719.
- Gribble F, Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol. 2019;15(4):226–237.
- Liu B, Wang W, Zhu X, et al. Response of gut microbiota to dietary fiber and metabolic interaction with SCFAs in piglets. Front Microbiol. 2018;9:2344.
- Wang M, Yang C, Wang Q, et al. Effects of dietary amylose-amylopectin ratio on growth performance and intestinal digestive and absorptive function in weaned piglet response to lipopolysaccharide. Animals (Basel). 2022;12(14):1833.
- Williams B, Verstegen M, Tamminga S. Fermentation in the large intestine of single-stomached animals and its relationship to animal health. Nutr Res Rev. 2001;14(2):207–228.
- Zhao L, Lou H, Peng Y, Chen S, Fan L, Li XJ. Elevated levels of circulating short-chain fatty acids and bile acids in type 2 diabetes are linked to gut barrier disruption and disordered gut microbiota. Diabetes Res Clin Pract. 2020;169:108418.
- Tang S, Zhang S, Zhong R, et al. Time-course alterations of gut microbiota and short-chain fatty acids after short-term lincomycin exposure in young swine. Appl Microbiol Biotechnol. 2021;105(21-22):8441–8456.
- Chen Y, Xie Y, Zhong R, et al. Effects of xylo-oligosaccharides on growth and gut microbiota as potential replacements for antibiotic in weaning piglets. Front Microbiol. 2021;12:641172.
- Wang X, Tsai T, Deng F, et al. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome. 2019;7(1):109.
- Wang Y, Xu L, Liu J, Zhu W, Mao S. A high grain diet dynamically shifted the composition of mucosa-associated microbiota and induced mucosal injuries in the colon of sheep. Front Microbiol. 2017;8:2080.
- Kyoung H, Lee J, Cho J, et al. Dietary glutamic acid modulates immune responses and gut health of weaned pigs. Animals (Basel). 2021;11(2):504.
- Nomoto R, Ishida-Kuroki K, Nakagawa I, Sekizaki T. Complete genome sequences of four Streptococcus parasuis strains obtained from saliva of domestic pigs in Japan. Microbiol Resour Announc. 2022;11(2):e0124521.
- Ding S, Azad M, Fang J, et al. Impact of sulfur-containing amino acids on the plasma metabolomics and intestinal microflora of the sow in late pregnancy. Food Funct. 2019;10(9):5910–5921.
- Mou Q, Yang H, Yin Y, Huang P. Amino acids influencing intestinal development and health of the piglets. Animals (Basel). 2019;9(6):302.