Abstract
The microbial groups, physico-chemical characteristics, proteolysis, lipolysis, and rheological properties over a 30-day ripening period of a semi-hard cheese from pasteurized goat's milk were investigated. The count of aerobic mesophilic flora was high in cheese throughout ripening with lactic acid bacteria being the main microbial group. Halophilic bacteria, yeast and molds showed initial low counts but maintained their levels relatively constant during the ripening period. The main biochemical modification of cheese during ripening was related to the extent of proteolysis. The water soluble nitrogen in the semi-hard cheese increased during ripening. Lipolysis also occurred throughout the ripening period, with the major constituents being the palmitic, oleic, myristic, capric, and lauric acids. The rheological study suggested that the most important factors influencing the texture of the goat cheese is the level of total solids, and the extent of protein degradation recorded as soluble nitrogen during the ripening period. Rheological properties of goat cheese showed a transformation from a soft and elastic consistency to a hard and brittle body as a function of aging.
INTRODUCTION
The purpose of aging in cheese is to develop specific flavor, structure, and texture qualities. These characteristics result from the activity of microorganisms and enzymes. For such development to take place, the cheese must be maintained under the conditions favorable to the desired growth and activity. The aging conditions can also result in objectionable changes if the original milk is contaminated with undesirable microorganisms or if improper manufacturing procedures are used. Thus knowledge of the main physicochemical, biochemical, and microbiological characteristics at various stages of ripening is required for the development of an acceptable product.
Cheese ripening involves several biochemical processes including proteolysis, lipolysis, and glycolysis. Proteolysis is the principal and most complex biochemical event occurring during the ripening process of most cheese varieties.Citation[1, Citation2] Proteolysis contributes to cheese ripening through a direct contribution to flavor via the formation of peptides and amino acids, and by changing the texture of cheese owing to breakdown of the protein network.Citation[1] Textural properties play a key role in consumer acceptance of cheese.Citation[3, Citation4, Citation5] The rheological characterization of cheeses is important as a means of determining structure and texture for quality and identity as a function of composition, processing techniques, and storage conditions. At room temperature and for a given manufacturing process, milk proteins contribute to firmness and milk fat provide smoothness to cheese.Citation[6]
Lipolysis also plays an important role in cheese flavor. Most of the free fatty acids (FFA) generated from lipolysis are precursors of volatile compounds, like methyl ketones, alkanones, lactones, etc.Citation[7] In addition, normal FFA such as hexanoic, octanoic, nonanoic and decanoic acids, and other branched fatty acids contribute by themselves to the “goaty” flavor of cheese.Citation[8, Citation9] Likewise, lipolysis can affect negatively the flavor by giving the cheese a rancid flavor, the result of the production of an excessive amount of volatile FFA released by the action of the lipoprotein lipase.Citation[2]
The quality of milk plays a very important role in the production of all types of cheese, affecting both yield and characteristics of the cheese.Citation[10] The effects of ripening on the chemical and physical characteristics of cheese have been studied by numerous scientists. However, most of this research was focused on cheese made from raw milk, with cheese made from pasteurized milk being rather neglected. Cheese made from raw milk tends to develop stronger flavors and to ripen quickly than cheese from pasteurized milk. Yet, most commercial cheese is produced from pasteurized milk, rather than raw milk, to eliminate pathogens. There is scanty information in the literature on cheese made from pasteurized goat's milk. The present study deals with the physicochemical, biochemical, and rheological changes effected by the microbial flora during ripening of cheese from pasteurized goat's milk.
MATERIALS AND METHODS
Cheese Manufacture and Sampling
Whole goat milk was collected from Sultan Qaboos University Agriculture Experimental Station (AES) from January to June. The milk was collected under hygienic conditions into 50 liters vats and immediately transported to Sultan Qaboos University Dairy plant. Milk was pasteurized immediately using the batch method (65°C, 30 min.) The pasteurized milk was then refrigerated at 4°C until used for cheese making for no longer than 12 hours.
Goat cheese was manufactured according to the standard protocol described by Trujillo et al.Citation[11] with some modifications. Pasteurized goat milk (40 L) was placed in 100 L vats and heated to 32°C. The milk was inoculated with 8 g of direct vat set lactic starter CH-N 11 (Mesophilic aromatic culture type LD consisting of Lactococcus lactis ssp. Lactis and Lactococcus lactis ssp. Cremoris; CHR-HANSEN). Milk was held for 10 minutes at 32°C and then 4 g of CaCl2.2H2O (food quality grade) and 1.2 g of chymosin powder (CHR-HANSEN) were added.
After 90 minutes of incubation, the formed curd was cut, drained, molded and pressed. Salting was performed by immersion in brine (19% NaCl solution) for 45 min. Cheese curds were ripened in a chamber at 14°C and 85% relative humidity. Blocks of cheese were turned every 2 days and covered with aluminum foil on day 8 following manufacture. The size of each block was approximately 130 mm in diameter and 60 mm high.
Microbial Study
Changes in microflora during ripening were studied. Cheese samples were homogenized in a blender and 10 g were dissolved in 90 ml of sterile 2% sodium citrate solution heated at 45°C.Citation[12] Appropriate dilutions were prepared and incubated in duplicates. Plate counts of total aerobic organisms were carried out using plate count agar (30°C for 48 h);Citation[13] lactococci using M17 (30°C for 72 h);Citation[14] lactobacilli using MRS medium (30°C for 24–36 h);Citation[15] halophilic flora using MSA agar (30°C for 48 h); and molds and yeasts using potato dextrose agar acidified with tartaric acid to pH 3.5 (30°C for 3 days).Citation[13]
Physical and Chemical Study
The development of physicochemical and biochemical characteristics during ripening were measured on the curd. Dry matter was determined by oven drying at 105°C.Citation[16] The pH was measured by immersing the electrode of a Beckman meter into a blend of 10 g of grated cheese with 50 ml of distilled water. Total and soluble nitrogen at pH 4.6 in 0.5 M trisodium citrate with a pH of 7.0 were measured using the method described by Gripon et al.Citation[17] The kinetics of global proteolysis were followed by the ratio of water soluble nitrogen (WSN) to total nitrogen (TN). The total fat and free fatty-acid content of cheese samples were determined according to the BÜCHI CaviezelR method using the Büchi fat and fatty acid determination system B-815/820.Citation[18] The method was based on a gas chromatographic technique. The sample and internal standard (tridecanoic acid) along with potassium hydroxide were added to n-butanol.
In the first phase, the fat from the sample matrix was extracted with the n-butanol solvent and was simultaneously saponified in the presence of potassium hydroxide. After the extraction was completed, the alkali salts of fatty acids were then converted to fatty acids by the addition of an acidic aqueous-salt solution that produces a two-phase system. An aliquot from the upper organic phase containing the fatty acids and an internal standard was then injected into the fatty acid determination system (B-820). The fatty acids were separated from the solvent by gas chromatography and were detected by flame ionization. The total fat and free-fatty acid contents were then calculated from the internal standards and the individual fatty acid peak areas. The fat content was automatically converted to triglyceride content using a predetermined factor. The results are presented as dry basis (g fatty acid/100 g dry solids).
Rheological Investigation
This was performed using the ARES (Advanced Rheometric Expansion System) from Rheometric Scientific, (Piscataway, NJ, USA) which is a controlled strain rheometer. ARES has two force rebalance transducers (FRT) covering a torque range of 1.962 × 10−6 to 0.196 N.m. FRT transducers are air-lubricated and essentially non-compliant thus ensuring that any inherent machine compliance was insufficient to significantly offset measured values from the cheese networks.
For precise control of the sample temperature, an air convection oven was used. The oven has a dual element heater with counter-rotating air flow covering a wide temperature range of −60 to 160°C. Samples were loaded onto the plate of the rheometer and analysed at ambient temperature (23°C). Parallel plate geometry of 40 mm diameter and 5 mm gap was used. Frequency sweeps of 1.6 × 10−2 to 16 Hz were obtained at regular time intervals throughout the 30 days of sample aging. In a second repeat of experiments, strain sweeps were carried out to identify the area of linear viscoelastic response of the sample and potential changes in their elasticity with aging. Thus readings of the rigidity/storage modulus (G’), viscous/loss modulus (G’’) and complex dynamic viscosity (η*) variation with frequency and applied deformation were obtained.
The variation of storage modulus as a function of applied deformation at a fixed frequency of oscillation (0.16 Hz) was also used to identify the onset of fracture in these materials. Within the linear viscoelastic region, double logarithmic plots of G’ vs. the applied strain (%) remain flat. The onset of non-linear viscoelasticity signifying irreversible fracture-related effects on the proteinaceous matrix is reflected in decreasing values of G’ at the upper range of experimental deformation. The point of deviation from linearity was taken, therefore, as the onset of fracture on shear and was plotted against time for the experimental period of ripening.
Statistical Analysis
Analysis of variance was done by GLM using SAS® System for Windows software, v 8e (SAS Institute Inc., Cary, NC, USA). Tukey's Studentized Range test was conducted to detect the significant differences.
RESULTS AND DISCUSSION
Development of Microbial Population During Ripening
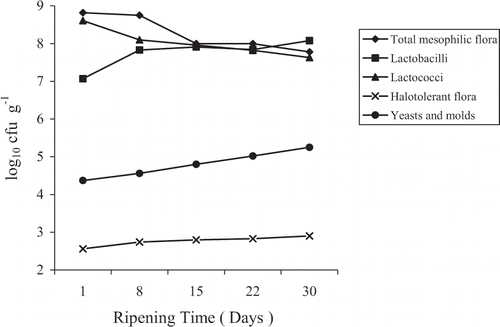
The number of total mesophilic flora on PCA, Lactococci on M17, Lactobacilli on MRS, halotolerant flora on MSA, yeasts and molds on PDA at different ripening times are shown in . It is apparent from the results that each group of microorganisms underwent a characteristic development in the cheese. Aerobic mesophilic flora and Lactococci showed a sharp decrease in their numbers during the first two weeks of ripening (about 1 log cycle decrease), and stabilized downwards their number thereafter. Lactobacilli showed a sharp increase in the first week (about 1 log cycle increase) and retained a slow increase thereafter. All other groups showed a constant increase in their counts until the end of the ripening period.
The high counts of aerobic mesophilic flora during the whole ripening period are a reflection of the influence of the starter bacteria inoculated into the cheese milk. The aerobic mesophilic count decreased by about 1 log cycle during the first 2 weeks but remained constant at high levels until the end of the ripening period. The decrease observed in the first 2 weeks was accompanied by a sharp drop in pH values (about 0.45 units in ), which is a consequence of the production of acid by microorganisms. This drop in pH is expected considering that lactic acid bacteria (mainly Lactococci and Lactobacilli) were also a dominant microbial group during the ripening process of the cheese.
Table 1 Changes in pH, total solids, and nitrogen fractions in goat's cheese during ripening.
The lactic flora remained high during the whole ripening process. The decrease in Lactococci count mainly at the onset of the ripening period is in accordance with other studies,Citation[19, Citation20] where starter counts decreased during ripening by 1 to 2 log cycles over 30 days. The decrease may be the result of competition with other flora. Halophilic flora comprising mainly Micrococci, coryneform bacteria and fecal Streptococci was reported by Lenoir,Citation[21] and Richard and ZadiCitation[22] as an important competitor of lactic acid bacteria. At the end of the ripening period, the Lactobacilli count became more important than that of Lactococci (). This result is rationalized by the fall in pH (), which favors the former; see also work by Mor-Mur et al.Citation[23] for Cendrat del Montsec, a Spanish goat's cheese.
The flora that better tolerates salt (Micrococci) grew moderately during ripening (). Micrococci showed initial low counts but maintained their levels upwards, a result also observed by Tornadijo et al.Citation[24] The weak growth is probably related to the low pH values observed in the cheese. This type of behavior can be further explained by the fact that being aerobic bacteria, Micrococci do not grow well at the low redox potential reached inside the cheese.Citation[24] They have a beneficial role in cheese ripening because they provide proteolytic, lipolytic and esterolytic activities.Citation[25]
Counts of yeasts and molds were low and remained almost constant throughout the ripening period. Besides their well-known role in the development of aroma and the modification of texture during the ripening of cheese, yeasts and molds act as the agents responsible for progressive surface-neutralization of cheese.Citation[26, Citation27] They metabolize residual lactic acid thus allowing the surface proliferation of Micrococci.
Compositional Characteristics During Ripening
The mean overall values for moisture, fat, protein, and pH at 1, 8, 15, 22, and 30 days of ripening are shown in . The pH of cheese () decreased progressively until day 22 of ripening and then increased. The initial decrease in pH should be attributed to the metabolic activity of such groups of microorganisms that show high growth rates in the curd as Lactococcus and Lactobacillus. A similar trend in pH was reported for La Serena cheeseCitation[28] and Cesar de Caceres cheeseCitation[29] during ripening.
Total solids increased continuously during the ripening period. The increase was due to surface evaporation and the exchange of volatile products (water, ammonia, fatty acids, etc.) between the cheese and its environment.Citation[27] At the end of ripening, total solids reached the mean value of 57.7 g/100 g of material considered to be normal in this type of cheese. The level of total solids is, of course, one of the determinants of texture in finished products.Citation[5, Citation26, Citation27]
Nitrogen compounds underwent noticeable changes during the ripening process of the goat cheese. Total nitrogen (TN) was maintained constant, whereas the remaining nitrogenous fractions varied. Protein degradation is clearly demonstrated by the definitive increase in water soluble nitrogen (WSN). also shows the WSN/TN ratio as a function of ripening time. At the beginning of ripening, the soluble nitrogen was 17.5% of the total nitrogen. The former showed a rapid increase in the first week of ripening from 17.5 to 24.9%, which continued unabated throughout the 30-day period. Results were comparable with those of the standard goat cheese,Citation[11] and Majorerro cheese.Citation[30] Soluble nitrogen components in cheese are produced mainly by the action of rennet (chymosin) at pH 4.6Citation[17, Citation21] but can also be produced by starter bacteria or plasmin.Citation[31]
Besides the obvious action of chymosin on the degradation of casein during ripening, the kinetics and nature of proteolysis in this kind of cheese depend on the action of proteases secreted by Mesophilic lactic acid bacteria. Thus, proteases secreted by Mesophilic Streptococci were a major contributor to proteolysis in several types of cheese.Citation[32] Many Micrococci also possess important proteolytic power.Citation[22, Citation33]
In contrast to proteolysis, the amount of fat varied relatively little during the ripening process of the cheese (). The lipids were not degraded much during the ripening of this type of cheese. There has been, however, considerable lipolysis of triglycerides to free fatty acids (FFA). Thus, total FFA content of cheese increased significantly (p < 0.05) during ripening, from about 10 mg g−1 fat on day 1 to 22.4 mg g−1 fat on day 30. These figures are close to those reported by Martin-Hernandez et al.,Citation[34] Carballo et al.,Citation[35] Vanbelle et al.Citation[36] The mild fat degradation may be explained by the fact that pasteurization destroys indigenous lipases of milk as well as the natural flora of this cheese. The latter consists basically of lactic acid bacteria, which have a very limited lipolytic activity.Citation[37] The main FFA observed during ripening were palmitic (C16), oleic (C18:1), myristic (C14), capric (C10), and steric (C18) acids, representing together about 74% of the total FFA content.
Table 2 Total fat and fatty acids composition of cheese during ripening (g/100 g dry matter).
Small and Large Deformation Textural Properties
According to , the most important factors that influence the texture of the goat cheese is the level of total solids and the extent of protein degradation recorded as soluble nitrogen during the ripening period. These two physico-chemical variations argue for opposite effects on the texture of the cheese, i.e. strengthening, with increasing solids content as opposed to weakening, with high values of WSN/TN ratio in the proteinaceous network. To identify the net effect of the two opposing mechanisms we performed small and large deformation dynamic oscillation experiments in the form of mechanical spectra and strain sweeps.
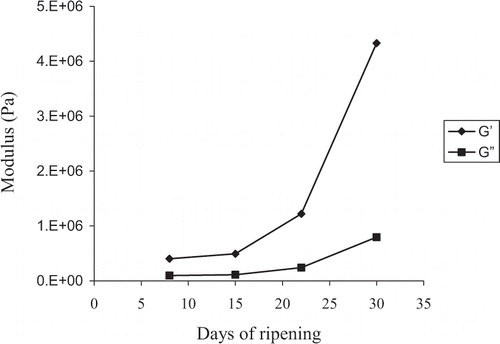
summarizes the variation of storage modulus (G’) and loss modulus (G’’) as a function of ripening obtained at 25°C and frequency of 0.16Hz. Data were obtained from mechanical spectra, which revealed solid-like structures (not shown here). Thus the storage modulus dominated over the loss modulus and complex viscosity descended rapidly with increasing frequency of oscillation. Furthermore, the traces of shear modulus showed modest frequency dependence. As shown in , structures are weaker at the beginning of ripening and enhance almost ten fold their rigidity at the end of the thirty-day period of observation (G’ values increase from 5 × 105 Pa to 4.5 × 106 Pa). These results argue that the increase in the levels of solids is the dominant effect on the texture of the goat cheese.
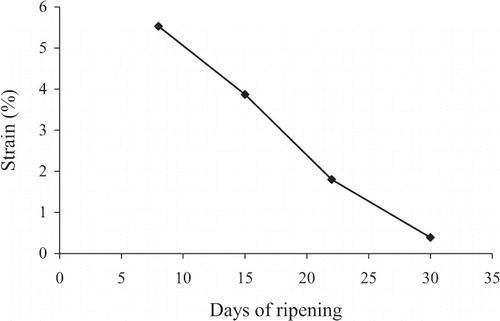
This is also reflected in the data of , which summarizes the strain variation of fracture points of the cheese during the 30-day ripening period (original strain sweeps are not shown here). In agreement with the increase in network strength with ripening, samples increase their brittleness, a result which is equivalent to a reduction in yield strain in large deformation properties. Thus catastrophic fracture commences at about 5.5% strain at the onset of experimentation and much lower (0.4% strain) at the end of the 30-day period. The final value is characteristic of rather brittle networks. Frequency sweeps yielding the results of were carried out at 0.1% strain, which according to , is well within the linear viscoelastic region of the samples.
Recent work on the rheological properties of Greek feta cheese showed a transformation from a soft and elastic consistency (yield stress: 2.3 kPa; yield strain: 18% deformation) to a hard and brittle body (yield stress: 6.9 kPa; yield strain: 7% deformation) as a function of aging from day 110 to day 300 of production.Citation[38] However, rheological analysis demonstrated the softening of Camembert cheese during ripening due to extensive proteolysis, which in this type of cheese becomes the dominant factor.Citation[39]
CONCLUSIONS
This is the third and final part of an investigation that attempted to map out the microbial, biochemical, and textural properties of popular cheese embodiments. These included Greek Feta, Camembert, and Omani goat cheese. Raw materials were pasteurized sheep, cow, and goat milk, respectively. Quantitative microbiology identified the main microorganisms and their characteristic growth in cheese. This varied from a uniform development in goat cheese, to gradation from the surface to the center of Camembert cheese. Fat degradation was modest, but proteolysis and variation in solid content was considerable. The relative kinetic rates of the last two processes determined the ultimate textural properties of the proteinaceous networks towards a hard and brittle (goat cheese and Feta) or soft (Camembert) material at the end of the ripening period.
REFERENCES
- Fox , P.F. 1989 . Proteolysis during cheese manufacture and ripening . J. Dairy Sci. , 72 : 1379 – 1400 .
- Grappin , R. and Beuvier , E. 1982 . Review: Possible implications of milk pasteurization on the manufacture and sensory quality of ripened cheese . International Dairy Journal , 7 : 751 – 761 . [CROSSREF]
- Ak , M.M. and Gunasekaran , S. 1992 . Stress-strain curve analysis of Cheddar cheese under uniaxial compression . J. Food Sci. , 57 : 1078 – 1081 .
- Bugaud , C. , Buchin , S. , Noël , Y. , Tessier , L. , Poshet , S. , Martin , B and Chambra , J.F. 2001 . Relationship between Abondance cheese texture, its composition and that of milk produced by cows grazing different types of pastures . Lait , 81 : 593 – 607 . [CROSSREF]
- Vassal , L. , Monnet , V. , Le Bars , D. , Roux , C. and Gripon , J.C. 1986 . Relation Entre le pH, la Composition Chimique et la Texture des Fromages de Type Camembert . Lait , 66 : 341 – 351 .
- Konstance , R.P. and Holsinger , V.H. 1992 . Development of rheological test methods for cheese . Food Technol. , 1 : 105 – 109 .
- Nájera , A.I. , Barrón , L.J.R. and Barcina , Y. 1993 . Review: Lipid fraction composition of cows, sheeps and goats cheese, and the influence on its quality . Revista Espaňola de Ciencia y technologia de Alimentos , 33 ( 4 ) : 345 – 363 .
- Rahmet , A. and Richter , R. 1996 . Formation of volatile free fatty acids during ripening of Cheddar-like goat cheese . Journal of Dairy Science , 79 : 717 – 724 .
- Le Qué , J.L. , Pierre , A. , Riaublanc , A. and Desmaizières , D. 1998 . Characterization of aroma compounds in the volatile fraction of soft goat cheese during ripening . Lait , 78 : 279 – 290 .
- Summer , A. , Franceschi , P. , Bollini , A. , Formaggioni , P. , Tosi , F. and Mariani , P. 2003 . Seasonal variations of milk characteristics and cheesemaking losses in the manufacture of Parmigiano-Reggiano cheese, Vet . Res. Comm. , 27 : 663 – 666 .
- Trujillo , A.J. , Royo , C. , Ferragut , V. and Guamis , B. 1999 . Ripening Profiles of Goat Cheese Produced from Milk Treated with High Pressure . Journal of Food Science , 64 ( 5 ) : 833 – 837 .
- Schmidt , J.L. and Lenoir , J. 1978 . Contribution à l'Etude de la Flore Levure du Fromage de Camembert. Son Evolution au Cours de la Maturation . Lait , 58 : 355 – 370 .
- Harold , V.L. 1976 . Compendium of Methods for the Microbiological Examination of Foods , Edited by: Speck , M.L. 10 – 104 . Washington, D.C. : American Public Health Association .
- Terzaghi , B.E. and Sandine , W.E. 1975 . Improved medium for lactic streptococci and their bacteriophages . Applied Microbiology , 29 : 807 – 813 . [INFOTRIEVE]
- De Man , J.C. , Rogosa , M. and Sharpe , M.E. 1960 . A medium for cultivation of Lactobacilli . Journal of Applied Bacteriology , 23 : 130 – 135 .
- International Dairy Federation . 1982 . “ Cheese and processed cheese ” . In Determination of the total solid content. Standard 4A , Brussels, , Belgium : International Dairy Federation .
- Gripon , J.C. , Desmazeaud , M.J. , Lebars , D. and Bergere , J.L. 1975 . Etude du Rôle des Micro-organismes et des Enzymes au Cours de la Maturation des Fromages: II. Influence de la Présure Commerciale . Lait , 55 : 502 – 516 .
- Gertz , C. and Fiebig , H.J. 2000 . Determination of fat content by the CaviezelR method (rapid method) . European Journal of Lipid Science and Technology , 102 : 154 – 158 . [CROSSREF]
- Buffa , M. , Guamis , B. , Pavia , M and Trujillo , A.J. 2001 . Lipolysis in cheese made from raw, pasteurized or high-pressure-treated goat's milk . International Dairy Journal , 11 : 175 – 179 . [CROSSREF]
- McSweeny , P.L.H. , Fox , P.F. , Lucey , J.A. , Jordan , K.N. and Cogan , T.M. 1993 . Contribution of the indigenous microflora to the maturation of cheddar cheese . Int. dairy J. , 3 : 613 – 634 . [CROSSREF]
- Lenoir , J. 1963 . La Flore Microbienne du Camembert et Son Evolution au Cours de la Maturation (1) . Lait , 43 : 262 – 270 .
- Richard , J. and Zadi , H. 1983 . Inventaire de la Flore Bactérienne Dominante des Camemberts Fabriqués Avec du Lait Cru . Lait , 63 : 25 – 42 .
- Mor-Mur , M. , Carretero , C. , Pla , R. and Guamis , B. 1994 . Microbiological changes during ripening of Cendrat del Montsec, a goat's milk cheese . Food Microbiology , 11 : 177 – 185 . [CROSSREF]
- Tornadijo , M.E. , Freson , J.M. , Bernardo , A. , Sarmiento , R.M. and Carballo , J. 1995 . Microbiological changes throughout the manufacturing and ripening of a Spanish goat's milk cheese (Armada variety) . Le Lait , 75 : 551 – 564 .
- Bhowmik , T and Marth , E.H. 1990 . Role of micrococcus and pediococcus species in cheese ripening: A review . J. Dairy. Sci. , 73 : 859 – 867 .
- Hassouna , M. and Guizani , N. 1995 . Evolution de la Flore Microbienne et des Caractéristiques Physico-chimiques au Cours de la Maturation du Fromage Tunisien de Type Camembert Fabriqué Avec du Lait Pasteurisé . Microbiolgy et Hygiene Alimentaire , 7 : 14 – 23 .
- Hassouna , M. , Nafti , A. and Ghrir , R. 1996 . L'affinage d'un Fromage à Pâte Molle et à Croûte Fleurie de Type Camembert au Lait Cru de Brebis: Aspects Microbiologiques et Physico-chimiques . Sciences des Aliments , 16 : 187 – 203 .
- Fernández del pozo , B. , Gaya , B. , Medina , M. , Rodriguez-Marin , M. and Nuňez , M. 1988 . Changes in chemical and rheological characteristics of La Serena ewe's milk cheese during ripening . J. Dairy Res. , 55 : 457 – 464 .
- Poulet , B. , Huertas , M. , Sanchez , A. , Caseres , P. and Larriba , G. 1991 . Microbial study of Cesar de Casares cheese throughout ripening . J. Dairy Res. , 58 : 231 – 242 .
- Martin-Hernández , M.C. , Juárez , M. and Ramos , M. 1992 . Biochemical characteristics of three types of goat cheese . J. Dairy Sci. , 75 : 1747 – 1752 .
- Visser , F.M.W. 1977 . Contribution of enzymes from rennet, starter bacteria and milk to proteolysis and flavour development in Gouda cheese. 4. Protein breakdown: a gel electrophoresis study . Neth. Milk. Dairy J. , 31 : 24 – 38 .
- Desmazeaud , M.J. , Gripon , J.C. , Le Bars , D. and Bergere , J.L. 1976 . Etude du rôle des Micro-organismes et des Enzymes au Cours de la Maturation des Fromages: III. Influence des Micro-organismse (Streptococcus lactis, Penicillium caseicolum et P. roqueforti) . Lait , 56 : 379 – 396 .
- Choisy , C. , Desmazeaud , M. , Gripon , J.C. , Lamberet , G. , Lenoir , J. and Tourneur , C. 1987 . Le fromage , 2e éd , Edited by: Eck , A. 62 – 100 . Lavoisier, , Paris : Techniques Et Documents .
- Martin-Hernández , M.C. , Juárez , M. and Ramos , M. 1988 . A ripening and storage study of soft goat cheese with penicillium candidum on the surface . Food Chemistry , 30 : 191 – 203 . [CROSSREF]
- Carballo , J. , Fresno , J.M. , Tuero , J.R. , Prieto , J.G. , Bernardo , A and Martin-Sarmiento , R. 1994 . Characterization and biochemical changes during the ripening of a Spanish hard goat cheese . Food Chemistry , 49 : 77 – 82 . [CROSSREF]
- Vanbelle , M. , Verback , W. and Foulon , M. 1978 . Composition en acides gras supérieurs de quelques types de fromages consommés en Belgique . Le Lait , 58 : 246 – 260 . 341–351
- Gutierrez , L.M. , Carballo , J. , Vidal , I. , Gonzalez Prieto , J. , Martin Srmiento , R. and Bernardo , A. 1988 . Evolucion de los principales grupos de microorganismos durante la elaboracion y maduracion del queso de Valdeteja . An. Fac. Vet. Leon. , 34 : 119 – 126 .
- Kasapis , S. and Boskou , D. 2001 . Rheological and sensory properties of popular Greek foodstuffs: A review . International Journal of Food Properties , 4 : 327 – 340 . [CROSSREF]
- Guizani , N. , Kasapis , S. , Al-Attabi , Z. and Al-Ruzeiki , M. 2002 . Microbiological, physicochemical and biochemical changes during ripening of camembert cheese made of pasteurized cow's milk . International Journal of Food Properties , 5 ( 3 ) : 483 – 494 . [CROSSREF]