Abstract
Some viral outbreaks have plagued the world since antiquity, including the most recent COVID-19 pandemic. The continuous spread and emergence of new viral diseases have urged the discovery of novel treatment options that can overcome the limitations of currently marketed antiviral drugs. Chalcones are natural open chain flavonoids that are found in various plants and can be synthesised in labs. Several studies have shown that these small organic molecules exert a number of pharmacological activities, including antiviral, anti-inflammatory, antimicrobial and anticancer. The purpose of this review is to provide a summary of the antiviral activities of chalcones and their derivatives on a set of human viral infections and their potential for targeting the most recent COVID-19 disease. Accordingly, we herein review chalcones activities on the following human viruses: Middle East respiratory syndrome coronavirus, severe acute respiratory syndrome coronavirus, human immunodeficiency, influenza, human rhinovirus, herpes simplex, dengue, human cytomegalovirus, hepatitis B and C, Rift Valley fever and Venezuelan equine encephalitis. We hope that this review will pave the way for the design and development of potentially potent and broad-spectrum chalcone based antiviral drugs.
Introduction
Nature is considered as a valuable source of medicine due to the existence of certain active ingredients and chemicals in various plant species. Numerous plant extracts were found to be useful remedies in different disease conditions [Citation1–3]. Among the well-studied phytochemicals are chalcones. Chalcones are considered the main precursors for flavonoids and isoflavonoids biosynthesis in plants [Citation4]. They are found in various plant species including fruits and vegetables [Citation4]. These medicinal molecules can also be synthesised in the lab in bulk quantities [Citation4]. A group of schemes and procedures have been described for the synthesis of these compounds. These methods include, but are not limited to, Claisen–Schmidt condensation, Aldol condensation, Suzuki reaction, Friedel–Crafts acylation, Witting reaction and photo-Fries rearrangement of phenyl cinnamate [Citation4].
Chalcones consist of an aromatic ketone and an enone that form a variety of biological agents [Citation5]. Their skeleton is made up of two aromatic rings with an aliphatic three-carbon chain linking them to form a linear or planar skeleton structure [Citation5]. They are also interconnected by conjugated double bonds and possess a delocalised π-electron system on the aromatic rings [Citation5]. Chalcones and their natural or synthetic derivatives (through some structural modifications of the chalcone rings) are known to have a wide range of numerous pharmacological actions comprising anti-inflammatory, anti-oxidant, antitumor, anti-tubercular, anti-viral, anti-malarial, anti-fungal and anti-bacterial activities [Citation6]. Several natural and synthetic or semi-synthetic chalcones have shown great medicinal bioactivity due to their actions against diverse targets [Citation6,Citation7]. Their anticancer activity was evident by targeting several molecules including P-glycoprotein (P-gp), aromatase, 5α-reductase, proteasome, vascular endothelial growth factor (VEGF) and other important factors [Citation6,Citation7]. Chalcones also showed promising beneficial activities against haematological, cardiovascular and obesity related diseases [Citation6,Citation7]. They have been reported to possess inhibitory effects on calcium (Ca2+)/potassium (K+) channel, angiotensin-converting enzyme (ACE), acyl-coenzyme A, thromboxane (TXA2 and TXB2), etc. [Citation6,Citation7]. Their excellent anti-inflammatory actions were proposed to be due to their inhibition of different targets like lipooxygenase (LOX), cyclooxygenase (COX), interleukins (ILs), nitric oxide synthase, prostaglandins (PGs), etc. [Citation8]. Additionally, chalcone molecules exert anti-diabetic effects through modulating agents of dipeptidyl peptidase-4 (DPP-4), peroxisome proliferator-activated receptor gamma (PPAR-Γ), α-glucosidase and tissue sensitivity [Citation9]. Their potential anti-viral activities have been well recognised via various targets including glyceraldehyde-3-phosphate dehydrogenase (GAPDH), topoisomerase-II, fumarate reductase, lactate dehydrogenase, several protein kinases, protein tyrosine phosphatase, human immunodeficiency virus (HIV-integrase (IN)/protease), lactate/isocitrate dehydrogenase, etc. [Citation10].
One of the major issues with several antiviral treatments is drug resistance that can emerge through mutations, genetic modifications and phenotypic changes [Citation11]. Thereby, the virus will no longer respond to the previous effective drug resulting in failure of controlling the disease, leading to higher risks of disease spreading, and high mortality rates [Citation11]. Consequently, the need for new antiviral agents became essential, thus, with the proven antiviral role of chalcones, they became a new attractive subject that have acquired the interest of the scientific community. We herein will go through different aspects of various chalcone based antiviral compounds, focussing particularly on their molecular antiviral activities and potential for treating COVID-19.
Viral infections and their therapeutic management
Viruses encompass a large cluster of pathogens that are accountable for causing severe infectious diseases and represent a major threat to the global health and economy. During 2019, the World Health Organization listed four different viruses among the 10 global health threats that require more attention, which included influenza, dengue, HIV and ebola viruses [Citation12]. Other high-threat viral infections requiring special attention and priority were also listed and included zika, haemorrhagic fevers, Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome (SARS) [Citation12]. Subsequently, during 2020 specifically, the world has faced the greatest health pandemic of the twenty-first century after the tremendous spread of the new COVID-19 disease triggered via SARS-CoV-2 coronavirus [Citation13]. This virus has spread to every continent and efforts to quarantine it are not yet effective [Citation13]. Unfortunately, SARS-CoV-2 has striking negative consequences on morbidity, mortality, healthcare systems capacity and world economy [Citation13,Citation14]. Viral infections are associated with deleterious consequences in the host body including cell death, stimulation of inappropriate response of immune system, disruption of cell function and cellular transformation [Citation15]. In immunocompetent patients, the majority of viral infections tend to resolve spontaneously [Citation15]. Additionally, antivirals are used in the management of a limited number of infectious diseases in order to reduce the virulence of the virus and minimise the course of illness [Citation16]. Vaccines are also used as a preventive measure against severe viral strains, but they are not always efficient and are still lacking for several types of viruses [Citation17].
Managing severe viral infections requires the timely and efficient use of therapeutic antiviral interventions to control the spread of the virus and reduce disease severity. Antiviral medications act principally by targeting either viral or cellular proteins [Citation18]. The first mechanism usually results in a specific targeted response with less side effects, but with a higher possibility for developing drug resistance [Citation18]. The second mechanism on the other hand, while presenting with less chances of developing drug resistance, provides a broad spectrum of antiviral activity and high toxicity profile [Citation18]. Most of the currently approved antiviral drugs function directly or indirectly by targeting different stages of the virus replication cycle as well as specific cellular and viral enzymes required for its replication [Citation19]. These targeted viral life cycle stages involve virus attachment and adsorption, cell fusion, synthesis of viral RNA or DNA and progeny virus release [Citation19]. Since viruses are obligate pathogens that use the host-cell machinery for their replication, finding drug targets that interact with viral replication is challenging [Citation18,Citation19]. Additionally, the current antivirals are associated with a group of limitations which include diminished efficacy, high toxicity, high costs and emergence of drug resistance [Citation18]. Therefore, discovering improved antiviral alternatives targeting viral diseases is highly required, especially with the developing concern of re-emergence of new viral strains and infections.
Chalcones as potential candidates for treating viral infections
Various studies have reported that different types of chalcones can act on important targets in diseases caused by viral infections. These promising diverse antiviral bioactions, make chalcone derivatives a favourable broad-spectrum candidate for targeting the most recent viral pandemic, COVID-19 and any other potentially emerging viral diseases. Their interesting biological activities have been studied in several scientific investigations as potential pharmacological agents capable of targeting a variety of human viruses including; MERS-CoV, severe acute respiratory syndrome-related coronavirus (SARS-CoV), HIV, influenza virus, human rhinovirus, herpes simplex virus (HSV), dengue virus (DEN), human cytomegalovirus (HCMV), hepatitis B virus (HBV), hepatitis C virus (HCV), Rift Valley fever (RVF) and Venezuelan equine encephalitis virus (VEEV), through their well discovered antiviral cellular targets and based on specific chalcone skeleton design [Citation20–30]. illustrates the main mechanisms of actions of chalcone derivatives that were reported in literature on different human viruses. Chalcones were shown to act on important viral molecular targets affecting different stages of viral replication cycle including; reverse transcriptase (RT) [Citation31,Citation32], IN [Citation22,Citation33–35], protease [Citation36–38], neuraminidase (NA) [Citation39], aminotransferases [Citation40], superoxide dismutase, glutathione peroxidase and other associated enzymes [Citation40]. They were also found to act on important receptors such as CXCR4 chemokine receptors [Citation41], US28 receptor of HCMV [Citation27] and capsid pocket inside viral protein 1 (VP1) in rhinovirus [Citation42]. Other important activities include targeting the kinase activity of epidermal growth factor receptor (EGFR) [Citation27], inhibition of DNA hybridisation [Citation30], mammalian target of rapamycin (mTOR) pathway [Citation43], blocking virus-mediated cell fusion and inhibiting the late stages of viral replication [Citation44]. In addition, chalcones were demonstrated by some studies to act as broad-spectrum covering agents on different viral infections and were shown to possess some preventive and prophylactic potential [Citation45]. Further clinical trials have supported the use of chalcone due to its low toxicity potential, making it an attractive option in the pharmaceutical industry [Citation46]. summarises the mechanisms of action of bioactive chalcone derivatives based on the viruses they were tested on, which are discussed in more details on subsequent text.
Figure 1. Mechanisms of action and targets of antiviral chalcone derivatives reported on different human viruses.
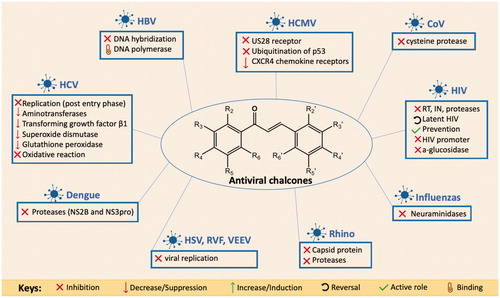
Table 1. Bioactive chalcone derivatives tested on different viral infections.
Chalcone derivatives tested on coronaviruses
Coronaviruses (CoVs) are enveloped viruses that belong to the Nidovirales order and Coronaviridae family, and are divided into four genera (α, β, γ and δ CoVs) [Citation67]. These pathogens have been found in bats, dogs, humans, as well as other mammals [Citation67]. There are seven species of CoVs identified to date that can induce diseases in humans [Citation68]. Four out of them (229E and NL63 α-CoVs; HKU1 and OC43 β-CoVs) trigger mild symptoms of common cold [Citation68]. The remaining three are β-CoVs (SARS-CoV, MERS-CoV and SARS-CoV-2) and are responsible for severe diseases [Citation68]. SARS first appeared in 2002–2003 and SARS-CoV was identified as its causative agent [Citation69]. This virus resulted in the viral outbreak that lasted from 2002 to 2003, which was the first pandemic of the twenty-first century originating from China and spreading to other countries [Citation69]. As per the Centers for Disease Control (CDC), SARS spread in 26 countries leading to 774 deaths and 8098 cases of infection [Citation70]. Around ten years later (2012), the Middle East respiratory syndrome emerged and a sixth coronavirus (MERS-CoV) was recognised in a patient in Saudi Arabia presenting with severe respiratory symptoms [Citation71]. This virus was reported in more than 27 countries across the Middle East, Asia, North Africa and Europe resulting in 2040 infections and 712 deaths [Citation71]. More recently, a seventh coronavirus (SARS-CoV-2) was identified in December 2019 in China and consequently spread worldwide resulting in a major health pandemic [Citation13]. The disease induced by SARS-CoV-2 was referred to as COVID-19 and is very closely related to the SARS disease identified in 2002 [Citation13]. The typical symptoms of SARS include dry cough, fever, shortness of breath and patients may progress to pneumonia in severe cases [Citation13]. As of 10 October 2020, the World Health Organization reported that 1,056,186 COVID-19 patients have died and 36,361,054 people are infected with SARS-COV-2 [Citation72]. A huge global effort is being undertaken in order to stop and control the current significant spread of COVID-19. There is no specific drug or vaccine to control the spread of CoVs; therefore, there is an ongoing research to identify and develop effective medications and new treatment options.
Chalcone pharmacophore represents a unique and attractive scaffold for the discovery and targeting of CoVs due to their significant anti-infective properties. Few chalcones were investigated with regards to their effect on CoVs infection, namely the SARS-CoV and MERS-CoV [Citation20,Citation21,Citation30,Citation47]. Only two studies investigated chalcones effect on SARS-CoV [Citation21,Citation47]. Park et al. synthesised and tested the antiviral activity of nine chalcone derivatives isolated from Angelica keiskei plant against SARS-CoV [Citation47]. Among these derivatives, an alkylated chalcones with methoxy and perhydroxyl substitutions were found to have potent inhibitory activities against important targets in SARS-CoV, which are cysteine proteases (i.e. papain‐like protease (PLpro) and a 3chymotrypsin‐like protease (3CLpro)) [Citation73]. Additionally, it showed an IC50 of 11.4 µM against 3CLpro and 1.2 μM against PLpro. Therefore, the substantial effect of chalones against these important proteases makes them attractive molecules for the development of anti-SARS drugs. On the other hand, two studies have explored chalcones activities against MERS-CoV [Citation20,Citation30]. MERS-CoV 3C-like protease (3CLpro) was explored as a target for chalones in a study published by Jo et al. [Citation20]. They reported two chalcone derivatives, isobavachalcone and helichrysetin, that exert prominent inhibitory activity against MERS-CoV 3CLpro (IC50: 35.85 and 67.04 μM, respectively) [Citation20]. In the other study, chloropyridine chalcone derivative exhibited a better inhibitory activity against viral replication and cellular growth (EC50: 3.2 μg/mL; CC50: 5.5 μg/mL; SI50: 1.7 μg/mL), indicating that the pyridyl functionality can enhance anti-MERS-CoV activity [Citation30]. Taken together, chalcone derivatives can target different types of CoVs, particularly their cysteine protease enzymes. This makes them a possible candidate for treating the emerging COVID-19 disease caused by SARS-CoV-2 virus. To date, no drug or vaccine has been approved for the treatment of COVID-19 and other coronavirus diseases and research is still ongoing. Therefore, more studies should be undertaken to test chalcone derivatives as potential candidates on SARS-CoV-2 and other CoVs.
Chalcone derivatives tested on human immunodeficiency virus
Acquired immunodeficiency syndrome is a life-threatening chronic disease caused by the HIV [Citation74,Citation75]. HIV is classified into two subtypes (HIV-1 and HIV-2), with HIV-1 being 24 times more common than HIV-2 [Citation74,Citation75]. According to the World Health Organization and the Joint United Nations Program on HIV and AIDS, 39 million patients died due to the AIDS epidemic and around 37 million patients were infected with HIV by the end of 2018 [Citation76,Citation77]. Therefore, treating HIV efficiently and effectively is a global health issue specially with increasing concerns of patients developing drug resistance. HIV/AIDS is currently treated mainly through the use of multiple antiretroviral drugs. These drugs are classified into six classes depending on their mechanisms that target different stages of the HIV life-cycle [Citation78]. These classes are: nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), chemokine receptor 5 (CCR5) antagonist, protease inhibitors (PIs), IN inhibitors and fusion inhibitors [Citation78].
Numerous natural and synthetic chalcone derivatives and analogues were tested on different viral infections. The vast majority of them were explored on HIV infection (with major focus on HIV-1 subtype). As summarised in , the main HIV targets that were investigated with chalcone were RT [Citation31,Citation32], IN [Citation22,Citation33–35] and protease [Citation36–38] enzymes. Some chalcones were investigated for their inhibitory potential on viral replication without specifying their exact targets [Citation49,Citation51]. Few chalcones were tested for their effects on RT enzyme [Citation31,Citation32]. For example, one study reported quinoline based chalcones as possible NNRTs [Citation31]. Based on their findings, chloro and bromo substituted chalcones increased RT inhibition (IC50 values of the most active molecules were 0.10 μg/mL and 0.11 μg/mL) [Citation31]. Chalcone derivatives were also evaluated against IN enzyme [Citation22,Citation33–35]. Among them, a derivative based on the chalcone pharmacophore had potently inhibited the IN mediated 3′ processing (IC50: 1.9 μM) and strand transfer (ST) processes (IC50: 0.6 μM) [Citation34]. Protease enzyme is another target that was studied for chalcone anti-HIV actions [Citation36–38]. Turkovic et al. reported the discovery of a very active chalcone derivative exhibiting a potent inhibitory activity towards protease enzyme (IC50 value of 0.001 μM) [Citation36].
Other tested anti-HIV activities of chalcones included preventive approaches [Citation45], latent HIV reversing [Citation48], HIV promoter activity inhibition [Citation52] and a-glucosidase inhibition [Citation54]. In a study testing chalcones therapeutic and preventive role on HIV, a chalcone with bromo and methoxy-substitutions was found to inhibit HIV infection potently in a dose-dependent manner in different HIV clinical isolates (e.g. anti-HIV IC50: 4.7 µM in TZM-bl-HIV infected cells) with no apparent toxicity [Citation45]. The ability to prevent HIV-1 infection was also investigated in the same study, where another chalcone derivative bearing bromo- and ortho-benzyl-substitutions was reported to exert both preventive and therapeutic responses [Citation45]. The effect on targeting latent viral reservoirs was also explored [Citation48]. Wu et al. investigated the effect of a new chalcone derivative [(E)-3-(5-(adamantan-1-yl)-2,4-bis (methoxymethoxy) phenyl)-1-(2-hydroxy-5-methylphenyl)prop-2-en-1-one] as a latency-reversing agent [Citation48]. The principle of LRAs action is based on reactivating the latent proviruses and consequently eliminating them [Citation48]. This chalcone derivative worked by activating HIV transcription by phosphorylating CDK9 at the T-loop and prompting the formation of Tat-SEC complex at the viral promoter [Citation48]. There are no LRAs currently in the market for HIV due to their high toxicity/low efficacy profiles. Therefore, this chalcone should be further examined for possible further development. In a different article, it was reported that a chalcone derivative had affected HIV gene expression at the transcriptional level by inhibiting HIV promoter activity by 80% [Citation52]. This significant activity was not observed on the cytomegalovirus (CMV) promoter, making this molecule selective towards the HIV promoter [Citation52]. Additionally, alpha-glycosidase inhibition has been reported as a possible mechanism to treat HIV [Citation79]. In a study exploring novel sulphonamide chalcone derivatives as alpha-glucosidase inhibitors, it was reported that a chalcone bearding dihydroxy substituent inhibited alpha-glucosidase non-competitively with a potent IC50 of 0.4 μM (inhibitory constant (Ki): 0.24 μM) [Citation54].
HIV-2 represents another distinct subtype that is far less common compared to HIV-1 [Citation74,Citation75]. However, around 1.5 million people are living with HIV-2 including those with dual HIV-1 and HIV-2 infection, yet it is attracting much attention [Citation74,Citation75]. Only few studies investigated the role of chalcones on HIV-2 [Citation50,Citation55]. In a study by Casano et al., three novel chalcone derivatives were found to have specific inhibition for HIV-2 multiplication, one compound was found to be six times more selective towards HIV-2 with IC50 of 47.7 μM [Citation50]. Additionally, para substitution was reported to increase the potency of this inhibition [Citation50]. Others synthesised chalcones within the same study exerted potent non-selective activities towards both HIV-1 and HIV-2 (e.g. IC50: 5.7 μM on both subtypes) [Citation50]. However, the majority of the chalcones were reported to be selective towards HIV-1 [Citation50].
Chalcone derivatives tested on influenza virus
Influenza is a viral infection with seasonal variations caused by RNA viruses of the Orthomyxoviridae family [Citation80]. Influenza A and B viruses are causative factors of death of many people worldwide [Citation80]. Influenza virus has evolved cellular mechanisms for exploiting human cell factors to boost its replication while suppressing host immune responses [Citation80]. Identification of these cellular techniques would help to widen the number of the anti-influenza drug targets. Current targets include two proteins; the M2 ion channel and NA [Citation81]. Neuraminidase is a large glycoprotein on the outside surface of viral particles. NA is one of the pathogenic factors and is responsible for the release of progeny virus from infected cells [Citation82]. Despite the emergence of many inhibitors of viral NA, including rimantadine, amantadine, oseltamivir and zanamivir, drug-resistant influenza viruses have been generating rapidly [Citation83]. Therefore, search for effective anti-influenza drugs against different influenza strains is given high priority. Throughout the developmental process of an anti-influenza screening program of natural products, numerous effective molecules were found to play a role in limiting the threat of resistant strains or enhancing the effectiveness of many anti-viral agents. NA became one of the major anti-viral targets of many natural and synthetic chalcones, as targeting it is crucial for the inhibition of the viral growth. 2′, 4′-dihydroxy-4-methoxy chalcone is derived from bioactive compounds in nature and presented a high activity in inhibiting H1N1 NA [Citation39]. In a different study, H9N2, H1N1, novel H1N1 and oseltamivir-resistant novel H1N1 cells were targeted by natural products obtained from the acetone extract of Glycyrrhiza inflata including isoliquiritigenin (IC50 (μg/mL) of 8.41 ± 0.39, 9.69 ± 0.37, 3.48 ± 0.19 and 3.42 ± 0.12, respectively) and echinantin (IC50 (μg/mL) of 5.80 ± 0.30, 5.70 ± 0.55, 2.49 ± 0.14 and 2.19 ± 0.06, respectively) [Citation23]. 2′-Hydroxy-4-methoxychalcone is also a synthetic compound designed based on quercetin and was found to effectively target H5N1 [Citation56]. In another study, 2-hydroxy-3-methyl-3-butenyl alkyl is a synthetic product that mainly targets H1N1 with IC50:12.3 μM [Citation59]. This compound exhibited the most potent inhibitory effect of NA activity in comparison to other chalcone substitutes; 6-hydroxyl-3,7-dimethyl-octa-2,7-dienyl > dimethylallyl > geranyl in a non-competitive inhibition manner [Citation59]. According to another study, H1N1 and H9N2 were also inhibited by natural products extracted from the root bark of Erythrina addisoniae including; licoagrochalcone A, abyssinone VI and 5′-prenylbutein [Citation57]. (E)-4,2′,4′-Trihydroxy-6′-methoxy-3′,5′-dimethylchalcone, 2′,4′-dihydroxy-6′methoxy-3′,5′-dimethylchalcone, 2′,4′dihydroxy-3′-methyl-6′-methoxychalcone and 2,2′,4′-trihydroxy-6′methoxy-3′,5′-dimethylchalcone are natural C-methylated products isolated from Cleistocalyx operculatus buds that exhibited major inhibition of the viral NAs from H1N1 and H9N2 strains [Citation58].
Chalcone derivatives tested on rhino virus
The rhinovirus is a common viral infectious pathogen in humans that is a predominant agent of the common cold and upper respiratory tract infections [Citation84]. Rhinovirus infection prefer to proliferate in warm temperatures of 33–35 °C, mainly in the nose [Citation85]. It belongs to the Picornaviridae family; which are small nonenveloped viruses with a single-stranded RNA [Citation84]. There are three species of rhinovirus (A, B and C) and include around 160 serotypes differentiated according to their surface proteins [Citation86]. Current antiviral agents against this virus were formed to target cell susceptibility by preventing virus binding and attachment, viral replication, virus uncoating and production of protein [Citation86]. Chalcones have been tested on a number of specific targets including binding sites, capsid pocket and viral proteases inside VP1, the latter was found to be the main effective target of several anti-rhinovirus chalcones. Ro 09-0410, was the main chalcone and one of the first isolated compounds [Citation24,Citation60,Citation61]. However, upon further testing on humans through clinical trials, it did not significantly reduce the incidence of infection or illness; therefore, several attempts have been made to produce various synthesised analogues related to Ro 09-0410 compound [Citation24]. The goal of identifying anti-rhinovirus agents was to develop compounds with higher therapeutic ratio to limit viral infectivity. Chalcone Ro 09-0410, 4′,6-dichloroflavan (DCF) and RMI-15,731(1-[5-tetradecyloxy-2-furanyl]-ethanone), were suggested by several studies for directly inactivating the virus [Citation62]. However, the binding site of the aforementioned compounds on the capsid was found to have slight conformational differences among viral serotypes [Citation62]. A minor change in the binding site was proposed since chalcones can bind to the DCF resistant rhino virus type, and no cross-resistance was seen between RMI and DCF [Citation62]. In another study, amide analogues where developed and among them; Ro 09-0535 ([4-methoxy-3H]) with IC50 of 0.0018 µg/mL, Ro 09-0696 (chalcones derivatives with methoxy (OMe) substitution) with IC50 of <0.0018 µg/mL, and Ro 09-0881 was shown to be 4.5–12 times more effective on several serotypes of the virus than Ro 09-0410 or other agents that target the capsid protein [Citation42]. An earlier study reported synergistic activities among certain drug combinations. DCF (dichloroflavan), chalcone Ro-09-0410, enviroxime and HuIFN (human interferon)-alpha 2, HuIFN-beta, HuIFN-beta X 401 and HuIFN-gamma, showed an activity against rhinovirus type 2 (RV2) and rhinovirus type 9 (RV9) [Citation87]. The combinations of HuIFN-gamma or HuIFN-alpha and enviroxime were of special interest [Citation87]. These results indicate that similar drug combinations are worthy of future clinical studies. Subsequent investigations emphasised the importance of closely monitoring the development of drug-resistant viruses during chemotherapy and antiviral treatment, as a single change in an amino acid is sufficient for the emergence of drug viral resistance [Citation88]. However, another study reported that use of chalcones is beneficial despite drug resistance. The study reported that chalcone Ro 09-0410 was capable of decreasing rhino virus infectivity in humans who were challenged with a drug resistance mutant. In the same study, a drug-dependent virus had completely lost its capability of infecting humans [Citation89]. Further studies should be conducted to determine the mechanism of action by which chalcones can overcome or reduce the extent of drug resistance.
Chalcone derivatives tested on herpes simplex virus
Herpes simplex virus belongs to the Herpesviridae family and is associated with various types of diseases [Citation90]. The most prevalent types that are associated with human infections are HSV-1 followed by HSV-2 [Citation91]. In 2016, it was estimated that 3.7 billion people were living with HSV-1 and 491.5 million individuals had HSV-2 [Citation91]. Latent HSV-1 infections can reactivate causing severe illnesses in all age groups including neonates [Citation90]. These infections include conjunctivitis, cutaneous or genital herpes, keratitis, eczema herpeticum or encephalitis [Citation90]. On the other hand, HSV-2 can cause aseptic meningitis, genital herpes and devastating neonates infections [Citation92]. HSV infections are managed through the use of antiviral agents, with acyclovir being the first-line treatment option [Citation92]. There is a range of promising virus and host-cell specific targets for HSV treatment [Citation93]. Examples include, viral polymerase and helicase–primase or host cell related targets such as cyclin-dependent kinases and cyclooxygenase-2 [Citation93].
Various natural and synthetic chalcone derivatives were tested against HSV focussing mainly on their inhibitory potency on viral replication [Citation25,Citation30,Citation53,Citation63,Citation64,Citation94]. A chalcone derivative isolated from Millettia leucantha has shown moderate inhibitory activity against HSV-1 and HSV-2 (IC50: 15.5 μg/mL against HSV-1 and IC50: 17.0 μg/mL against HSV-2) [Citation63]. In another study, a biphenyl substituted cyano chalcone derivative has also shown a good inhibition against HSV-1 (EC50: >6.00 μM) [Citation30]. In a third study by Ali et al., a group of chalcone derivatives were tested for their broad spectrum activities against different viruses [Citation25]. Among them, 5-(4′-chlorophenyl)-3-(4″-hydroxy-3″-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl-4-pyridyl methanone had the best replication inhibitory activity against HSV-1 and HSV-2 (minimum cytotoxic concentration: 200 μg/mL; minimum inhibitory concentration: >8 μg/mL against HSV-1 and 40 μg/mL against HSV-2) [Citation25]. This chalcone derivative has shown promising broad spectrum activities against other viruses including vesicular stomatitis, vaccinia, para-influenza-3, sindbis, reovirus-1, coxsackie and Punta Toro viruses [Citation25]. This is one of select studies investigating the role of chalcone derivatives as broad spectrum antiviral agents [Citation25,Citation30,Citation94]. Therefore, more studies investigating chalcone derivatives against multiple virus subtypes are highly required in order to distinguish broad spectrum chalcones from those having specific activities against certain viruses. Additionally, more research should be done focussing on chalcones effects on more specific HSV targets.
Chalcone derivatives tested on dengue virus
Dengue is an infection caused by the Arthropod-borne DEN [Citation95]. It can present as dengue fever (DF) or dengue haemorrhagic fever (DHF)/dengue shock syndrome (DSS) [Citation96]. Dengue virus belongs to the Flaviviridae family and is caused by four variants of viruses including DEN-1, DEN-2, DEN-3 and DEN-4 [Citation96]. Dengue present 3–14 days after the infection with symptoms of high fever, muscle and joint pains, and rash, with expected recovery taking 2–7 days [Citation96]. It is also considered an endemic disease in more than 100 countries [Citation97]. Flaviviridae family and DEN consist of a single positive-sense 1-kb RNA [Citation96]. Translation of the genome results into a single polyprotein made of three structural and seven non-structural (NS) proteins [Citation98]. This polyprotein yields a sequential order C-prM-E-NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5 [Citation98]. Upon gene expression, proteolytic processing of the synthesised polyprotein whether co- or post-translational is required [Citation99]. This processing results in the cis and trans-cleavage of polyprotein precursor by both host proteases or virus protease complex NS2B/NS3 alongside its cofactor NS2B [Citation99]. Although the efficacy of vaccines like the recently developed ChimeriVax is currently under evaluation, to date; there is no known vaccine approved for human use as a protective measure against dengue [Citation100]. However, there are a number of evolving promising therapeutic strategies against this disease.
Dengue virus protein targets were examined in several trials and including virus protease (NS2B-NS3pro), methyltransferase (MTase), helicase (NS3 helicase), virus envelope and protein RNA-dependent RNA polymerase (RdRp) [Citation101]. Despite several trials, NS3pro became an important and attractive target for potential therapeutic agents in addition to some chalcone compounds which were used in such trials against DF/DHF [Citation101]. 4-Hydroxypanduratin A is a natural product that was tested against dengue-2 virus and showed a competitive inhibition towards NS3 protease [Citation26]. In another study, panduratin A was also shown to behave similarly with the Ki value of 25 μM [Citation26]. Cardamonin, also known as dihydroxymethoxychalcone, is a synthetic compound that exhibited a non-competitive inhibition against DEN-2 NS2B/NS3 activities [Citation65]. There are two proposed mechanisms by which dengue virus (DENV2) NS2B/NS3 protease is targeted for inhibition [Citation102]. A promising compound can either destabilise electronic density to the catalytic triad residues NS3-ASP75, NS3-HIS51 and NS3-SER135 [Citation102]. Conversely, this enzyme can be inhibited by a ligand which can also interrupt the C-terminal movement of NS2B, which is needed for conversion between the open and closed conformations [Citation102]. Upon exploring these binding sites by multiple molecular dynamics simulations, chalcones were found to be one of the top potential drugs acting via both aforementioned inhibitory mechanisms [Citation102]. Nevertheless, further investigations on the allosteric pocket is needed for more drug discovery potentials.
Chalcone derivatives tested on human cytomegalovirus
Although there is currently a number of available drugs for cytomegalovirus (HCMV) infection treatment, the side effects of these medications including their toxicity and directed teratogenicity make their use in certain populations like pregnant women contraindicated [Citation103]. Moreover, drug resistance is considered an important problem in this disease management, especially in patients who are immunocompromised and are in severe need for such medications. There are several recent promising trials for antiviral medications that are able to combat HCMV infections with less drawbacks including toxicity and resistance. The genome of HCMV, is made of double stranded DNA that encodes for specific receptors, vital G protein coupled receptors (vGPCRs), of which US28 receptor is considered an attractive target [Citation104]. The US28 receptor processes a ligand-independent signalling pathways in which it can activate cascades to facilitate virus survival, invasion, oncogenesis and variety of diseases including cardiovascular disease through these constitutive signalling pathways [Citation104]. The potential role of US28 receptor in viral pathogenesis establishes a potent infection in immunocompromised hosts as well as immunocompetent hosts through latent infections that facilitate the viral dissemination and invasiveness [Citation104].
Known biological activities of flavonoids and their abundance in plants makes them attractive candidates for targeting HCMV virus as they are generally considered as effective and potentially safe antivirals for use in different patients; however, validation of their safety in specific populations like pregnant women are further needed [Citation105–107]. Of those flavonoids, chalcones became an ideal platform for their relatively easy synthesis and their biological effects in inhibiting a number of known enzymes; therefore, they became a potential tool for the treatment of HCMV infections [Citation105]. 5-(Benzyloxy)-2-(5-bromo-2-methoxyphenyl)-4H-chromen-4-one, is one of these chalcones that was found to inhibit the US28 receptor of HCMV with an EC50 of 3.5 μM [Citation27]. In 2004, xanthohumol 1, a prenylated chalcone showed moderate antiviral activity against HCMV through its action in downregulating CXCR4 chemokine receptors [Citation41]. Other chalcones were also found to be effective in targeting the kinase activity of EGFR and thus preventing HCMV entry into cells and limiting cellular activation [Citation27]. HCMV infection also induces an upregulation of p53, this process is a common target of trans-4-iodo, 4′-boranyl-chalcone as ubiquitination of p53 in infected cells is inhibited by the chalcone [Citation66]. In a study, biphenyl substituted cyano derivative was found to be effective against both resistant and non-resistant strain with high potency [Citation30]. Thus, due to their unique structure, strong activity and potential low toxicity profile, chalcones are expected to be further involved in future investigations as antiviral agents against HCMV.
Chalcone derivatives tested on hepatitis B virus
Hepatitis B virus is a virus that attacks the liver and induces hepatitis B infection [Citation108]. It is a small DNA virus that belongs to the Hepadnaviridae family [Citation108]. Hepatitis B is usually a short-term infection; however, for some patients, it may transform into a chronic illness [Citation108]. Chronic hepatitis B is associated with serious complications like liver cancer and cirrhosis [Citation108]. As of 2015, the World Health Organization reported that around 257 million individuals were living with chronic hepatitis B infection and that 887,000 died of it [Citation109]. There are some medications that can help with the management of hepatitis B including nucleoside/nucleotide analogues and interferon-based therapy [Citation110]. However, none of them is effective in eradicating the infection [Citation110]. The best preventive strategy is hepatitis B vaccination [Citation110]. Various studies have tested different types of molecules as potential treatment options for hepatitis B, including chalcone derivatives.
Few studies only investigated chalcones role on HBV [Citation28,Citation30]. Patil et al. synthesised a group of substituted aryl/heteroaryl derived thienyl chalcones and reported that the more rigid, less bulky thiophenylindenone derivative was the most potent at inhibiting DNA hybridisation in vitro (EC50: 4.2 μM; good selectivity index: >24) [Citation30]. These results demonstrate that chalones can be a potentially good candidates for inhibiting the replication of DNA viruses. In a study by Mathayan et al., they investigated the anti-HBV effects of the P. pinnata seeds, which contains some chalcone derivatives [Citation28]. At a concentration of 5 mg/mL, P. pinnata extract significantly inhibited HBV replication (virus concentration of 0.18 pg/mL; p < .001) with no apparent toxicity [Citation28]. On the other hand, molecular docking studies showed that two chalone derivatives (isopongachromene and glabaarachalcone) present in P. pinnata bind to the HBV DNA polymerase protein [Citation28]. However, further studies are needed in order to confirm the real effects of chalcone derivatives in vitro by isolating and testing them separately from the plant extract, especially with the promising virtual screening study results that merit further in vitro confirmation.
Chalcone derivatives tested on hepatitis C virus
Hepatitis C virus is a member of the Flaviviridae family [Citation111]. The heterogenicity of the viral genetic material has been used to classify the HCV virus into seven genotypes and more than 67 subtypes [Citation111]. The viral genome consists of a single stranded positive-sense RNA [Citation111]. This genetic material/genome is used to create a precursor (poly protein), which is made up of three thousands amino acids that need to be processed in the post-translational phase by the intracellular proteases in order to form mature proteins including core, E1, E2, a putative ion channel p7, in addition to NS proteins NS2, NS3, NS4A, NS4B, NS5A and NS5B [Citation111]. These structural and NS proteins are essential for the viral pathogenesis and aid in its infectivity, carcinogenesis and several other pathological processes [Citation111]. Besides its well-known effects on liver metabolic processes including lipids and glucose metabolic disorders, it has been well-known for being one of the major causative agents of chronic hepatitis, cirrhosis and hepatocellular carcinomas [Citation111]. There are a number of standard agents used for the treatment of HCV infection including pegylated interferon regiments, ribavirin and other direct-acting agents [Citation111]. The need for alternative drugs development for HCV infection treatment became a well-studied topic due to clinical and economic purposes. The cost of these medications as well as drug resistance emergence is commonly encountered. In this regard, complementary medicine aims to develop antiviral agents that are strong and effective inhibitors of HCV infection.
Chalcones and their compounds have been studied as effective substitutes because of their ability to inhibit viral translation and replication of HCV. Plants like Glycyrrhiza uralensis and G. glabra have been considered as good candidates in medicinal plants evolution [Citation29]. Several chalcones extracted from the aforementioned plants have been examined for their activity as anti HCV medications [Citation29]. A well discovered target is the mTOR pathway through the inhibition of ribosomal protein 6 (rps6) phosphorylation and its kinase [Citation43]. Licochalcone A and isoliquiritigenin are natural products targeting HCV genotype 2a (J6/JFH1P47) with an IC50 of 2.5 mg/mL and 3.7 mg/mL, respectively [Citation29]. Their activity is witnessed through the inhibition of HCV virus subgenomic RNA replication and protein synthesis [Citation29]. Xanthohumol (XN) is another well-known chalcone for its biological activities in protecting the liver by antiviral mechanisms [Citation40]. XN activity was monitored in HCV infected cells, and was shown to significantly decrease aminotransferases, transforming growth factor β1 expression and hepatic steatosis score in cells exposed to XN in comparison to their control [Citation40]. XN was also shown to significantly decrease the activity of superoxide dismutase, glutathione peroxidase and other associated enzymes [Citation40]. Therefore, XN is capable of affectively decreasing hepatic damage through the inhibition of oxidative reaction and manipulating the apoptosis pathways [Citation40]. Upon further investigations, it was shown to decrease liver fibrosis through its inhibitory activity on stellate cell function [Citation40]. Studies on medicinal plants are promising and can potentially yield more effective agents against HCV infective strains including resistant ones.
Chalcone derivatives tested on Rift Valley fever
Rift Valley fever virus (RVFV) is an arbovirus that belongs to the Phenuiviridae family and is transmitted mainly through mosquitoes [Citation112]. RVFV is accountable for a zoonosis disease which affects mainly domesticated animals in sub-Saharan Africa including cattle, sheep, camels and goats alongside animals in other countries [Citation113]. Humans can also get infected with RVFV through bites from infected mosquitoes or contact with infected animals’ blood or body fluids [Citation113]. However, transmission of RVFV through human-to-human contact was not documented to date [Citation113]. Similar to other arboviral infections such as dengue, zika and chikungunya, RVF infection by RVFV is emerging globally [Citation114]. According to the CDC, there is no available approved antiviral medications against RVF and the disease is usually self-limiting [Citation115]. However, severe cases can only be managed through supportive care [Citation115]. Therefore, identifying therapeutic options that are specific to RVFV is urgently needed. Some synthetic chalcone analogues were explored for their anti-RVFV activities [Citation30,Citation94]. Interestingly, a cyclopropylquinoline analogue was found to be 28 times more potent (EC50: 0.39 μg/mL) as compared to the conventional antiviral drug, ribavirin (EC50: 11 μg/mL) in terms of inhibiting RVFV viral replication [Citation30]. However, more investigations are needed to identify specific RVFV molecular targets of chalcone derivatives. Particularly, host-cell related pathways (e.g. transcriptional and mitochondrial processes) utilised by RVFV for its replication, are promising therapeutic targets for anti-RVFV drug development [Citation116].
Chalcone derivatives tested on Venezuelan equine encephalitis virus
Venezuelan equine encephalitis virus belongs to the Togaviridae family and affects both equines and humans [Citation117]. VEE outbreaks are known throughout Latin America and examples of affected countries include Venezuela, Peru, Colombia, Costa Rica, Ecuador, Mexico, El Salvador, Panama, as well as USA [Citation117]. Around 14 subtypes of VEEV were identified with subtype I varieties A, B and C being the most associated with epidemics in humans and equids [Citation117]. VEE is mostly misdiagnosed as dengue infection which makes the estimation of its specific burden on health and economy more difficult [Citation117]. VEEV may cause encephalitis in humans and common signs and symptoms of VEE involve headache, fever, tremors, nausea and vomiting [Citation118]. The main management strategy to control VEE is through vaccination against VEEV [Citation117]. However, there is no FDA-approved drug available for targeting this virus. Patil et al. have investigated the role of a group of chalcone derivatives on VEEV [Citation30]. The most potent in inhibiting VEEV viral replication was a cyclopropylquinoline analogue (EC50 >2.8 μg/mL) [Citation30]. Unfortunately, its cytotoxic concentration has a similar potency (CC50 2.8 μg/mL), making it unselective towards the virus as compared to the host cell (SI50 0 μg/mL) [Citation30]. Other investigated chalcone derivatives have all shown the same unselectively (SI50 0 μg/mL). Therefore, more attempts to improve the structure of these derivatives are needed.
Conclusions
Viral infections are one of the most common human diseases and are considered the leading cause of a variety of illnesses with high morbidity and mortality. A great number of antivirals were developed and are widely used around the world. Despite their broad range of coverage, the rapidly evolving antiviral resistance limits their efficacy to combat viral infections. In addition to high rate of drug resistance development and emergence of new viral strains, the need for alternative antivirals has increased over the years necessitating the extraction and synthesis of novel antiviral agents, of which chalcones became one of the main candidates. Chalcones are widely biosynthesised in plants and play a significant role in defending the body against pathogens and insects. Numerous chalcone derivatives were investigated for their antiviral activities and found to possess interesting and remarkable effects on modulating several anti-infective molecular targets which makes chalcone derivatives a potentially effective and safe broad spectrum antiviral agents. However, additional investigations should be conducted in order to study chalcones specific mechanisms and targets in vitro and in vivo as most reports thus far were conducted in vitro, without further determining their specific molecular targets. In addition, there are very limited clinical studies on chalcones as antiviral therapies. Therefore, in order to establish the viability of chalcones as potential antiviral agents in human diseases more in vivo investigations are needed.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Funding
References
- Ali I, Suhail M, Naqshbandi M, et al. Role of unani medicines in cancer control and management. Curr Drug Ther. 2019;14(2):92–113.
- Nabavi SM, Marchese A, Izadi M, et al. Plants belonging to the genus Thymus as antibacterial agents: from farm to pharmacy. Food Chem. 2015;173:339–347.
- Ahmad MF, Ahmad FA, Ashraf SA, et al. An updated knowledge of Black seed (Nigella sativa Linn): review of phytochemical constituents and pharmacological properties. J Herb Med. 2021;25:100404.
- Bukhari SN, Jasamai M, Jantan I. Synthesis and biological evaluation of chalcone derivatives (mini review). Mini Rev Med Chem. 2012;12(13):1394–1403.
- Rani A, Anand A, Kumar K, et al. Recent developments in biological aspects of chalcones: the Odyssey continues. Expert Opin Drug Discov. 2019;14(3):249–288.
- Batovska DI, Todorova IT. Trends in utilization of the pharmacological potential of chalcones. Curr Clin Pharmacol. 2010;5(1):1–29.
- Zhou B, Xing C. Diverse molecular targets for chalcones with varied bioactivities. Med Chem (Los Angeles). 2015;5(8):388–404.
- Kar Mahapatra D, Asati V, Bharti SK. An updated patent review of therapeutic applications of chalcone derivatives (2014–present). Expert Opin Ther Pat. 2019;29(5):385–406.
- Otvos SB, Hsieh CT, Wu YC, et al. Continuous-flow synthesis of deuterium-labeled antidiabetic chalcones: studies towards the selective deuteration of the alkynone core. Molecules. 2016;21(3):318.
- Mahapatra DK, Bharti SK, Asati V. Chalcone scaffolds as anti-infective agents: structural and molecular target perspectives. Eur J Med Chem. 2015;101:496–524.
- Irwin KK, Renzette N, Kowalik TF, et al. Antiviral drug resistance as an adaptive process. Virus Evol. 2016;2(1):vew014.
- WHO. Ten threats to global health in 2019; 2019. Available from: https://www.who.int/news-room/feature-stories/ten-threats-to-global-health-in-2019
- Pal M, Berhanu G, Desalegn C, et al. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an update. Cureus. 2020;12(3):e7423.
- Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance – United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(24):759–765.
- Baron S, Fons M, Albrecht T. Viral pathogenesis. In: Baron S, editor. Medical microbiology. 4th ed. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 45. PMID: 21413306.
- De Clercq E, Li G. Approved antiviral drugs over the past 50 years. Clin Microbiol Rev. 2016;29(3):695–747.
- Ravanfar P, Satyaprakash A, Creed R, et al. Existing antiviral vaccines. Dermatol Ther. 2009;22(2):110–128.
- De Clercq E. Strategies in the design of antiviral drugs. Nat Rev Drug Discov. 2002;1(1):13–25.
- Lou Z, Sun Y, Rao Z. Current progress in antiviral strategies. Trends Pharmacol Sci. 2014;35(2):86–102.
- Jo S, Kim H, Kim S, et al. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem Biol Drug Des. 2019;94(6):2023–2030.
- Lin CW, Tsai FJ, Tsai CH, et al. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antiviral Res. 2005;68(1):36–42.
- Monserrat JP, Al-Safi RI, Tiwari KN, et al. Ferrocenyl chalcone difluoridoborates inhibit HIV-1 integrase and display low activity towards cancer and endothelial cells. Bioorg Med Chem Lett. 2011;21(20):6195–6197.
- Dao TT, Nguyen PH, Lee HS, et al. Chalcones as novel influenza A (H1N1) neuraminidase inhibitors from Glycyrrhiza inflata. Bioorg Med Chem Lett. 2011;21(1):294–298.
- Al-Nakib W, Higgins PG, Barrow I, et al. Intranasal chalcone, Ro 09-0410, as prophylaxis against rhinovirus infection in human volunteers. J Antimicrob Chemother. 1987;20(6):887–892.
- Ali MA, Shaharyar M, De Clercq E. Synthesis of 5-(4-hydroxy-3-methylphenyl)-5-(substituted phenyl)-4, 5-dihydro-1H-1-pyrazolyl-4-pyridylmethanone derivatives with anti-viral activity. J Enzyme Inhib Med Chem. 2007;22(6):702–708.
- Kiat TS, Pippen R, Yusof R, et al. Inhibitory activity of cyclohexenyl chalcone derivatives and flavonoids of fingerroot, Boesenbergia rotunda (L.), towards dengue-2 virus NS3 protease. Bioorg Med Chem Lett. 2006;16(12):3337–3340.
- Kralj A, Nguyen MT, Tschammer N, et al. Development of flavonoid-based inverse agonists of the key signaling receptor US28 of human cytomegalovirus. J Med Chem. 2013;56(12):5019–5032.
- Mathayan M, Jayaraman S, Kulanthaivel L, et al. Inhibition studies of HBV DNA polymerase using seed extracts of Pongamia pinnata. Bioinformation. 2019;15(7):506–512.
- Adianti M, Aoki C, Komoto M, et al. Anti-hepatitis C virus compounds obtained from Glycyrrhiza uralensis and other Glycyrrhiza species. Microbiol Immunol. 2014;58(3):180–187.
- Patil V, Patil SA, Patil R, et al. Exploration of (hetero)aryl derived thienylchalcones for antiviral and anticancer activities. Med Chem. 2019;15(2):150–161.
- Hameed A, Abdullah MI, Ahmed E, et al. Anti-HIV cytotoxicity enzyme inhibition and molecular docking studies of quinoline based chalcones as potential non-nucleoside reverse transcriptase inhibitors (NNRT). Bioorg Chem. 2016;65:175–182.
- Mathaiyan M, Suresh A, Balamurugan R. Binding property of HIV p24 and Reverse transcriptase by chalcones from Pongamia pinnata seeds. Bioinformation. 2018;14(6):279–284.
- Sharma H, Patil S, Sanchez TW, et al. Synthesis, biological evaluation and 3D-QSAR studies of 3-keto salicylic acid chalcones and related amides as novel HIV-1 integrase inhibitors. Bioorg Med Chem. 2011;19(6):2030–2045.
- Deng J, Sanchez T, Al-Mawsawi LQ, et al. Discovery of structurally diverse HIV-1 integrase inhibitors based on a chalcone pharmacophore. Bioorg Med Chem. 2007;15(14):4985–5002.
- Deng J, Kelley JA, Barchi JJ, et al. Mining the NCI antiviral compounds for HIV-1 integrase inhibitors. Bioorg Med Chem. 2006;14(11):3785–3792.
- Turkovic N, Ivkovic B, Kotur-Stevuljevic J, et al. Molecular docking, synthesis and anti-HIV-1 protease activity of novel chalcones. Curr Pharm Des. 2020;26(8):802–814.
- Cheenpracha S, Karalai C, Ponglimanont C, et al. Anti-HIV-1 protease activity of compounds from Boesenbergia pandurata. Bioorg Med Chem. 2006;14(6):1710–1714.
- Xu HX, Wan M, Dong H, et al. Inhibitory activity of flavonoids and tannins against HIV-1 protease. Biol Pharm Bull. 2000;23(9):1072–1076.
- Malbari K, Gonsalves H, Chintakrindi A, et al. In search of effective H1N1 neuraminidase inhibitor by molecular docking, antiviral evaluation and membrane interaction studies using NMR. Acta Virol. 2018;62(2):179–190.
- Yang M, Li N, Li F, et al. Xanthohumol, a main prenylated chalcone from hops, reduces liver damage and modulates oxidative reaction and apoptosis in hepatitis C virus infected Tupaia belangeri. Int Immunopharmacol. 2013;16(4):466–474.
- Buckwold VE, Wilson RJ, Nalca A, et al. Antiviral activity of hop constituents against a series of DNA and RNA viruses. Antiviral Res. 2004;61(1):57–62.
- Ninomiya Y, Shimma N, Ishitsuka H. Comparative studies on the antirhinovirus activity and the mode of action of the rhinovirus capsid binding agents, chalcone amides. Antiviral Res. 1990;13(2):61–74.
- Mateeva N, Eyunni SVK, Redda KK, et al. Functional evaluation of synthetic flavonoids and chalcones for potential antiviral and anticancer properties. Bioorg Med Chem Lett. 2017;27(11):2350–2356.
- Wang Y, Liu TX, Wang TY, et al. Isobavachalcone inhibits pseudorabies virus by impairing virus-induced cell-to-cell fusion. Virol J. 2020;17(1):39.
- Cole AL, Hossain S, Cole AM, et al. Synthesis and bioevaluation of substituted chalcones, coumaranones and other flavonoids as anti-HIV agents. Bioorg Med Chem. 2016;24(12):2768–2776.
- Sahu NK, Balbhadra SS, Choudhary J, et al. Exploring pharmacological significance of chalcone scaffold: a review. Curr Med Chem. 2012;19(2):209–225.
- Park JY, Ko JA, Kim DW, et al. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J Enzyme Inhib Med Chem. 2016;31(1):23–30.
- Wu J, Ao MT, Shao R, et al. A chalcone derivative reactivates latent HIV-1 transcription through activating P-TEFb and promoting Tat-SEC interaction on viral promoter. Sci Rep. 2017;7(1):10657.
- Wu JH, Wang XH, Yi YH, et al. Anti-AIDS agents 54. A potent anti-HIV chalcone and flavonoids from genus Desmos. Bioorg Med Chem Lett. 2003;13(10):1813–1815.
- Casano G, Dumetre A, Pannecouque C, et al. Anti-HIV and antiplasmodial activity of original flavonoid derivatives. Bioorg Med Chem. 2010;18(16):6012–6023.
- Pan W, Liu K, Guan Y, et al. Bioactive compounds from Vitex leptobotrys. J Nat Prod. 2014;77(3):663–667.
- Uchiumi F, Hatano T, Ito H, et al. Transcriptional suppression of the HIV promoter by natural compounds. Antiviral Res. 2003;58(1):89–98.
- El-Subbagh HI, Abu-Zaid SM, Mahran MA, et al. Synthesis and biological evaluation of certain alpha, beta-unsaturated ketones and their corresponding fused pyridines as antiviral and cytotoxic agents. J Med Chem. 2000;43(15):2915–2921.
- Seo WD, Kim JH, Kang JE, et al. Sulfonamide chalcone as a new class of alpha-glucosidase inhibitors. Bioorg Med Chem Lett. 2005;15(24):5514–5516.
- Ali MA, Yar MS, Siddiqui AA, et al. Synthesis and anti-HIV activity of N′-nicotinoyl-3-(4′-hydroxy-3′-methylphenyl)-5-[substituted phenyl]-2-pyrazolines. Acta Pol Pharm. 2007;64(5):423–428.
- Chintakrindi AS, Martis EA, Gohil DJ, et al. A computational model for docking of noncompetitive neuraminidase inhibitors and probing their binding interactions with neuraminidase of influenza virus H5N1. Curr Comput Aided Drug Des. 2016;12(4):272–281.
- Nguyen PH, Na M, Dao TT, et al. New stilbenoid with inhibitory activity on viral neuraminidases from Erythrina addisoniae. Bioorg Med Chem Lett. 2010;20(22):6430–6434.
- Dao TT, Tung BT, Nguyen PH, et al. C-Methylated flavonoids from Cleistocalyx operculatus and their inhibitory effects on novel influenza A (H1N1) neuraminidase. J Nat Prod. 2010;73(10):1636–1642.
- Park JY, Jeong HJ, Kim YM, et al. Characteristic of alkylated chalcones from Angelica keiskei on influenza virus neuraminidase inhibition. Bioorg Med Chem Lett. 2011;21(18):5602–5604.
- Ishitsuka H, Ninomiya YT, Ohsawa C, et al. Direct and specific inactivation of rhinovirus by chalcone Ro 09-0410. Antimicrob Agents Chemother. 1982;22(4):617–621.
- Ahmad ALM, Dowsett AB, Tyrrell DAJ. Studies of rhinovirus resistant to an antiviral chalcone. Antiviral Res. 1987;8(1):27–39.
- Ishitsuka H, Ninomiya Y, Suhara Y. Molecular basis of drug resistance to new antirhinovirus agents. J Antimicrob Chemother. 1986;18(Suppl. B):11–18.
- Phrutivorapongkul A, Lipipun V, Ruangrungsi N, et al. Studies on the chemical constituents of stem bark of Millettia leucantha: isolation of new chalcones with cytotoxic, anti-herpes simplex virus and anti-inflammatory activities. Chem Pharm Bull (Tokyo). 2003;51(2):187–190.
- Brandao GC, Kroon EG, Duarte MG, et al. Antimicrobial, antiviral and cytotoxic activity of extracts and constituents from Polygonum spectabile Mart. Phytomedicine. 2010;17(12):926–929.
- Heh CH, Othman R, Buckle MJ, et al. Rational discovery of dengue type 2 non-competitive inhibitors. Chem Biol Drug Des. 2013;82(1):1–11.
- Chen Z, Knutson E, Wang S, et al. Stabilization of p53 in human cytomegalovirus-initiated cells is associated with sequestration of HDM2 and decreased p53 ubiquitination. J Biol Chem. 2007;282(40):29284–29295.
- Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23.
- Rabaan AA, Al-Ahmed SH, Haque S, et al. SARS-CoV-2, SARS-CoV, and MERS-COV: a comparative overview. Infez Med. 2020;28(2):174–184.
- Cheng VC, Lau SK, Woo PC, et al. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20(4):660–694.
- CDC. Severe acute respiratory syndrome (SARS) basics fact sheet; 2020. Available from: https://www.cdc.gov/sars/about/fs-sars.html
- Chafekar A, Fielding BC. MERS-CoV: understanding the latest human coronavirus threat. Viruses. 2018;10(2):93.
- WHO. WHO coronavirus disease (COVID-19) dashboard; 2020. Available from: https://covid19.who.int/
- Ghosh AK, Xi K, Johnson ME, et al. Progress in anti-SARS coronavirus chemistry, biology and chemotherapy. Annu Rep Med Chem. 2007;41:183–196.
- Arien KK, Abraha A, Quinones-Mateu ME, et al. The replicative fitness of primary human immunodeficiency virus type 1 (HIV-1) group M, HIV-1 group O, and HIV-2 isolates. J Virol. 2005;79(14):8979–8990.
- Gottlieb GS, Raugi DN, Smith RA. 90-90-90 for HIV-2? Ending the HIV-2 epidemic by enhancing care and clinical management of patients infected with HIV-2. Lancet HIV. 2018;5(7):e390–e399.
- WHO. HIV/AIDS; 2019. Available from: https://www.who.int/health-topics/hiv-aids/#tab=tab_1
- UNAIDS. 90-90-90: treatment for all; 2018. Available from: https://www.unaids.org/en/resources/909090
- Sierra-Aragon S, Walter H. Targets for inhibition of HIV replication: entry, enzyme action, release and maturation. Intervirology. 2012;55(2):84–97.
- Robina I, Moreno-Vargas AJ, Carmona AT, et al. Glycosidase inhibitors as potential HIV entry inhibitors? Curr Drug Metab. 2004;5(4):329–361.
- Lofgren E, Fefferman NH, Naumov YN, et al. Influenza seasonality: underlying causes and modeling theories. J Virol. 2007;81(11):5429–5436.
- Min JY, Subbarao K. Cellular targets for influenza drugs. Nat Biotechnol. 2010;28(3):239–240.
- McAuley JL, Gilbertson BP, Trifkovic S, et al. Influenza virus neuraminidase structure and functions. Front Microbiol. 2019;10:39.
- McKimm-Breschkin JL. Influenza neuraminidase inhibitors: antiviral action and mechanisms of resistance. Influenza Other Respir Viruses. 2013;7(Suppl. 1):25–36.
- Greenberg SB. Respiratory consequences of rhinovirus infection. Arch Intern Med. 2003;163(3):278–284.
- Foxman EF, Storer JA, Fitzgerald ME, et al. Temperature-dependent innate defense against the common cold virus limits viral replication at warm temperature in mouse airway cells. Proc Natl Acad Sci U S A. 2015;112(3):827–832.
- Bochkov YA, Gern JE. Rhinoviruses and their receptors: implications for allergic disease. Curr Allergy Asthma Rep. 2016;16(4):30.
- Ahmad ALM, Tyrrell DAJ. Synergism between anti-rhinovirus antivirals: various human interferons and a number of synthetic compounds. Antiviral Res. 1986;6(4):241–252.
- Domingo E, Sheldon J, Perales C. Viral quasispecies evolution. Microbiol Mol Biol Rev. 2012;76(2):159–216.
- Yasin SR, Al-Nakib W, Tyrrell DA. Pathogenicity for humans of human rhinovirus type 2 mutants resistant to or dependent on chalcone Ro 09-0410. Antimicrob Agents Chemother. 1990;34(6):963–966.
- Kukhanova MK, Korovina AN, Kochetkov SN. Human herpes simplex virus: life cycle and development of inhibitors. Biochemistry (Mosc). 2014;79(13):1635–1652.
- James C, Harfouche M, Welton NJ, et al. Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bull World Health Organ. 2020;98(5):315–329.
- Whitley R. Herpes simplex virus. In: Booss ACTaJ, editor. Handbook of clinical neurology. Vol. 123. Netherlands: Elsevier; 2014. p. 252–263.
- Coen DM, Schaffer PA. Antiherpesvirus drugs: a promising spectrum of new drugs and drug targets. Nat Rev Drug Discov. 2003;2(4):278–288.
- Patil SA, Patil V, Patil R, et al. Identification of novel 5,6-dimethoxyindan-1-one derivatives as antiviral agents. Med Chem. 2017;13(8):787–795.
- Mairuhu AT, Wagenaar J, Brandjes DP, et al. Dengue: an arthropod-borne disease of global importance. Eur J Clin Microbiol Infect Dis. 2004;23(6):425–433.
- Martina BEE, Koraka P, Osterhaus ADME. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 2009;22(4):564–581.
- WHO. Dengue and severe dengue; 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue
- Silva EM, Conde JN, Allonso D, et al. Dengue virus nonstructural 3 protein interacts directly with human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and reduces its glycolytic activity. Sci Rep. 2019;9(1):2651.
- Niyomrattanakit P, Winoyanuwattikun P, Chanprapaph S, et al. Identification of residues in the dengue virus type 2 NS2B cofactor that are critical for NS3 protease activation. J Virol. 2004;78(24):13708–13716.
- Guirakhoo F, Pugachev K, Zhang Z, et al. Safety and efficacy of chimeric yellow Fever-dengue virus tetravalent vaccine formulations in nonhuman primates. J Virol. 2004;78(9):4761–4775.
- Powers CN, Setzer WN. An in-silico investigation of phytochemicals as antiviral agents against dengue fever. Comb Chem High Throughput Screen. 2016;19(7):516–536.
- Mukhametov A, Newhouse EI, Aziz NA, et al. Allosteric pocket of the dengue virus (serotype 2) NS2B/NS3 protease: in silico ligand screening and molecular dynamics studies of inhibition. J Mol Graph Model. 2014;52:103–113.
- Congenital cytomegalovirus infection: update on treatment: scientific impact paper no. 56. BJOG. 2018;125(1):e1–e11.
- Lee S, Chung YH, Lee C. US28, a virally-encoded GPCR as an antiviral target for human cytomegalovirus infection. Biomol Ther (Seoul). 2017;25(1):69–79.
- Evers DL, Chao C-F, Wang X, et al. Human cytomegalovirus-inhibitory flavonoids: studies on antiviral activity and mechanism of action. Antiviral Res. 2005;68(3):124–134.
- Elkhalifa D, Siddique AB, Qusa M, et al. Design, synthesis, and validation of novel nitrogen-based chalcone analogs against triple negative breast cancer. Eur J Med Chem. 2020;187:111954.
- Beltramino R, Penenory A, Buceta AM. An open-label, randomized multicenter study comparing the efficacy and safety of Cyclo 3 Fort versus hydroxyethyl rutoside in chronic venous lymphatic insufficiency. Angiology. 2000;51(7):535–544.
- Liang TJ. Hepatitis B: the virus and disease. Hepatology. 2009;49(5 Suppl.):S13–S21.
- WHO. Hepatitis B key facts 2020; 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
- Liu SH, Seto WK, Lai CL, et al. Hepatitis B: treatment choice and monitoring for response and resistance. Expert Rev Gastroenterol Hepatol. 2016;10(6):697–707.
- Echeverría N, Moratorio G, Cristina J, et al. Hepatitis C virus genetic variability and evolution. World J Hepatol. 2015;7(6):831–845.
- Wright D, Kortekaas J, Bowden TA, et al. Rift Valley fever: biology and epidemiology. J Gen Virol. 2019;100(8):1187–1199.
- WHO. Rift Valley fever 2018; 2018. Available from: https://www.who.int/news-room/fact-sheets/detail/rift-valley-fever
- Weaver SC, Charlier C, Vasilakis N, et al. Zika, chikungunya, and other emerging vector-borne viral diseases. Annu Rev Med. 2018;69:395–408.
- CDC. Rift Valley fever (RVF) treatment 2020; 2020. Available from: https://www.cdc.gov/vhf/rvf/treatment/index.html
- Pinkham C, Ahmed A, Bracci N, et al. Host-based processes as therapeutic targets for Rift Valley fever virus. Antiviral Res. 2018;160:64–78.
- Aguilar PV, Estrada-Franco JG, Navarro-Lopez R, et al. Endemic Venezuelan equine encephalitis in the Americas: hidden under the dengue umbrella. Future Virol. 2011;6(6):721–740.
- Gardner CL, Burke CW, Tesfay MZ, et al. Eastern and Venezuelan equine encephalitis viruses differ in their ability to infect dendritic cells and macrophages: impact of altered cell tropism on pathogenesis. J Virol. 2008;82(21):10634–10646.
