ABSTRACT
Many forest reserves in Bangladesh have been converted to protected areas (PAs) to conserve the forests resources from further depletion. This study has investigated if such initiatives have improved the state of biodiversity of these PAs amid tremendous anthropogenic pressure on forest resources. We have assessed the phytosociological attributes of the PAs in the country through a case study at Kaptai National Park (KNP) and compared the attributes with those of the adjacent areas and of the tropical forests across the world. We have identified 52 species belonging to 45 genera and 28 families. The most dominant species in KNP was Dipterocarpus spp. and the adjacent area was dominated by Tectona grandis. Unexpectedly, the Shannon-Wiener index of KNP has dropped down from 2.98 in 2000 to 0.90 in 2014. However, in terms of relative density, relative dominance, and relative frequency, KNP was better than the adjacent areas. In contrast, the mean Shannon-Wiener index in KNP (0.90) was smaller than that in the tropical countries (2.99). We recommend strengthening effective comanagement of PAs and enabling nonforestry income generation activities for the forest-dependent people so that the biodiversity of the PAs can be enriched while people’s livelihoods are ensured.
Introduction
Forests cover approximately 25% (Hirakuri, Citation2003) to 31% (Adams, Citation2012) of the world’s landmass and are critical in meeting human needs for water, food, shelter, medicine, fuelwood, fodder, and timber (Adams, Citation2012). They also provide a wide range of environmental services including biodiversity conservation, watershed protection, soil protection, and climate change mitigation (Hirakuri, Citation2003; Landell-Mills & Porras, Citation2002). In the last several decades, deforestation and biodiversity losses were on the rise throughout the globe. Declaring protected areas (PAs) has been viewed as the most effective and widespread strategy to stop and reverse such defacing situation of the forest and wetland biodiversity in the world (Kramer, Schaik, & Johnson, Citation1997; Lewis, Citation1996; Mukul, Citation2007; Mulongoy & Chape, Citation2004). Protected areas such as national parks and wildlife sanctuaries form the front line in the campaign to conserve biodiversity (Chape, Blyth, Fox, & Spalding, Citation2003). Not just only for biodiversity conservation, PAs are increasingly becoming more popular destinations for wildlife tourists of national and international origins (Chape et al., Citation2003; Scherr, White, & Kaimowitz, Citation2004; Tuxill & Nabhan, Citation2001).
However, many of the world’s PAs are important not only for their biodiversity but also for their natural resources that many local people depend on for their livelihoods (Cavendish, Citation2000; Falconer & Arnold, Citation1989). Many social scientists believe that, without active participation of the local people in PA management and increased economic incentives for their collaboration in conservation, there is little chance for PAs to be conserved and local resources to be sustainably managed (Masozera & Alavalapati, Citation2004). They also argue that the conservation of biodiversity in PAs will be more challenging if local community is heavily dependent on these areas for energy, nutrition, medicine, and other subsistence needs (Masozera & Alavalapati, Citation2004). Thus, local people’s support and involvement for PA management, especially in developing countries, has been considered as an important element for nature conservation in recent years, (Nagothu, Citation2003; Wells & Mcshane, Citation2004). Comanagement of PAs under the broad canopy of community-based natural resource management is an emerging agenda for conservation policy in many developing countries of the world. This idea has also been widely promoted by various international conservation agencies (Fisher, Citation2003; Jeanrenaud, Citation2002; Kothari, Pathak, & Vania, Citation2000). Comanagement can play an effective role for reversing the degradation of biodiversity resources into conservation pools for the improvement of biodiversity status of PAs (Klooster & Masera, Citation2000; Mbile et al., Citation2005; Mukul & Quazi, Citation2007; Sekhar, Citation2003).
In line with the international trends of protected area declarations, the Bangladesh Government has also taken various initiatives to declare many of its reserved forest areas into PAs to conserve the remaining floral and faunal diversity of the country (Brown & Durst, Citation2003). Presently, there are 49 PAs in Bangladesh of which 17 have so far been taken under the umbrella of comanagement (Bangladesh Forest Department [BFD], Citation2016). PAs cover only 1.8% of the country’s land area (BFD, Citation2016) and only 11.08% of Bangladesh forests (Mukul, Uddin, Uddin, Khan, & Marzan, Citation2008). In Bangladesh, even though people can have partial supports for their livelihoods from forest resources mostly through an act of forest sanitation, PAs are practically free from commercial removal of timber and nontimber forest products (Mukul, Manzoor Rashid, Uddin, & Khan, Citation2016). However, due to poor socio-economic background, such definition of PAs turns to a hypothetical conception in Bangladesh and traditionally people living in or around the PAs exploit various forest resources from the PAs for their subsistence (Mukul et al., Citation2016; Mukul, Uddin, Manzoor Rashid, & Fox, Citation2010). Therefore, conflicts occurred between PA managers and the forest dependent people living in and around the PAs. In the face of such a situation, comanagement has been adopted in PAs in Bangladesh (Mukul, Herbohn, Manzoor Rashid, & Uddin, Citation2014; Mukul, Rashid, Quazi, Uddin, & Fox, Citation2012).
Uddin, Steinbauer, Jentsch, and Mukul (Citation2013) have investigated the correlation between the richness of indigenous and exotic species in and around Satchari National Park, another rich PA in the northeastern part of Bangladesh. They reported an inverse relationship between the two in the surrounding area while there was no significant relationship between them in the national park area. However, they considered all forest disturbance events as factors for ecological succession which ultimately helped the invasive growth of exotic species in the PAs. Thus, how the legal tools and anthropogenic pressure on forest resources could shape the bioreserves in and around PAs has not been sufficiently addressed. That being said, we hypothesized that there should be considerable heterogeneity in the degree of anthropogenic pressure on forest produces in the inner part of a PA, on its legal boundary, and in the adjacent forest areas. Thus, we also assumed that the phytosociological attributes in these three zones had significant differences among them. In addition, since Bangladesh shares the climatic conditions similar to that in other tropical countries in the world, we were also interested to evaluate the biodiversity status in the PAs of Bangladesh with that in the tropical forests in other parts of the world. Hence, we took up this study aiming to examine whether the attributes and indices of floristic biodiversity of the PAs in Bangladesh were at least as good as that in the tropical forests of its adjacent areas and other countries. The study recommends involving people into forest management decisions while ensuring them nonforestry income sources. This strategy is expected to help the policy makers develop an effective management plan for the declared PAs in the country so that the objectives of such declarations could be met.
Methods
Study site
Like the other comanaged PAs in Bangladesh, Kaptai National Park (KNP), a tropical evergreen to semievergreen forest that harbors 412 floral species (Rahman, Roy, Anik, & Fardusi, Citation2013), was also declared as a PA in September, 1999 with an aim to protect its depleting flora and fauna (BFD, Citation2016). Prior to the PA declaration, its legal status was a reserved forest. Its conversion from the “reserved” to the “protected” status has shrunk local people’s scopes for their livelihoods from PA resources. As has been observed in the other PAs in the country, the interests of KNP administration and the KNP-dependent people differed to a great extent. Given this similarity, we assumed that, KNP could be a better representative of the typical PAs in Bangladesh in terms of bioresources and the anthropogenic pressure on these resources. Hence, we considered KNP as a case to study the general biodiversity situations of PAs in Bangladesh.
Administratively, KNP belongs to Kaptai Forest Range under the management of Rangamati South Forest Division, Bangladesh. It is approximately 57 km away from and to the northeast of Chittagong City (). The area located at 22° 29.991´ N and 92° 10.722´ E in the Chittagong Hill Tracts (CHTs) of Bangladesh. It covers an area of 5,464 ha. The level of valley bottoms range from 32 to 90 m above the mean sea level and the maximum elevation is about 500 m (Uddin & Hassan, Citation2012). It falls under tropical monsoon climate with annual average rainfall of 254 cm (BFD, Citation2010; Rahman, Citation2004) to 290 cm (Uddin & Hassan, Citation2012), average maximum temperature of 25.5°C, and average minimum temperature of 15.6°C. December is the driest month here with almost no rainfall and with an average humidity of 74% (BFD, Citation2010; Rahman, Citation2004). Historically, KNP is famous for the reason that the first Tectona grandis plantation in Bangladesh was established here in 1871. As early as 1873, this massive plantation was initiated by local forest management that resulted in much of today’s beautiful growth (Hossain, Alam, & Miah, Citation2008).
Sampling framework
Kaptai National Park is divided into two ranges: Kaptai Range and Karnaphuli Range. Kaptai Range consists of Kaptai Sadar Beat, Kamalyachari Beat, Shuknachari Beat, Bangchari Beat, and Rampahar Beat. On the other hand, Karnaphuli Range has Karnaphuli Sadar Beat, Kaptaimukh Beat, Patachari Beat, Chakua Beat, Chitmorom Beat, Fringkhiong Beat, Kalmichara Beat, Brick Field Camp, and Fringkhiong Depot. To examine if the biodiversity health of KNP was different from that in its adjacent areas, we had to collect data from inner part of, on, and from outside the administrative boundary of KNP. Thus, the study site was divided into three strata—namely, INSIDE, ON, and OUTSIDE of the KNP boundary for the measurement of biodiversity attributes and indices. The INSIDE consisted of the villages that were in the core zone of KNP. The villages thus selected were Bangchari, Bot Toli, Kaptai Sadar Beat, and Rampahar Beat. The ON zone consisted of the villages that were situated on the administrative boundary of KNP. The ON zone included the villages—namely, Chowdhury Chara Para, Aara Chara Para, and Chakua Para. The OUTSIDE zone contained the villages around KNP that had entirely private forest ownership while the IN and ON zones contained only public forests. From the OUTSIDE zone, we studied Kodalia Beat, Pomora, and Rassel Ecopark villages. The INSIDE, ON, and OUTSIDE zones were at least 1 km apart from each other. However, the width of the OUTSIDE zone was kept limited to 4 km outward.
A total of 66 sample plots, each measuring 20 m × 20 m, were identified for studying the phytosociological attributes and biodiversity indices. Of the 66 plots, 22 plots were from INSIDE, 22 plots from ON, and 22 plots from OUTSIDE zone. The plots were selected at an interval of 500 m from each other. In each of these plots, we counted the number of trees, seedlings, and saplings by species. We also measured diameter at breast height (DBH) and total height of all trees in these plots. Again, a plot of size 2 m × 2 m was inscribed in the center of each of the 20 m × 20 m plots for the study of natural regeneration status. Thus, total number of regeneration plots was also 66. We counted the number of naturally growing seedlings of all available species in each of the regeneration plots.
Quantitative framework
Biodiversity indices
The species were identified using the standard methods or characteristics enunciated in Prain (Citation1903), Brandis (Citation1906), Heinig (Citation1925), Das and Alam (Citation2001), and Dey (Citation2006). The vernacular names of the species were determined in consultation with local people and taxonomists. We calculated the phytosociological attributes and diversity indices for all the 66 plots we have identified. Major phytosociological attributes such as species density, relative density, frequency, relative frequency, relative dominance, and importance value index; and important biodiversity indices such as Simpson index, species evenness index, Shannon-Wiener index were also calculated for each of the INSIDE, ON, OUTSIDE zones using standard methods stated in .
Table 1. Specifications of phytosociological parameters and diversity indices for the floral species available in Kaptai National Park.
Comparing biodiversity indices
Due to the legal regulation, the three zones (INSIDE, ON, OUTSIDE) had three distinct types of anthropogenic disturbances. However, a specific zone was under the same degree of disturbance due to people’s similar access to that zone. Thus, we assumed that, the phytosociological attributes (DBH, height, number of trees, number of seedlings, number of saplings, and number of species) at a certain zone were more or less homogeneous. In contrast, there was considerable heterogeneity among those attributes between each pair of zones. That being said, we planned to verify whether the phytosociological attributes of the plant species at different zones relative to the administrative boundary of KNP were different. For this, we conducted a one-way ANOVA to test the null hypothesis, H0: Mi,INSIDE = Mi,ON = Mi,OUTSIDE; where, Mi’s were the means of phytosociological attribute i at the specific zones relative to the administrative boundary of KNP. The rejection of the null hypothesis would lead to conclude that the means were not equal at the designated zones.
In addition to comparing the biodiversity indices in KNP with those in its adjacent areas, we wanted to compare them with those in other tropical countries in the world. The ground for such comparison was that the tropical forest in the study area enjoys the bioclimatic and geographic characteristics similar to those in other tropical countries. The area has a tropical monsoon climate with an annual average rainfall of 254 cm (BFD, Citation2010; Rahman, Citation2004). It is under prolonged hot (30–40°C) and humid (75–85% relative humidity) summer and rainy seasons. The area has relatively a short winter (November–January) which is characterized by a cold (15.6–25.5°C) and dry (average maximum humidity of 74%) weather with almost no rainfall (BFD, Citation2010; Rahman, Citation2004). However, to facilitate such comparison, we gathered the values of biodiversity indices from existing studies conducted in different tropical forests across the world. The mean values of the biodiversity indices in KNP were statistically compared with the mean biodiversity indices of the tropical forests across the world. We tested the null hypothesis that the mean value of each of the three biodiversity indices obtained in the studies conducted in the tropical countries of the world did not significantly differ from the mean of the corresponding index obtained in our study using the t-statistic:
where, Mi and μi were the mean of the biodiversity index i obtained in the studies conducted in other tropical countries and in our study, respectively; σi was the standard error of mean of the index i obtained in the studies conducted in the tropical countries.
Results and discussion
Overall species composition
represents the overall composition of the plant species available in the study area. We have identified 52 plant species belonging to 45 genera and 28 families. Of the 52 plant species, 44 were tree, one shrub, four herb, and three vine species. A similar study conducted by Biswas and Misbahuzzaman (Citation2010) in the natural Dipterocarp forest in the same geographic area came up with similar outcomes. They reported 66 tree species belonging to 27 families and 45 genera. However, the boundary zone (ON) had the highest number of species (43) followed by the core zone (INSIDE) and outer zone (OUTSIDE) with 28 and nine species, respectively (). Even though the diversity of species was the highest in the ON zone, the number of individual plants was the highest (711) in the INSIDE zone of KNP. It was followed by the OUTSIDE and ON zones with 396 and 352 individual plants, respectively. The OUTSIDE zone was dominated mostly by Tectona grandis monoculture and hence the diversity of species in this zone was quite poor. The ON zone was being patrolled by the forest department officials more closely and frequently than other zones. Thus, the diversity was the highest here. However, this zone was dominated by big trees, and hence the number of individual trees was smaller in this area compared to that in the INSIDE zone.
Table 2. Overall distributions of flora in different zones of Kaptai National Park boundary.
Phytosociological attributes and diversity indices
Relative dominance
Inside the KNP, the highest dominance (52.72%) was occupied by the Dipterocarpus spp.—an ecologically adapted and economically important indigenous tree species in Bangladesh. In the INSIDE zone, Dipterocarpus spp was followed by Tectona grandis, Syzygium grandis, Syzygium cumini, and Protium serratum, respectively, with the relative dominance of 16.48, 8.97, 4.33, and 3.969% (). In the ON zone Tectona grandis was followed by the Dipterocarpus spp. (15.69%), Bischofia javanica (13.15%), Syzygium cumini (6.73%) and Lagerstroemia speciosa (6.42%). However, we found an opposite pattern in the relative dominance of the species available in the OUTSIDE zone of KNP. Here Tectona grandis was distantly followed by Artocarpus chama and Dipterocarpus spp. with 2.91 and 2.19% relative dominance, respectively (). Our findings were in line with the study of Mamun, Hossain, Hossain, and Alam (Citation2015), where they found the highest relative dominance (21.04%) for the Dipterocarpus spp. in Chunati natural forests though the degree of dominance of Dipterocarpus spp. in this forest was less than that in KNP. Similarly, Uddin and Misbahuzzaman (Citation2007) reported that Dipterocarpus spp. was the single dominant species in Dulhazara Safari Park in Cox’s Bazar Forest Division. The similarity in these studies justified the consistency of our findings. Unlike the INSIDE zone, Tectona grandis was the most dominant species in the ON and OUTSIDE zones with the relative dominance of 38.31 and 91.27%, respectively (). While the INSIDE and ON had a relatively mixed composition of species, 91.27% basal area of the OUTSIDE zone was occupied by Tectona grandis alone. The dominance of Tectona grandis in the OUTSIDE zone of KNP might have a number of explanations including its better growth in the region and the high value of its timber in the local timber markets. Since the Tectona grandis forest in the OUTSIDE zone was under private ownership, illicit removal of Tectona grandis was not common. This might have caused the OUTSIDE zone to have unique dominance of Tectona grandis.
Table 3. Comparison of relative dominance of different species available in the plots in different zones of Kaptai National Park boundary.
Relative frequency
From our study, we found the highest value of relative frequency (8.51%) for the Dipterocarpus spp. tree in the INSIDE zone of KNP (). Thus, it can be inferred that Dipterocarpus spp. was the highly dispersed tree species over the entire INSIDE zone of KNP. It was followed by the Protium serratum and Syzygium grandis, which attained the relative frequency of 6.38 and 5.67%, respectively. However, Tectona grandis was found as most dispersed tree species with the highest relative frequency of 13.51 in the ON zone of KNP. This was followed by the relative frequency of Microcos paniculata (7.21%) and Protium serratum (5.40%), respectively. In case of OUTSIDE zone, we found Tectona grandis as the mostly dispersed planted species, which had the relative frequency of 48.31%. The Tectona grandis dispersal was attributed to massive Tectona grandis monoculture in the forest areas adjacent to KNP. Swietenia mahagoni, Artocarpus heterophyllus, Dipterocarpus spp., and Albizia procera were comparatively less dispersed tree species over the entire OUTSIDE zone of KNP with the relative frequency of 10.35, 10.35, 6.90, and 6.90%, respectively. It indicates that the floral distribution in KNP was higher compared to that in the adjacent areas. As explained already, the private ownerships in the OUTSIDE zone were more inclined to raise Tectona grandis plantations with a view to produce valuable timber rather than just caring about creating environmental benefits through biodiversity enrichment.
Similar findings were reported in a study conducted in Dulhazara Safari Park in Cox’s Bazar Forest Division by the Uddin and Misbahuzzaman (Citation2007), where they reported the highest relative frequency (13.75%) for the Diperocarpus spp. Overall, both the number of species and their frequencies were higher in the two zones, INSIDE and ON, of the KNP compared to those in the OUSIDE zone. The study aimed to identify whether the floral diversity of KNP was better than that in its adjacent areas. The findings as reported in came up with the conclusion that, KNP was better protected compared to its surrounding areas. The objectives of forest management were also different in and outside the KNP boundary. While the KNP managers were engaged more on enriching the forest biodiversity in KNP, the private owners outside KNP were more concerned about producing valuable timber of Tectona grandis. This might have caused the differences in biodiversity status in the INSIDE and OUTSIDE zones.
Relative density
describes the relative density of plant species in the INSIDE, ON, and OUTSIDE zones of KNP. Dipterocarpus spp. again was identified to have scored the highest relative density in the INSIDE (33.47%) and ON (16.32%) zones of the KNP boundary. This was followed by the relative density of Tectona grandis, Vitis spp., and Protium serratum with the relative density of 14.64, 7.74, and 6.07%, respectively, in the INSIDE zone of KNP. Thus, it appears that, there was mixed diversity of floral species inside the KNP. Similar mixture of species diversity was also found in the ON zone of the KNP. Here, Dipterocarpus spp was closely followed by Tectona grandis. However, the number of species and the number of individual plants were the highest in the ON zone. A similar study was conducted by Mamun et al. (Citation2015) in Chunati, a different forest area with the same physiographic conditions in southeastern Bangladesh. They also reported that Dipterocarpus spp. was the most dominant species in that forest area, and it was followed by Tectona grandis. Thus, our study outcomes were consistent with the findings of Mamun et al. (Citation2015).
The floral diversity was the highest on the administrative boundary of KNP. As discussed earlier, the ground for the boundary region of KNP to have the highest plant diversity was that, it was under stringent patrolling of the Forest Department staffs. Unlike the INSIDE and ON zones of the KNP, the OUTSIDE area was very poor in plant density. In the OUTSIDE zone, Tectona grandis alone had the relative density of 89.69%. Besides, the OUTSIDE zone was clearly dominated with monoculture of Tectona grandis while the ON and INSIDE zones of the KNP were occupied with 43 and 28 species, respectively. Thus, as far as relative density was concerned, the ON zone of KNP was richer in plant biodiversity compared to the INSIDE and OUTSIDE zones. So, we could conclude that, the management of KNP as a protected area has made clear difference in enriching the biodiversity resource of KNP.
Importance value index (IVI)
The most important species growing naturally in the INSIDE zone of KNP was Dipterocarpus spp. with an IVI of 94.71 (). It was followed by Tectona grandis, Syzygium grandis, and Protium serratum. Similar IVI pattern, with a little exception, was exhibited by the plant species on the ON zone. Here Tectona grandis was the most important species followed by Dipterocarpus spp. with the IVI of 67.30 and 36.51, respectively. In contrast, the OUTSIDE zone was monotonically dominated by Tectona grandis with an extremely high IVI of 229.27. Overall, this finding enforces the notion that the diversity status of the floral community of KNP was better than that of the floral community growing outside the KNP administrative boundary. These outcomes were supplemented by Mamun et al. (Citation2015). They reported Dipterocarpus spp. to have the highest IVI (25.10) and it was followed by Tectona grandis with the IVI of 16.46 in the natural forest of Chunati.
Table 4. Importance value index (IVI) of floral species at different zones relative to the administrative boundary of Kaptai National Park.
Our study has identified a total of 52 plant species in KNP which was higher than that of Ukhia Forest Range in Cox’s Bazar. Ahmed and Haque (Citation1993) identified 38 tree species in Ukhia Forest Range. On the contrary, the species diversity of KNP was poorer compared to that of Bamu Reserve Forest of Cox’s Bazar. Hossain, Hossain, and Alam (Citation1997) reported 85 tree species in Bamu Reserve Forest. Thus, we conclude that, although KNP looked to be having better biodiversity status compared to its adjacent areas, its species diversity status was moderate compared to that in the other protected forests in the southeastern region of Bangladesh. KNP biodiversity indices compared () portrays a comparative status of species biodiversity indices at and around KNP boundary. The Shannon-Wiener index was the highest, 1.35, in the ON zone. It was closely followed by the same index (1.1) in the INSIDE zone. However, the Shannon-Wiener index was the lowest in the OUTSIDE zone. Since a larger Shannon-Wiener index indicates greater species diversity, these results clearly are in support of KNP to have been in better biodiversity health compared to that in the adjacent areas. We studied KNP splitting it into two regions—ON boundary and INSIDE boundary—hypothesizing that the biodiversity conditions at the ON zone would be better than that at the INSIDE zone on the ground that, boundary regions are at constant vigilance of the Forest Department staffs. As expected, the Shannon-Wiener index was the highest and so did the species diversity at the ON zone of the KNP.
Figure 2. Biodiversity indices of floral composition at different zones relative to the administrative boundary of Kaptai National Park.
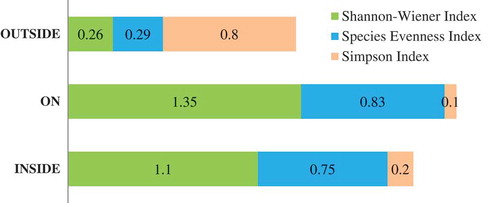
Again, states that the Simpson index has been the lowest (0.10) in the ON zone and the highest (0.80) in the OUTSIDE zone. Since a smaller Simpson index denotes greater species diversity, this result is also in line with the results exhibited by Shannon-Wiener index. This study also investigated the degree of evenness of the species distribution across the KNP and in the adjacent areas. The evenness index was the highest (0.83) at the ON zone followed closely by the index (0.75) in the INSIDE zone and distantly in the OUTSIDE zone. By comparing these three major biodiversity indices, we can conclude that the ON region of the KNP has better biodiversity status and it was closely followed by the INSIDE zone. However, biodiversity status of the OUTSIDE region was too poor to be compared with that either in the ON or INSIDE zone.
The above results did not confirm whether the biodiversity status of KNP itself was rich compared to its counterparts in other tropical countries. According to Kent and Coker (Citation1992), a tropical forest community is said to be rich if it has a Shannon-Wiener index of 3.5 and above. Having said this, we can conclude that, even though KNP was in better biodiversity status compares to its adjacent areas, the overall biodiversity of KNP was quite below the global expectation. The findings of a similar study conducted by Nath, Hossain, and Alam (Citation2000) in the same study area (the then Sitapahar Reserve Forest) immediately after it had been declared a PA also confirms this claim. They found a Shannon-Wiener index of 2.98 (), which was much higher compared to that reported in our study (1.10–1.35). The implication of this result is that, even though KNP got its legal status as a PA, its floral diversity degraded over time as a result of anthropogenic disturbances. The disturbance is possibly attributed to the exclusion of the local people from exercising their traditional rights on the KNP resources for their livelihoods. However, it was expected that the situation should improve since comanagement was adopted here in 2009.
Table 5. Comparison of biodiversity indices in the study area with that in the other tropical forests in the world.
compares the key biodiversity indices of our study area with that in the other tropical forests in the world. We found the Shannon-Wiener index ranging between 1.10 and 1.35. A similar study (Mishra & Garkoti, Citation2016) reported a Shannon-Wiener index of 1.34 in Nepal. This confirms that, the species diversity in the PAs of Bangladesh resembles the species diversity in Nepal. However, the Shannon-Wiener index obtained in our study was much lower in comparison to that in the forests of other tropical countries such as India (1.99–4.27), Myanmar (2.41), Malaysia (5.61), Cameroon (3.87), and Uganda (2.91; ). In light of this, we conclude that KNP as a protected area had poorly diverse floral composition compared its counterparts in the tropical countries.
We found the value of 0.75–0.83 for the species evenness index. Since this range is closer to 1.00, we can conclude that, the species were more or less evenly distributed in our study area. However, studies conducted in India reported the species evenness indices between 0.46 and 0.99 (). It means that some PAs in India had better evenness index compared to that in Bangladesh while some performed poorly. A study conducted in Cameroon reported an evenness index of 0.90 and one in Malaysia with the evenness index of 0.22. Thus, the evenness index in Bangladesh PAs lies between that in Cameroon and Malaysia. Overall, the evenness index in the PAs of Bangladesh was similar to that in the other tropical countries. In our study, we estimated the Simpson index that fell within the range of 0.10–0.20. In India, Myanmar, Nepal, and Malaysia this index was respectively 0.02–0.90, 0.84, 0.52, and 0.15 (). Since the Simpson index in our study was one of the lowest, we conclude that the species diversity richness was quite satisfactory in the PAs of Bangladesh compared to that in other tropical forests in the world.
While provides specific values of the biodiversity indices of the tropical forests across the world, statistically confirms whether the biodiversity indices obtained in our study significantly differed from that obtained in studies conducted in other tropical countries. We rejected the null hypothesis that the mean value of Shannon-Wiener indices obtained in the studies conducted in the tropical countries of the world did not significantly differ from that in our study at 1% level. That means the Shannon-Wiener index of the tropical forests in other countries (mean = 2.99) was significantly higher than that in the KNP (mean = 0.90). However, we failed to reject the null hypotheses that the species evenness index and Simpson index at KNP did differ from those in other tropical countries (). Thus, using these two, we cannot conclude whether the biodiversity health of KNP was better or worse than that in the other tropical countries. Overall, the biodiversity status at KNP was inferior compared to that in other tropical countries.
Table 6. Comparing biodiversity indices of Kaptai National Park with that of other tropical forests in the world.
Analysis of variance for different floristic attributes
depicts the variation in the floristic attributes of the plant species growing in the INSIDE, ON, and OUTSIDE zones of KNP. The F-statistic was significant at 1% level for DBH. This led to the rejection of the null hypothesis that the mean DBH of the tree species growing in three zones of KNP was the same. That means the mean DBH of the tree species growing inside KNP was not equal to that growing outside KNP. Similarly, the F-statistics for height, number of trees, and number of species were statistically significant at the 1% level and that of the number of seedlings was significant at the 5% level. It denotes that the biodiversity status of KNP flora differed in most of the floristic indicators from that of the flora growing outside the KNP. If we combine this result with the results obtained in through , we finally conclude that the plant diversity of KNP was better than that in its adjacent areas. That means that the management intensity of KNP as a PA has, to some extent, improved its biodiversity health. The field experience confirms that, the plantations outside the KNP were mostly monocultures with Tectona grandis as opposed to mixed culture species plots inside the boundary. Thus, the variation in the floristic attributes of the plants in these monoculture plantations was lower compared to those growing inside KNP.
Table 7. Analysis of variance (ANOVA) for different floristic attributes at different zones in relation to the administrative boundary of Kaptai National Park.
Conclusion
This study has estimated various phytosociological attributes of the floral community at KNP to evaluate the biodiversity conservation efforts for PAs in Bangladesh. Biodiversity health of KNP was better than that in the adjacent areas in all the phytosociological attributes. The reason for such better condition of KNP bioreserves might have a number of explanations. The adjacent forest areas are owned by private owners who might be more interested in planting commercially valuable timber species such as Tectona grandis, Swietenia mahagoni, and Artocarpus chama rather than just caring about the species diversity. Thus, the IVI for Tectona grandis has gradually increased from the central part of KNP to its boundary area, and again, from the boundary area to the adjacent private forest area. An exact reverse situation was observed in case of Dipterocarpus spp. The reason might be that the KNP falls in the natural Dipterocarpus spp. belt of the southeastern Bangladesh. Again, the KNP is basically a natural forest where Tectona grandis, an exotic species, was introduced as early as in 1871. Since it is a natural forest, it was expected that its species diversity should be richer than that in the adjacent forest areas. However, we suggest that the people around the KNP should be motivated about the importance of biodiversity and natural environment so that, a shift from “a Tectona grandis dominated less diversified plant community” to “a more diversified rich plant community” could be made possible. This motivation can effectively be done through the activities of the comanagement committee where the Bangladesh Forest Department and the local communities can work together for biodiversity conservation.
Even though the biodiversity indices of KNP were better than those in the adjacent forest areas, it was not a proof that KNP itself was in good shape. This study has compared the biodiversity indices of the PAs in Bangladesh with those in the tropical regions of the world taking KNP as a case. As measured through Shannon-Wiener index, the PAs in Bangladesh was significantly poorer compared to that in the forests of tropical countries. However, Simpson index and species evenness index were testifying that PAs in Bangladesh were competitive with those in the tropical countries of the world in terms of biodiversity status. Overall, the biodiversity situation at KNP was degrading over time. Shannon-Wiener index showed a rapid decline in the study area from as high as 2.98 in 2000 to as low as 1.1 in 2014. Thus, it can be inferred that, the biodiversity health of KNP has deteriorated over time despite its legal status change in September, 1999 from a reserve forest to a protected forest. The reason for such degradation might be sourced to excessive and destructive anthropogenic pressure on the KNP resources for the livelihood needs of the local people. Unless the livelihood demands of the local people for forest resources could be met, biodiversity conservation initiatives could not successful. Generating nonforestry income from alternative sources could be an effective option to reduce anthropogenic pressure on forest resources. This ultimately is expected to help conserve the biodiversity of KNP in particular and PAs across the country as a whole.
Another way of improving the biodiversity status of the PAs was to involve people in the management of the forest resources in the PAs. With this view, the local people were included in the KNP management system through comanagement approach in 2009. Yet, as this study focuses, the deteriorating face of KNP could not take a turn towards improvement. It might either be too early to capture such changes or the comanagement system might not be working properly. For the former, the comanagement activities should be speeded up, and for the latter, the faults with the co-management system, if any, should be identified and fixed. Again, creating awareness about biodiversity conservation should be an important task on the forest managers’ table to revert the existing defacing situation of the PAs where local people are heavily dependent on PA resources.
Without an active consideration for ensuring the local livelihoods, reduction of anthropogenic pressure on PA resources might not be possible. However, combining the two—making people take part in forest management activities and ensuring them with potential livelihood supports through nonforestry income generation activities—is expected to yield the best result in biodiversity conservation efforts in the PAs of Bangladesh. An active and effective comanagement committee should work for creating and conserving bioresources while supporting the local livelihoods. In the end, we expect that the legal status change from reserved forest to protected forest coupled with effective comanagement of KNP would help conserve the depleting biodiversity resources of KNP and all other PAs in Bangladesh in days to come.
Acknowledgments
The authors appreciate the recommendations and suggestions provided by the anonymous reviewers and the editors which have improved the overall quality of the manuscript.
References
- Adams, E. A. (2012). World forest area still on the decline. Europe, 989(998), 1–5.
- Ahmed, G. U., & Haque, S. M. S. (1993). Percentage distribution of species and diameter class in natural forest of Bangladesh. The Chittagong University Studies, Part II, 17(1), 109–113.
- Aye, Y., Pampasit, S., Umponstira, C., Thanacharoenchanaphas, K., & Sasaki, N. (2014). Floristic composition, diversity and stand structure of tropical forests in Popa Mountain Park. Journal of Environmental Protection, 5(17), 1588–1602. doi:10.4236/jep.2014.517150
- Bangladesh Forest Department (BFD). (2010). State of protected areas of Bangladesh. Dhaka, Bangladesh: Ministry of Environment and Forest, Government of the People’s Republic of Bangladesh.
- Bangladesh Forest Department (BFD). (2016). Protected areas of Bangladesh. Retrieved from http://www.bforest.gov.bd/
- Bhuyan, P., Khan, M. L., & Tripathi, R. S. (2003). Tree diversity and population structure in undisturbed and human-impacted stands of tropical wet evergreen forest in Arunachal Pradesh, Eastern Himalayas, India. Biodiversity and Conservation, 12(8), 1753–1773. doi:10.1023/A:1023619017786
- Biswas, S. R., & Misbahuzzaman, K. (2010). Tree species diversity and regeneration traits of the dominant species in a Dipterocarp forest in Bangladesh: Implications for conservation. The International Journal of Biodiversity Science and Management, 4(2), 8–91.
- Brandis, D. (1906). Indian trees. Delhi, India: Periodical Experts Book Agency.
- Brown, C., & Durst, P. B. (2003). State of forestry in Asia and the Pacific— Status, changes and trends. Bangkok, Thailand: FAO-RAP.
- Cavendish, W. (2000). Empirical regularities in the poverty-environment relationship of rural households: Evidence from Zimbabwe. World Development, 28(11), 1979–2003. doi:10.1016/S0305-750X(00)00066-8
- Chape, S. S., Blyth, L. F., Fox, P., & Spalding, M. (Eds.). (2003). United Nations list of protected areas. Cambridge, UK: UNEP-WCMC.
- Colwell, R. K. (2009). Biodiversity: Concepts, patterns, and measurement. Princeton, NJ: Princeton University Press.
- Dallmeier, F., Kabel, M., & Rice, R. (1992). Methods for long-term biodiversity inventory plots in protected tropical forests. In F. Dallmeier (Ed.), Long-term monitoring of biological diversity in tropical forest areas: Methods for establishment and inventory of permanent plots (pp. 11–46). Paris, France: UNESCO.
- Das, D. K., & Alam, M. K. (2001). Trees of Bangladesh. Chittagong, Bangladesh: Bangladesh Forest Research Institute.
- Dey, T. K. (2006). Useful plants of Bangladesh (Vol. 2). Chittagong, Bangladesh: The Add. Communication Press.
- Falconer, J., & Arnold, J. M. (1989). Household food security and forestry: An analysis of socio-economic issues. Rome, Italy: Food and Agriculture Organization of the United Nations.
- Fisher, R. J. (2003). Innovations, persistence and change: Reflections on the state of community forestry. The community forestry current innovations and experiences. Bangkok, Thailand: FAO-Regional Office for Asia and the Pacific, Regional Community Forestry Training Center (RECOFTC) and FAO-Regional Office for Asia and the Pacific (FAO-RAP).
- Gopalakrishna, S. P., Kaonga, M. L., Somashekar, R. K., Suresh, H. S., & Suresh, R. (2015). Tree diversity in the tropical dry forest of Bannerghatta National Park in Eastern Ghats, Southern India. European Journal of Ecology, 1(2), 12–27. doi:10.1515/eje-2015-0013
- Hayat, M. A., Kudus, K. A., Faridah-Hanum, I., Noor, A. A., & Nazre, M. (2010). Assessment of plant species diversity at Pasir Tengkorak Forest Reserve, Langkawi Island, Malaysia. Journal of Agricultural Science, 2(1), 31–38. doi:10.5539/jas.v2n1p31
- Heinig, R. L. (1925). List of plants of Chittagong Collectorate and Hill Tracts. Darjeeling, India: The Bengal Government Branch Press.
- Hirakuri, S. R. (2003). Can law save the forests? Lessons from Finland and Brazil. Bogor, Indonesia: Centre for International Forestry Research.
- Hossain, M. K., Alam, M. K., & Miah, M. D. (2008). Forest restoration and rehabilitation in Bangladesh. In D. K. Lee (Ed.), Keep Asia green (IUFRO World Series, Vol. 20, pp. 21–66). Vienna, Austria: IUFRO.
- Hossain, M. K., Hossain, M., & Alam, M. K. (1997). Diversity and structural composition of trees in Bamu Reserve Forest of Cox’s Bazar Forest Division, Bangladesh. Bangladesh Journal of Forest Science, 26(1), 31–35.
- Jeanrenaud, S. (2002). People-oriented approaches in global conservation: Is the leopard changing its spots? London, UK: International Institute for Environment and Development (IIED) and Brighton, UK: Institute for Development Studie (IDS).
- Kent, M., & Coker, P. (1992). Vegetation description and analysis. London, UK: Belhaven Press.
- Klooster, D., & Masera, O. (2000). Community forest management in Mexico: Carbon mitigation and biodiversity conservation through rural development. Global Environmental Change, 10, 259–272.
- Kothari, A., Pathak, N., & Vania, F. (Eds). (2000). Where communities care: Community-based wildlife and ecosystem management in South Asia. London, UK: Kalpavriksh, Pune, India and IIED.
- Kramer, R., Schaik, C. V., & Johnson, J. (1997). Last stand: Protected areas and the defense of tropical biodiversity. New York, NY: Oxford University Press.
- Krebs, C. J. (1999). Ecological methodology (Vol. 620). Menlo Park, CA: Benjamin/Cummings.
- Kumar, A., Marcot, B. G., & Saxena, A. (2006). Tree species diversity and distribution patterns in tropical forests of Garo Hills. Current Science, 91(10), 1370–1381.
- Landell-Mills, N., & Porras, I. T. (2002). Silver bullet or fools’ gold?: A global review of markets for forest environmental services and their impact on the poor. London, UK: International Institute for Environment and Development.
- Lewis, C. (Ed). (1996). Managing conflicts in protected areas. Gland, Switzerland: Keystone Center and IUCN.
- Magurran, A. E. (1988). Ecological diversity and measurement. Princeton, NJ: Princeton University Press.
- Mamun, A., Hossain, M. A., Hossain, M. K., & Alam, S. (2015). Quantifying diversity and composition of tree species in secondary hill forests of Chunati Forest, Chittagong, Bangladesh. Indian Forester, 141(5), 566–572.
- Masozera, M. K., & Alavalapati, J. R. R. (2004). Forest dependency and its implications for protected areas management: A case study from the Nyungwe Forest Reserve, Rwanda. Scandinavian Journal of Forest Research, 19(Suppl. 4), 85–92. doi:10.1080/14004080410034164
- Mbile, P., Vabi, M., Meboka, M., Okon, D., Arrey-Mbo, J., Nkongho, F., & Ebong, E. (2005). Linking management and livelihood in environmental conservation: Case of the Korup National Park Cameroon. Journal of Environmental Management, 76, 1–13.
- Michael, P. (1990). Ecological methods for field and laboratory investigation. New Delhi, India: Tata Mc Graw Hill.
- Mishra, B. K., & Garkoti, S. C. (2016). Species diversity and regeneration status in Sabaiya Collaborative Forest, Nepal. In N. J. Raju (Ed.), Geostatistical and geospatial approaches for the characterization of natural resources in the environment: Challenges, processes and strategies (Vol. 2, pp. 427–433). Cham, Switzerland: Springer.
- Misra, R. (1968). Ecology workbook. New Delhi, India: Oxford and IBH Publishing.
- Mukul, S. A. (2007). Biodiversity conservation strategies in Bangladesh: The state of protected areas. Tigerpaper, 34, 28–32.
- Mukul, S. A., Herbohn, J., Manzoor Rashid, A. Z. M., & Uddin, M. B. (2014). Comparing the effectiveness of forest law enforcement and economic incentives to prevent illegal logging in Bangladesh. International Forestry Review, 16(3), 363–375. doi:10.1505/146554814812572485
- Mukul, S. A., Manzoor Rashid, A. Z. M., Uddin, M. B., & Khan, N. A. (2016). Role of non-timber forest products in sustaining forest-based livelihoods and rural households’ resilience capacity in and around protected area: A Bangladesh study. Journal of Environmental Planning and Management, 59(4), 628–642. doi:10.1080/09640568.2015.1035774
- Mukul, S. A., & Quazi, S. A. (2007). Communities in conservation: Changing protected area management and enhanced conservation in Bangladesh. In R. N. Leslie (Ed.), The future of forests in Asia and the Pacific: Outlook for 2020 (pp. 143–159). Chiang Mai, Thailand: Food and Agriculture Organization of the United Nations.
- Mukul, S. A., Rashid, A. M., Quazi, S. A., Uddin, M. B., & Fox, J. (2012). Local peoples’ responses to co-management regime in protected areas: A case study from Satchari National Park, Bangladesh. Forests, Trees and Livelihoods, 21(1), 16–29. doi:10.1080/14728028.2012.669132
- Mukul, S. A., Uddin, M. B., Manzoor Rashid, A. Z. M., & Fox, J. (2010). Integrating livelihoods and conservation in protected areas: Understanding role and stakeholders’ views on the prospects of non-timber forest products, A Bangladesh case study. International Journal of Sustainable Development & World Ecology, 17(2), 180–188. doi:10.1080/13504500903549676
- Mukul, S. A., Uddin, M. B., Uddin, M. S., Khan, M., & Marzan, B. (2008). Protected areas of Bangladesh: Current status and efficacy for biodiversity conservation. Proceedings of the Pakistan Academy of Sciences, 45(2), 59–68.
- Mulongoy, K. J., & Chape, S. (Eds). (2004). Protected areas and biodiversity: An overview of key issues. Montreal, Canada: CBD Secretariat and Cambridge, UK: UNEP-WCMC.
- Nagothu, U. S. (2003). Local people’s attitudes towards conservation and wildlife tourism around Sariska Tiger Reserve, India. Journal of Environmental Management, 69, 339–347. doi:10.1016/j.jenvman.2003.09.002
- Nangendo, G., Stein, A., Gelens, M., De Gier, A., & Albricht, R. (2002). Quantifying differences in biodiversity between a tropical forest area and a grassland area subject to traditional burning. Forest Ecology and Management, 164(1), 109–120. doi:10.1016/S0378-1127(01)00603-X
- Nath, T. K., Hossain, M. K., & Alam, M. K. (2000). Assessment of tree species diversity of Sitapahar Forest Reserve, Chittagong Hill Tracts (South) Forest Division, Bangladesh. Indian Forester, 126, 16–21.
- Ndah, N. R., Andrew, E. E., & Bechem, E. (2013). Species composition, diversity and distribution in disturbed Takamanda Rainforest, South West, Cameroon. African Journal of Plant Science, 7(12), 577–585. doi:10.5897/AJPS2013.1107
- Panda, P. C., Mahapatra, A. K., Acharya, P. K., & Debata, A. K. (2013). Plant diversity in tropical deciduous forests of Eastern Ghats, India: A landscape level assessment. International Journal of Biodiversity and Conservation, 5(10), 625–639.
- Parthasarathy, N., & Karthikeyan, R. (1997). Plant biodiversity inventory and conservation of two tropical dry evergreen forests on the Coromandel coast, south India. Biodiversity & Conservation, 6(8), 1063–1083. doi:10.1023/A:1018328016810
- Parthasarathy, N., & Sethi, P. (1997). Trees and liana species diversity and population structure in a tropical dry evergreen forest in south India. Tropical Ecology, 38(1), 19–30.
- Pielou, E. C. (1966). Species-diversity and pattern-diversity in the study of ecological succession. Journal of Theoretical Biology, 10(2), 370–383. doi:10.1016/0022-5193(66)90133-0
- Prain, D. (1903). Bengal plants (Vol. 1, pp. 240–625). Calcutta, India: Botanical Survey of India.
- Rahman, M. H., Roy, B., Anik, S. I., & Fardusi, M. J. (2013). Ecotourism and protected area conservation in Bangladesh: A case study on understanding the visitors views on prospects and development. Journal of Forest Science, 29(1), 15–28. doi:10.7747/JFS.2013.29.1.15
- Rahman, M. M. (2004). Forest resources of Bangladesh with reference to conservation of biodiversity and wildlife in particular for poverty alleviation. Retrieved from http://www.fao.org/docrep/008/ae537e/ae537e0k.htm
- Scherr, S. J., White, A., & Kaimowitz, D. (2004). A new agenda for forest conservation and poverty reduction: Making markets work for low-income producers. Washington, DC: Forest Trends.
- Sekhar, N. U. (2003). Local people's attitudes towards conservation and wildlife tourism around Sariska Tiger Reserve, India. Journal of Environmental Management, 69, 339–347.
- Shukla, R. S., & Chandel, P. S. (2000). Plant ecology and soil science (9th ed.). New Delhi, India: S. Chand.
- Simpson, E. H. (1949). Measurement of diversity. Nature, 163(4148), 688. doi:10.1038/163688a0
- Thakur, A. S., & Khare, P. K. (2006). Species diversity and dominance in tropical dry deciduous forest ecosystem. Journal of Environmental Research And Development, 1(1), 26–31.
- Tripathi, K. P., Tripathi, S., Selven, T., Kumar, K., Singh, K. K., Mehrotra, S., & Pushpangadan, P. (2004). Community structure and species diversity of Saddle Peak forests in Andaman Island. Tropical Ecology, 45(2), 241–250.
- Tuxill, J. D., & Nabhan, G. P. (2001). People, plants, and protected areas: A guide to in situ management (Vol. 3). Chicago, IL: Earthscan.
- Uddin, M. B., Steinbauer, M. J., Jentsch, A., & Mukul, S. A. (2013). Do environmental attributes, disturbances, and protection regimes determine the distribution of exotic plant species in Bangladesh forest ecosystem? Forest Ecology and Management, 303, 72–80. doi:10.1016/j.foreco.2013.03.052
- Uddin, S. M., & Misbahuzzaman, K. (2007). Tree species diversity in Dulhazara Safari Park of Bangladesh. Malaysian Applied Biology, 36(2), 33.
- Uddin, S. N., & Hassan, M. A. (2012). Angiosperm flora of Rampahar Reserve Forest under Rangamati District in Bangladesh. I. Liliopsida ( Monocots). Bangladesh Journal of Plant Taxonomy, 19(1), 37–44. doi:10.3329/bjpt.v19i1.10940
- Velho, N., & Krishnadas, M. (2011). Post-logging recovery of animal-dispersed trees in a tropical forest site in north-east India. Tropical Conservation Science, 4, 405–419.
- Wells, M. P., & Mcshane, T. O. (2004). Integrating protected area management with local needs and aspirations. Ambio, 33(8), 513–519. doi:10.1579/0044-7447-33.8.513