?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A large part of the Northeast Atlantic cod caught with trawls is landed frozen, processed, and sold thawed. Many trawlers have adapted a fishing practice termed ‘buffer towing’, causing probability for poor exsanguination and fillet redness. Here, the effect of buffer towing upon color and hemoglobin concentration in cod loin section is studied, and the development of TVB-N during chilled storage of thawed cod. No significant differences were proven for redness or hemoglobin concentration in loin from cod exposed to regular haul-back or buffer towing. Neither were significant differences found in TVB-N levels during chilled storage at 0 and 4°C.
KEYWORDS:
Introduction
Thawed, or ‘refreshed’, cod fillets have been available in supermarkets in many countries as a response to consumer demand for continuous availability (Martinsdottir and Magnusson Citation2001; Sørensen et al. Citation2020). A study by Altintzoglou et al. (Citation2012) showed that consumers in England may prefer thawed over fresh cod fillets.
The use of frozen and thawed cod may also be beneficial for the seafood industry. The cod fisheries in Norway are highly seasonal, with most cod being caught during the first 4 months of the year. The large volume of fish caught during a limited period leads to processing capacity challenges in the land-based industry; a challenge that can be met by applying fish frozen at sea and subsequent thawing (Erikson et al. Citation2021; Roiha et al. Citation2018; Svendsen et al. Citation2022).
During the last four years (2018–2021), 52% of cod landed in Norway was landed frozen, and 81% of this was caught by trawl (The Norwegian Directorate of Fisheries Citation2022; Magnusson and Martinsdottir Citation1995). As described by Brinkhof et al. (Citation2018), the dense aggregations of Northeast Atlantic cod (Gadus morhua) in the Barents Sea have led to a fishing practice among many trawlers termed ‘buffer towing’. In order to secure a continuous supply of fish and avoid stops during processing, the trawl is redeployed immediately after taking the catch onboard. If the desired volume of fish is caught before the catch from the previous haul is processed, the trawl is lifted off the seabed and towed at a given depth at low speed (∼1–2 knots), until the production capacity of the onboard factory is restored. Brinkhof et al. (Citation2018) showed that this practice has a negative impact on fish quality, and cod subjected to buffer towing had an increased probability for poor exsanguination and increased fillet redness compared to regular (direct) haul-back.
In the study by Brinkhof et al. (Citation2018), a fillet index evaluating the redness in the whole fillet was used. However, fish processors today commonly produce different fillet products like loins and tails, whereof cod loins are more expensive than cod fillets (Sogn-Grundvag et al. Citation2013). Olsen et al. (Citation2008) and Jensen et al. (Citation2022) showed uneven distribution of heme-pigment in different parts of the fillets when studying the effects of stress and bleeding upon blood content in cod fillets, and generally, there were lower concentrations of hemoglobin in loins. However, when lifting the trawl off the seabed, the rapid decompression causes the swim bladder to expand and eventually rupture (Humborstad and Mangor-Jensen Citation2013; Midling et al. Citation2012), which may result in bruises in the loin part of the fillet adjacent to the swim bladder. Thus, cod caught by demersal trawling, both with and without buffer towing, may have more blood in the loins compared to cod not exposed to barotrauma.
Studies have shown that thawed cod fillets may have longer shelf-life after thawing compared to fresh cod fillets (Guldager et al. Citation1998; Magnusson and Martinsdóttir Citation1995; Skjerdal et al. Citation1999), and the reason is probably inactivation of spoilage bacteria by freezing and frozen storage (Bøknæs et al. Citation2000; Skjerdal et al. Citation1999; Sørensen et al. Citation2020). However, the storage stability of frozen fish and the shelf life of chilled fish may be reduced both due to residual blood in the muscle leading to oxidation (Eliasson et al. Citation2020; Larsson et al. Citation2007; Richards and Hultin Citation2002; Secci and Parisi Citation2016) and increased microbial growth (Sternisa et al. Citation2018). The objectives of the present work were thus to compare the amounts of residual blood in cod loin after trawling with and without buffer towing during catch and to determine whether buffer towing affected the shelf life, as measured by total volatile basic nitrogen (TVB-N), of thawed cod fillets.
Materials and methods
Catch and sample preparation
Atlantic cod was caught with the research trawler R/V ‘Helmer Hanssen’ (63.8 m, 4080 HP) as described by Brinkhof et al. (Citation2018) in the central part of the Barents Sea (N 74°59‘–N 75°26’, E 30°54‘–E31°17’) November 2016. The trawl setup was similar to commercial fisheries, and the trawl was a standard two-panel Alfredo 3 fish trawl with a standard codend (8 mm polyethylene twine, 150 mm nominal mesh size). To reduce catch-size variations between hauls, the codend was set to allow capture of around 2 metric tons of fish. A total of 6 hauls was conducted (3 pairs), alternating between hauls with and without buffer towing. Catch data for each haul are shown in .
Table 1. Overview of the hauls conducted showing the towing start time, towing time, haul type, depth, mean buffer towing depth, the percentage depth reduction during buffer towing, number of fish caught, and average length of fish.
Immediately after catch, the fish was bled by cutting the isthmus and exsanguinated in running sea water (ca. 50 L/min) for 30 min. Next, the fish were beheaded, gutted, and cleaned, laid in 50 kg blocks in commercial vertical plate freezers, frozen to a core temperature of −18°C, and finally packed in commercial type laminated paper bags and stored at −30°C until landing. On land, the fish was thawed in tanks containing 1000 L of chilled water (1°C) for 24 h and then further thawed on ice for additional 24 h at 0-1°C.
After thawing, a total of 144 fish, 24 randomly chosen fish from each of the three hauls from each of the two trawling procedures, were manually filleted. Fillets from seven fish from each haul were subjected to color measurements and determination of hemoglobin, while fillets from 17 fish were applied to chilled storage and analyses of total volatile nitrogen.
Color measurement
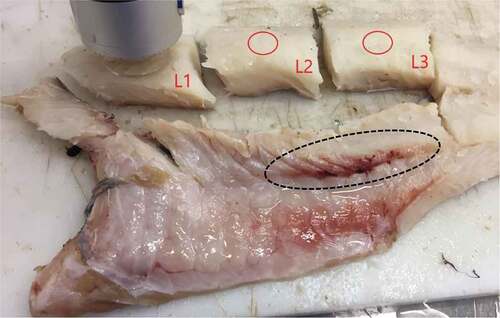
Color was measured in triplicate on the anterior (L1), middle (L2), and posterior (L3) part of the loin by the CIE L*a*b* system using a colorimeter (Minolta Chroma Meter CR-200, Minolta Camera Co. Ltd., Osaka, Japan). shows the position of the colorimeter and measurement area on L1, and the red circles indicate the position of the colorimeter on L2 and L3. Whiteness (W*) was calculated as . To reduce the source of error due to contamination of residual blood in the neck flesh after decapitation, the flesh closest to the neck cut was not included when sampling the three loin sections.
Measurement of hemoglobin
The middle (L2) and posterior (L3) parts of the loin were cut and used for determination of hemoglobin as a measure of residual blood. The muscle was minced twice using an electric meat mincer (Bosch Pro Power 2200 W, Germany) and then by hand-blender (Wilfa AS, Norway) for 30 seconds. Hemoglobin content in minced muscle was assessed in triplicate according to Chaijan and Undeland (Citation2015).
Chill storage and analyses of TVB-N
The left-side fillets from five fish from each of the three hauls, from each of the two trawling procedures, were used as control groups, N = 15 for each trawling procedure. Fillets from the remaining 12 fish from each of the three hauls, from each of the two trawling procedures, were used in the storage experiment, N = 72 for each trawling procedure. The fillets were divided into four experimental groups, consisting of either left- or right-side fillets from six fish from each haul (N = 18), as illustrated in . The fillets were stored individually in non-sealed plastic bags (PA/PE 120µ) at two different temperatures: on ice at 0°C and in a cold cabinet at 4°C. After chilled storage, the fillets were frozen at −30°C for simultaneous analyses of TVB-N.
Table 2. Time and temperature conditions for chilled storage of thawed cod fillets. N = 18 in the storage experiment, six fillets from each of the three hauls using each trawl procedure. As control, five left-side fillets from each of the three hauls using each trawl procedure were used.
Prior to analyses of TVB-N, the fillets were thawed at 1°C for approximately 18 h. The fillets were skinned, and loin was cut and homogenized in a food processor (Kenwood FP 110 300 W, United Kingdom). TVB-N was determined in triplicate by the Kjeldahl method (Tecator Citation1983) and expressed as mg TVB-N per 100 g fish muscle.
Statistics
To test for differences in color and hemoglobin content between loins from cod caught using regular haul-back and buffer towing techniques and between different parts of the loins, two-tailed unpaired t-tests in Excel were used.
The TVB-N-data were analyzed using IBM SPSS Statistics (Version 26) predictive analytics software. The significance of any difference between the groups or the impact of buffer towing was determined using one-way analysis of variance (ANOVA), generally followed by a post hoc Tukey’s test. P-values lower than 0.05 are regarded as statistically significant. The TVB-N-results are given as mean ± confidence interval (CI).
Results and discussion
Color and hemoglobin
In the present work, only slightly higher and non-significant redness (a*) and hemoglobin concentration were demonstrated in loin from cod exposed to buffer towing compared to regular haul-back (). In contrast, Brinkhof et al. (Citation2018) proved that buffer towing significantly reduced the quality of cod. They showed that cod subjected to buffer towing had an increased relative probability of 209% for fillet redness compared to cod subjected to regular (direct) haul-back, as assessed by visual evaluation of whole fillets. There is not necessarily a contradiction between these results. Olsen et al. (Citation2008) and Jensen et al. (Citation2022) showed that cod loins contain significantly less residual blood than belly in both unstressed and stressed fish. Thus, one possible explanation is that the buffer towing increases redness and residual blood in the whole fillet, as demonstrated by Brinkhof et al. (Citation2018), while the loin part of the fillet is less affected.
Table 3. Color parameters and hemoglobin content of the different loin sections in fish caught by regular haul-back and buffer towing. Values are presented as mean ± SD. N = 21, seven fillets from each of the three hauls using each trawl procedure. Different letters in the same column indicate significant differences (p < 0.05) between loin sections.
also shows that there are differences in color parameters between different loin sections within both experimental groups. For cod exposed to regular haul-back, the anterior part of the loin, L1, is significantly darker (lower L*) and significantly more red (higher a*) than the middle (L2) and posterior (L3) part of the loin. For cod exposed to buffer towing, L1 is significantly darker and significantly redder in flesh color than L3. The red discoloration of L1 could be due to contamination of residual blood on the surface of the neck flesh after decapitation or spontaneous blood coagulation in the wound after decapitation (Olsen et al. Citation2014; Tavares-Dias and Oliveira Citation2009).
Since the swim bladder is located along L2 and L3, the hemoglobin content was measured in these sections to investigate whether the different trawl procedures affected swim bladder rupture and ecchymosis differently. As shown in , there were only slightly and non-significantly higher levels of hemoglobin in the loin sections from buffer-towed cod. Thus, more severe barotrauma caused by buffer towing could not be proven. When comparing the hemoglobin content in L2 and L3, a significantly higher concentration was found in the posterior loin section (L3) compared to the middle (L2) in cod both from regular haul-back and buffer-towing. The higher levels in L3 are probably due to the swim bladder being located more adjacent to L3 than L2 (). Thus, L3 is more subjected to ecchymosis when the swim bladder ruptures. Similar differences were not detected when measuring the color parameters in the middle of L2 and L3 (), indicating that the increased concentration of hemoglobin in L3 is probably due to more blood in the lower part of the loin, adjacent to the swim bladder.
TVB-N
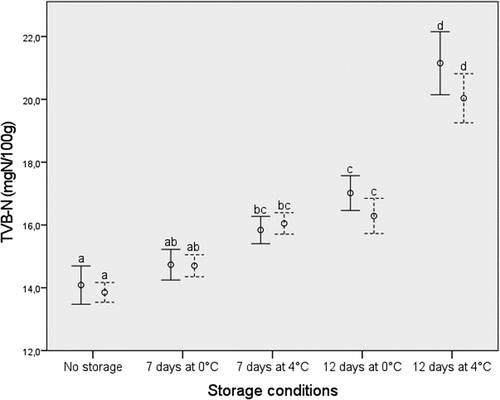
As seen by the overlapping confidence intervals in , there were no significant differences in shelf life of thawed cod caught by regular haul-back and buffer towing as measured by TVB-N. On the other hand, as expected, both storage time and storage temperature had a significant effect on the TVB-N-levels. During chilled storage for up to 12 days at 0 or 4°C, none of the fillets exceeded the EU critical limit of 35 mg-N TVBN/100 g (EC Citation2008). The TVB-N content is in accordance with what has been reported in previous studies for cod frozen for a few months prior to thawing and ice storage (Roiha et al. Citation2018; Sørensen et al. Citation2020; Vyncke Citation1983) and lower than for fish stored frozen for one week (Vyncke Citation1983). The reason for the low TVB-N content is probably inactivation of spoilage bacteria by freezing and frozen storage (Bøknæs et al. Citation2000; Skjerdal et al. Citation1999; Sørensen et al. Citation2020).
Figure 2. TVB-N mean values (mgN/100 g) with 95% confidence intervals in frozen-thawed Atlantic cod fillets stored for 0 days, 7 days at 0°C and 4°C, and 12 days at 0°C and 4°C. Cod from regular haul-back are shown as whole black line, while cod from buffer towing are shown by dotted line. Different letters indicate significant differences.
As discussed in the color and hemoglobin section, there is an apparent contradiction between the results found in the present experiment and that reported previously by Brinkhof et al. (Citation2018). But when taking into account that the loin has been shown to contain less residual blood than other fillet parts in both stressed and unstressed fish (Jensen et al. Citation2022; Olsen et al. Citation2008), it is reasonable to assume that color and hemoglobin content in loin is less affected by buffer towing than the fillet as a whole. In addition, it is known that the fish quality is reduced both by increased catch size and towing time (Digre et al. Citation2017; Olsen et al. Citation2013; Rotabakk et al. Citation2011; Veldhuizen et al. Citation2018). Compared to commercial trawl fisheries, where catches easily exceed 10 tonnes and more (Olsen et al. Citation2013), the catch size in this study is limited, as are the number of investigated individuals. Thus, possible differences in fish quality due to different trawling procedures may have been concealed in this experiment.
Conclusion
No significant increased redness (a*) and hemoglobin concentration were proven in loin from cod exposed to buffer towing compared to cod caught by regular haul-back. The posterior part of the loin has higher levels of hemoglobin, indicating ecchymosis in loin caused by rupture of the swim bladder.
Regarding shelf-life of thawed cod fillets, no significant differences were found in TVB-N levels during chilled storage at 0 and 4°C for cod from regular haul-back and buffer towing.
Acknowledgments
We are grateful to Jesse Brinkhof and the crew of RV “Helmer Hanssen” for valuable help during work at sea. We appreciate the effort from NOFIMA Tromsø by setting up the laboratory facilities for our experiments, and we thank Sjurdur Joensen and Tatiana N. Ageeva for assistance during the experiments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Altintzoglou T, Nøstvold BH, Heide M, Østli J, Egeness FA. 2012. The influence of labelling on consumers’evaluations of fresh and thawed cod fillets in England. Br Food J. 114(11):1558–70.
- Bøknæs N, Osterberg C, Nielsen J, Dalgaard P. 2000. Influence of freshness and frozen storage temperature on quality of thawed cod fillets stored in modified atmosphere packaging. LWT-Food Sci Technol. 33(3):244–48.
- Brinkhof J, Larsen R, Herrmann B, Olsen SH. 2018. Assessing the impact of buffer towing on the quality of Northeast Atlantic cod (Gadus morhua) caught with a bottom trawl. Fisheries Res. 206:209–19.
- Chaijan M, Undeland I. 2015. Development of a new method for determination of total haem protein in fish muscle. Food Chem. 173:1133–41.
- Digre H, Rosten C, Erikson U, Mathiassen JR, Aursand IG. 2017. The on-board live storage of Atlantic cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) caught by trawl: fish behaviour, stress and fillet quality. Fish Res. 189:42–54.
- EC. 2008. Commission Regulation (EC) No 1022/2008 of 17 October 2008 amending Regulation (EC) No 2074/2005 as regards the total volatile basic nitrogen (TVB-N) limits. European Commission. 277:18–20. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008R1022andfrom=en
- Eliasson S, Arason S, Margeirsson B, Palsson OP. 2020. Onboard evaluation of variable water flow and recirculation effects on bleeding of Atlantic Cod (Gadus morhua). Foods. 9(11):1519.
- Erikson U, Uglem S, Greiff K. 2021. Freeze-chilling of whitefish: effects of capture, on-board processing, freezing, frozen storage, thawing, and subsequent chilled storage-A review. Foods. 10(11):2661.
- Guldager HS, Bøknæs N, Østerberg C, Nielsen J, Dalgaard P. 1998. Thawed cod fillets spoil less rapidly than unfrozen fillets when stored under modified atmosphere at 2°C. J Food Prot. 61(9):1129–36.
- Humborstad OB, Mangor-Jensen A. 2013. Buoyancy adjustment after swimbladder puncture in cod Gadus morhua: an experimental study on the effect of rapid decompression in capture-based aquaculture. Mar Biol Res. 9(4):383–93.
- Jensen TK, Tobiassen T, Heia K, Møllersen K, Larsen RB, Esaiassen M. 2022. Effect of codend design and postponed bleeding on hemoglobin in cod fillets caught by bottom trawl in the Barents Sea demersal fishery. J Aquat Food Prod Technol. 31(8):775–84.
- Larsson K, Almgren A, Undeland I. 2007. Hemoglobin-mediated lipid oxidation and compositional characteristics of washed fish mince model systems made from cod (Gadus morhua), herring (Clupea harengus), and salmon (Salmo salar) muscle. J Agric Food Chem. 55(22):9027–35.
- Magnusson H, Martinsdottir E. 1995. Storage quality of fresh and frozen-thawed fish in ice. J Food Sci. 60(2):273–78.
- Martinsdottir E, Magnusson H. 2001. Keeping quality of sea-frozen thawed cod fillets on ice. J Food Sci. 66(9):1402–08.
- Midling K O, Koren C, Humborstad OB, Saether BS. 2012. Swimbladder healing in Atlantic cod (Gadus morhua), after decompression and rupture in capture-based aquaculture. Mar Biol Res. 8(4):373–79.
- The Norwegian directorate of fisheries 2022, statbank; [accessed 2022 Mar 29]. https://www.fiskeridir.no/Tall-og-analyse/Statistikkbanken.
- Olsen SH, Joensen S, Tobiassen T, Heia K, Akse L, Nilsen H. 2014. Quality consequences of bleeding fish after capture. Fisheries Res. 153:103–07.
- Olsen SH, Sørensen NK, Larsen R, Elvevoll EO, Nilsen H. 2008. Impact of pre-slaughter stress on residual blood in fillet portions of farmed Atlantic cod (Gadus morhua) — Measured chemically and by Visible and Near-infrared spectroscopy. Aquaculture. 284(1–4):90–97.
- Olsen SH, Tobiassen T, Akse L, Evensen TH, KØ Midling. 2013. Capture induced stress and live storage of Atlantic cod (Gadus morhua) caught by trawl: consequences for the flesh quality. Fish Res. 147:446–53.
- Richards MP, Hultin HO. 2002. Contributions of blood and blood components to lipid oxidation in fish muscle. J Agric Food Chem. 50(3):555–64.
- Roiha IS, Á Jónsson, Backi CJ, Lunestad BT, Karlsdóttir MG. 2018. A comparative study of quality and safety of Atlantic cod (Gadus morhua) fillets during cold storage, as affected by different thawing methods of pre-rigor frozen headed and gutted fish. J Sci Food Agric. 98(1):400–09.
- Rotabakk BT, Skipnes D, Akse L, Birkeland S. 2011. Quality assessment of Atlantic cod (Gadus morhua) caught by longlining and trawling at the same time and location. Fish Res. 112(1–2):44–51.
- Secci G, Parisi G. 2016. From farm to fork: Lipid oxidation in fish products. A review. Ital J Anim Sci. 15(1):124–36.
- Skjerdal OT, Esaiassen M, Løkken GB. 1999. The quality and marketing possibilities can be improved by optimising the freezing conditions. In Kennedy C, editor. Proceedings of the fifth plenary meeting in the concerted action project ‘‘The preservation of frozen food quality and safety throughout the distribution chain’’. Leeds: University of Leeds. p. 89–92.
- Sogn-Grundvag G, Larsen TA, Young JA. 2013. The value of line-caught and other attributes: an exploration of price premiums for chilled fish in UK supermarkets. Marine Policy. 38:41–44.
- Sørensen JS, Ørnfeld-Jensen O, Bøknæs N, Mejlholm O, Jessen F, Dalgaard P. 2020. Thawed and chilled Atlantic cod (Gadus morhua L.) from Greenland - Options for improved distribution. LWT-Food Sci Technol. 131:109473.
- Sternisa M, Dvorak P, Lunda R, Linhartova Z, Mozina SS, Mraz J. 2018. Bleeding of common carp (Cyprinus carpio) improves sensory quality of fillets and slows oxidative and microbiological changes during refrigerated Aerobic storage. Food Technol Biotech. 56(4):524–32.
- Svendsen ES, Widell KN, Tveit GM, Nordtvedt TS, Uglem S, Standal I, Greiff K. 2022. Industrial methods of freezing, thawing and subsequent chilled storage of whitefish. J Food Eng. 315:110803.
- Tavares-Dias M, Oliveira SR. 2009. A review of the blood coagulation system of fish. Braz J Biosci. 7:205–24.
- Tecator. 1983. Determination of total volatile basic nitrogen (TVBN) in fresh and deep frozen fish. Helsingborg, Sweden: Foss Tecator.
- Veldhuizen LJL, Berentsen PBM, De Boer IJM, Van De Vis JW, Bokkers EAM. 2018. Fish welfare in capture fisheries: a review of injuries and mortality. Fish Res. 204:41–48.
- Vyncke W. 1983. Shelf-life of thawed cod fillets kept in ice. Z Lebensm Unters. 177(1):19–21.