ABSTRACT
Background
Polycystic ovary syndrome (PCOS) is a metabolic and reproductive disorder. Current research findings present conflicting views on the effects of different PCOS phenotypes on outcomes in pregnancy and for newborns.
Methods
This research study followed the guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA). A thorough search of literature was carried out using the Cochrane Menstrual Disorders and Subfertility Group trials register, Web of Science, and EMBASE databases from their start to December 2023. The search focused on studies examining the links between hyperandrogenic and non-hyperandrogenic PCOS phenotypes and risks in pregnancy and neonatology. Odds ratios (ORs) and 95% confidence intervals (CIs) were computed using either a fixed-effects or random-effects model.
Results
Our analysis incorporated 10 research studies. Expectant mothers with a hyperandrogenic PCOS subtype had increased ORs for gestational diabetes mellitus (GDM) and preeclampsia (PE) compared to those with a non-hyperandrogenic PCOS subtype, with respective values of 2.14 (95% CI, 1.18–3.88, I2 = 0%) and 2.04 (95% CI, 1.02–4.08, I2 = 53%). Nevertheless, no notable differences were detected in ORs for outcomes like preterm birth, live birth, miscarriage, cesarean delivery, pregnancy-induced hypertension, small for gestational age babies, large for gestational age newborns, and neonatal intensive care unit admissions between pregnant women with hyperandrogenic PCOS phenotype and those without.
Conclusions
This meta-analysis highlights that the presence of hyperandrogenism heightens the risks of GDM and PE within the PCOS population. Healthcare providers ought to be aware of this connection for improved patient management.
Introduction
Polycystic ovary syndrome (PCOS) is a metabolic and reproductive disorder linked with a range of clinical characteristics (Citation1). Its prevalence is estimated to range from 6% to 17%, depending on the diagnostic criteria and population under scrutiny (Citation2–7). The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group suggests classifying women with PCOS for research purposes based on their phenotype, as follows: androgen excess + ovulatory dysfunction + polycystic ovarian morphology (Phenotype A); androgen excess + ovulatory dysfunction (Phenotype B); androgen excess + polycystic ovarian morphology (Phenotype C); and ovulatory dysfunction + polycystic ovarian morphology (Phenotype D) (Citation8).
Polycystic Ovary Syndrome (PCOS) is a metabolic disorder that affects fertility due to dysfunctional ovulation (Citation9). In some cases, medications are required to induce ovulation in the presence of this metabolic condition (Citation10,Citation11). This disorder is known to increase the risks associated with pregnancy for women, as well as for their babies (Citation12).
Various studies consistently demonstrate an elevated risk of pregnancy complications for women with PCOS compared to those without the condition. Yu et al. (Citation13) conducted a comprehensive review of multiple observational studies focusing on the obstetric risks associated with PCOS. Their findings revealed a nearly threefold increase in the risk of gestational diabetes and preeclampsia (PE), as well as a 50% higher likelihood of preterm delivery among women with PCOS. Additionally, based on the Barker hypothesis, which suggests that early life adversities can contribute to later health issues (Citation14). The meta-analysis conducted by Yu et al. highlighted a negative impact of a mother’s PCOS diagnosis on her child’s health based on six included studies (Citation13). However, no significant association was found between PCOS diagnosis and abnormal fetal growth.
It is essential to define specific diagnostic characteristics of PCOS when studying pregnancy complications in women with this condition. The diverse range of hormonal and metabolic abnormalities across various PCOS phenotypes can have a significant impact on both obstetric and neonatal outcomes (Citation15,Citation16). Nonetheless, the impact of different PCOS phenotypes on these outcomes remains a topic of debate in current research. A recent study conducted by Christ et al. found that women with hyperandrogenism before pregnancy had a higher risk of developing preeclampsia and delivering prematurely. Elevated levels of insulin and testosterone in the bloodstream were linked to adverse pregnancy outcomes (Citation17). Another study by the same research group, which focused on women undergoing PCOS phenotypic evaluation, showed a connection between the severity of hyperandrogenism and the development of gestational diabetes (Citation18). Conversely, a study in Denmark found no discernible differences in obstetric outcomes when examining the potential effects of hyperandrogenism (Citation19).
The objective of this meta-analysis was to synthesize all available evidence and investigate possible relationships between hyperandrogenic PCOS phenotypes (Phenotype A+B+C) and non-hyperandrogenic PCOS phenotype (Phenotype D) in terms of obstetric and neonatal outcomes.
Materials and methods
Inclusion and exclusion criteria
Inclusion criteria
The meta-analysis research criteria were as follows: (1) Study Design: Case-control, cross-sectional, or cohort studies. (2) Study Focus: All original research evaluating the links between negative obstetric outcomes or complications and PCOS, with or without excessive levels of male hormones. PCOS diagnosis adhered to the Rotterdam criteria established by the European Society for Human Reproduction and Embryology and the American Society for Reproductive Medicine. (3) Outcome Measures: Odds ratios (ORs), relative risks, hazard ratios, or standardized incidence ratios along with their respective 95% confidence intervals (CIs).
Exclusion criteria
The exclusion criteria for studies in the meta-analysis were the following: (1) Unavailability of full-text papers. (2) Poor content quality including lack of significant information or inadequate data integrity.
Information retrieval
This systematic review and meta-analysis followed the guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) protocol. A thorough literature search was conducted using the Web of Science, Cochrane Menstrual Disorders and Subfertility Group trials register, and EMBASE databases. The search covered the period from the inception of these databases up to December 2023. Additionally, reference lists of relevant primary and review articles were checked to identify any articles not found in electronic searches. The searches were not limited by language or publication type.
The search employed the following keywords: “hyperandrogen” OR “hyperandrogenism” OR “hyperandrogenic” OR “hyperandrogenisation” AND “polycystic ovary” OR “polycystic ovary syndrome” OR “PCOS” OR “Stein-Leventhal syndrome” AND “pregnancy outcome” OR “obstetric outcome” OR obstetric complications.’
Study selection
The abstract and title screening of the electronic searches were conducted independently by two reviewers (X.G. and Y.Y.). Full manuscripts for all citations believed to meet the predefined criteria were obtained. Final decisions on inclusion or exclusion were based on a thorough review of the full manuscripts. If duplicates were found, the most recent and comprehensive versions were chosen. Any discrepancies in inclusion were resolved through discussion or arbitration by a third reviewer (R.J.).
Evaluation of document quality and data extraction
The quality of the literature was assessed using the Cochrane risk of bias tool. Two researchers (X.G. and Y.Y.) independently reviewed, extracted data, and evaluated the quality of the literature based on the predefined criteria for inclusion and exclusion. Data extraction forms included basic information, patient characteristics, and sample sizes. Any disagreements between reviewers were resolved through discussion or arbitration by a third reviewer (R.J.).
Analysis of statistics
The meta-analysis was performed using Review Manager 5.3 software from the Cochrane Library. A fixed- or random-effects model was chosen based on study characteristics. Statistical heterogeneity was assessed using the chi-square test and represented with the I2 index. If the heterogeneity test result was I2 ≤50%, indicating no significant heterogeneity, the fixed-effect model was used. If heterogeneity was present, a thorough investigation was conducted. If a valid reason was found, a subgroup analysis was carried out; otherwise, a random-effects model was applied. Odds ratios (ORs) were used to represent variables, with 95% confidence intervals (CIs) for interval estimates. Statistical significance was determined at p < 0.05.
Results
Characteristics of the primary research
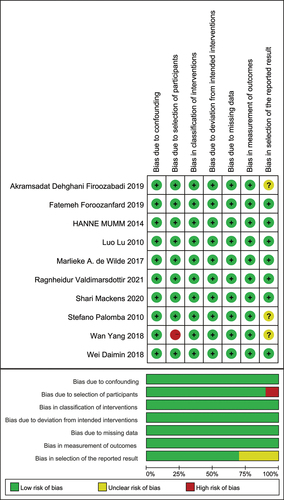
At the start of the investigation, a total of 934 relevant articles were identified. After a thorough review and screening process, 135 articles were chosen for retrieval (see ). The study followed the PRISMA guidelines, and the diagram illustrating the literature identification and selection process is shown in . All 10 studies were ultimately included in the final meta-analysis (Citation18–27). An overview of the trial specifics for the included studies can be found in . The results of the assessment of literature quality are depicted in . Together, these 10 studies involved 2485 cases.
Figure 1. Preferred reporting items for systematic reviews and meta-analyses flow diagram of study selection.
Table 1. Basic characteristics of the included studies.
Measurement of bias risk
The bias risk was evaluated for each of the 10 studies included. Of these, five studies (Citation19,Citation21,Citation22,Citation26,Citation27) were prospective, and the remaining five studies (Citation18,Citation20,Citation23–25) were retrospectively planned. Certain biases were noted in terms of selection (participants and reported outcomes). A summary of the bias risk evaluation is available in .
Systematic review results
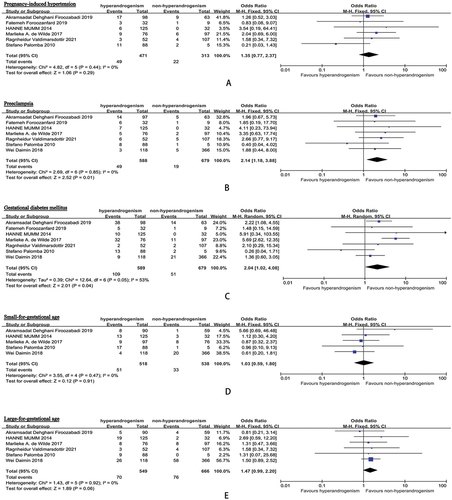
The meta-analysis results can be seen in . The incidence of PE in the hyperandrogenism group was 8.3%, compared to 2.8% in the non-hyperandrogenism group. The odds ratio (OR) for PE in pregnant individuals with hyperandrogenic PCOS phenotype versus those with non-hyperandrogenic PCOS phenotype was 2.14 (95% CI, 1.18–3.88, I2 = 0%). Moreover, the prevalence of pregnancy-induced hypertension (PIH) in the hyperandrogenism cohorts was 10.4%, while it was 7.0% in the non-hyperandrogenism cohorts. The OR for PIH in pregnant individuals with hyperandrogenic PCOS phenotype versus those with non-hyperandrogenic PCOS phenotype was 1.35 (95% CI, 0.77–2.37, I2 = 0%). Additionally, the rate of gestational diabetes mellitus (GDM) in the hyperandrogenism groups was 18.5%, in contrast to 7.5% in the non-hyperandrogenism groups. The OR for GDM in pregnant individuals with hyperandrogenic PCOS phenotype versus those with non-hyperandrogenic PCOS phenotype was 2.04 (95% CI, 1.02–4.08, I2 = 53%).
Figure 3. Pregnancy outcomes comparison: hyperandrogenic PCOS phenotype (polycystic ovary syndrome) versus non-hyperandrogenic PCOS phenotype (A: miscarriage; B: live-birth; C: preterm; D: caesarean section; E: admission to the neonatal intensive care unit).
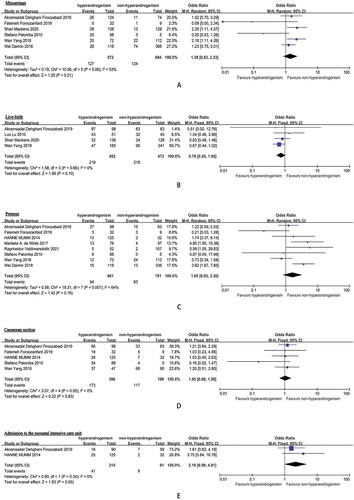
Figure 4. Pregnancy complications comparison: hyperandrogenic PCOS phenotype (polycystic ovary syndrome) versus non-hyperandrogenic PCOS phenotype (A: pregnancy-induced hypertension; B: preeclampsia; C: gestational diabetes mellitus; D: small-for-gestational age; E: large-for-gestational age).
In the cohorts with hyperandrogenism, the miscarriage rate was 22.2%, while it stood at 17.8% in the non-hyperandrogenism cohorts. The OR for miscarriage in pregnant individuals with the hyperandrogenic PCOS phenotype compared to those with the non-hyperandrogenic PCOS phenotype was 1.39 (95% CI, 0.83–2.33, I2 = 53%). Furthermore, the total live birth rate in the hyperandrogenism cohorts was 48.5%, while it was 46.4% in the non-hyperandrogenism group. The OR for live birth in pregnant individuals with hyperandrogenic PCOS phenotype compared to those with non-hyperandrogenic PCOS phenotype was 0.76 (95% CI, 0.55–1.05, I2 = 0%).
In terms of preterm birth, the overall rate in the hyperandrogenism groups was 14.2%, whereas it was 8.3% in the groups without hyperandrogenism. The OR for preterm birth in pregnant women with hyperandrogenic PCOS phenotype versus those with non-hyperandrogenic PCOS phenotype was 1.65, with a 95% CI of 0.83–3.30 and an I2 statistic of 64%. Additionally, the total rate of cesarean section (CS) in the hyperandrogenism groups was 44.4%, contrasting with 61.9% in the non-hyperandrogenism groups. The odds ratio for CS in pregnant women with hyperandrogenic PCOS phenotype compared to those with non-hyperandrogenic PCOS phenotype was 1.05, with a 95% CI of 0.69–1.58 and an I2 of 0%.
In the hyperandrogenism groups, the overall rate for a small for gestational age (SGA: birth weight percentile <10%) diagnosis was 9.8%, while it was 6.1% in the non-hyperandrogenism groups. The OR for SGA in pregnant women with hyperandrogenic PCOS phenotype compared to those with non-hyperandrogenic PCOS phenotype was 1.03, with a 95% CI of 0.59–1.80 and an I2 of 0%. Additionally, the total rate for a large for gestational age (LGA: birth weight percentile >10%) diagnosis in the hyperandrogenism groups was 12.8%, in comparison to 11.4% in the non-hyperandrogenism group. The OR for LGA in pregnant women with hyperandrogenic PCOS phenotype compared to those with non-hyperandrogenic PCOS phenotype was 1.47, with a 95% CI of 0.99–2.20 and an I2 of 0%. Moreover, the overall rate of admission to the neonatal intensive care unit (NICU) in the hyperandrogenism groups was 19.1%, while it was 9.9% in the non-hyperandrogenism groups. The OR for NICU admission in pregnant women with hyperandrogenic PCOS phenotype compared to those with non-hyperandrogenic PCOS phenotype was 2.18, with a 95% CI of 0.99–4.81 and an I2 of 0%.
There were no substantial variances discovered in the odds ratios pertaining to miscarriage, live birth, preterm birth, CS, pregnancy-induced hypertension (PIH), SGA, LGA, and admission to the NICU when comparing expectant mothers with hyperandrogenic PCOS phenotype to those with non-hyperandrogenic PCOS phenotype.
Publication bias evaluation
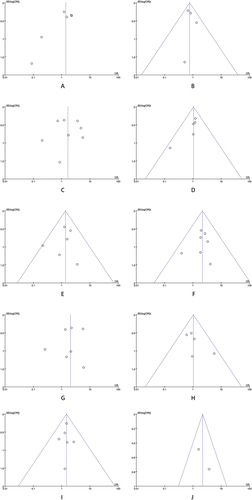
The lack of publication bias was revealed by the results of the funnel plot test (see ). It is important to highlight that half of the studies included in the analysis utilized medical registry databases, thus increasing the potential for coding errors.
Figure 5. Funnel plot of comparison: hyperandrogenic PCOS phenotype (polycystic ovary syndrome) versus non-hyperandrogenic PCOS phenotype (A: miscarriage; B: live-birth; C: preterm; D: caesarean section; E: pregnancy-induced hypertension; F: preeclampsia; G: gestational diabetes mellitus; H: small-for-gestational age; I: large-for-gestational age; J: admission to the neonatal intensive care unit).
Discussion
This research conducts the first comprehensive review and meta-analysis of existing studies examining the links between obstetric/neonatal complications and PCOS, with or without excessive levels of male hormones, in female individuals. Expectant mothers with the hyperandrogenic PCOS variation demonstrated higher chances of developing preeclampsia and gestational diabetes mellitus when contrasted with those having the non-hyperandrogenic PCOS form.
The relationship between PCOS, particularly with hyperandrogenism, and the heightened risks of PE and GDM is still not fully comprehended, and multiple potential explanations are feasible. PE is a complicated and demanding complication of pregnancy distinguished by the emergence of hypertension and proteinuria in the third trimester. PE can swiftly advance to severe complications, presenting dangers to both the mother and fetus (Citation28). The etiology of PE remains uncertain, with potential mechanisms (Citation29) such as placental ischemia (Citation30), generalized multi-system vasoconstriction (Citation31), oxidative stress (Citation32), micro-emboli (Citation33), dysfunction of endothelial cells (Citation34), and systemic inflammatory response (Citation35–37). The precise interaction of these elements in the setting of PCOS, particularly hyperandrogenic traits, necessitates further examination.
GDM is a common medical issue that occurs during pregnancy, with its underlying causes still not completely clear (Citation38). Both GDM and PE are mainly initiated by the release of inflammatory proteins and dysfunction of fat cells. More studies are needed to fully understand how these disorders develop and interact, especially in relation to PCOS and its excess androgen features (Citation39).
Hyperandrogenism is believed to have a direct impact on the emergence of pregnancy complications. Androgens, in this scenario, might play a role in irregular placental structure, disturbances in early trophoblast invasion and placentation, and adjustments in cervical remodeling and myometrial function (Citation40,Citation41). The placenta contains multiple variants of the androgen receptor, and the activation of this receptor may be linked to the occurrence of unfavorable pregnancy results. These potential mechanisms underscore hyperandrogenism’s complex role in influencing different parts of the reproductive process, bringing to light its effect on pregnancy results. Additional investigation is necessary to uncover the exact pathways in which hyperandrogenism contributes to these changes in pregnancy physiology (Citation42–45).
Hyperinsulinemia is frequently observed in women diagnosed with PCOS, and the potential impact it can have on obstetric and perinatal complications has been an area of limited investigation (Citation46). The prevalence of hyperinsulinemia is notably higher in cases where hyperandrogenic characteristics are dominant, further worsened by obesity and significantly heightened during pregnancy among women with PCOS. Insulin, within this context, plays a role in establishing a prothrombotic and profibrotic environment, intensifying vascular vasoconstriction and ultimately resulting in an escalation of blood pressure. A deeper comprehension of the intricate interplay between hyperinsulinemia and pregnancy outcomes in women with PCOS is crucial for informed clinical decision-making (Citation46).
Furthermore, the hyperandrogenic/hyperinsulinemic environment demonstrates a pro-inflammatory influence (Citation46,Citation47), characterized by increases in serum levels of C-reactive protein, white blood cells, and inflammatory cytokines and cell adhesion molecules (Citation48). In addition, impaired glucose control, often associated with endothelial dysfunction and inflammation, may heighten the negative consequences of hyperandrogenism (Citation49,Citation50). This inflammatory setting, combined with adverse placental modifications linked to PCOS – including changes in spiral arteries, placental vascular abnormalities, and inflammation – can contribute to an unfavorable pregnancy outcome and potentially establish a well-documented transgenerational impact. Recognizing the interconnected pathways and inflammatory reactions within this complex setting is vital for understanding the diverse implications on pregnancy and future generations (Citation44,Citation47).
The intricate pathophysiology involved in fetal growth restriction is characterized by inadequate placental development, which is particularly evident in a significant number of cases involving SGA infants, especially among women with hypertensive disorders that occur before term delivery. This association is also seen in cases that result in negative outcomes for the infant at birth. However, in the current investigation, no notable distinctions were observed between the two cohorts in terms of odds ratios for various outcomes such as miscarriage, successful birth, premature delivery, cesarean section, PIH, SGA, LGA, and admission to the NICU. Noteworthy findings from a study conducted by Yang and colleagues highlight the importance of basal levels of androstenedione as a significant predictor with moderate accuracy in determining the miscarriage rate (Citation22). Their data suggested that the predictive ability of basal androstenedione levels for miscarriage was slightly more robust compared to body mass index. Further exploration in this area is necessary to obtain a more thorough comprehension of the subject matter.
Despite the overall high quality of the studies included, as indicated by their positive quality assessment scores, this meta-analysis has limitations that warrant careful interpretation of its findings. First, publication bias assessment was not possible due to the limited number of studies included. Second, the criteria for HA involves symptomatology and biochemistry, potentially leading to variability among the studies. Additionally, certain biological factors that could impact outcomes may not have been accounted for. Furthermore, being an observational study meta-analysis, it can only show a relationship and not causation. These limitations highlight the importance of further research and a nuanced understanding of the results.
In conclusion, this meta-analysis underlines the association between hyperandrogenism in individuals with PCOS and an increased risk of PE and GDM. Recognizing this link is essential for healthcare providers, emphasizing the need for heightened vigilance in managing pregnancies in individuals with PCOS, especially those showing hyperandrogenic traits.
Abbreviation
| CIs | = | confidence intervals |
| CS | = | caesarean section |
| GDM | = | gestational diabetes mellitus |
| LGA | = | large for gestational age |
| NICU | = | neonatal intensive care unit |
| ORs | = | odds ratios |
| PCOS | = | polycystic ovary syndrome |
| PE | = | preeclampsia |
| PIH | = | pregnancy-induced hypertension |
| PRISMA | = | the Preferred Reporting Items for Systematic Review and Meta-Analyses |
| SGA | = | small for gestational age |
Author contributions
Conception and design: Xiaohan Guo, Ruoan Jiang. Acquisition and data: Xiaohan Guo, Yingsha Yao, and Ruoan Jiang. Analysis and interpretation of data: Yingsha Yao, Ting Wang, and Juanhong Wu. Drafting of the manuscript: Xiaohan Guo, Yingsha Yao. Critical revision of the manuscript for important intellectual content: Ruoan Jiang. Statistical analysis: Ruoan Jiang, Yingsha Yao, and Ruoan Jiang. Supervision: Ruoan Jiang.
Article highlights
Polycystic ovary syndrome (PCOS) is a reproductive and metabolic condition. The effect of different phenotypes of PCOS on obstetrical and neonatal outcomes is controversial based on current research results.
We performed a systematic review of the literature, using web of science, Cochrane Menstrual Disorders and Subfertility Group trials register and EMBASE, from their dates of inception to December 2023. The study included studies that reported the associations between hyperandrogenism-PCOS or non-hyperandrogenism-PCOS and obstetric/neonatal risk.
Our meta-analysis demonstrates an increased risk of PE and GDM among PCOS with hyperandrogenism pregnant women. Physicians should be aware of this association.
Availability of data and material
Further inquiries can be directed to the corresponding author/s.
Consent for publication
The authors declare that they agree with the publication of the article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Shrivastava S, Conigliaro RL. Polycystic ovarian syndrome. Med Clin North Am. 2023;107(2):227–12. doi: 10.1016/j.mcna.2022.10.004
- Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8(1):41. doi: 10.1186/1741-7015-8-41
- March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–551. doi: 10.1093/humrep/dep399
- Farhadi-Azar M, Behboudi-Gandevani S, Rahmati M, et al. The prevalence of polycystic ovary syndrome, its phenotypes and cardio-metabolic features in a community sample of Iranian population: Tehran lipid and glucose study. Front Endocrinol (Lausanne). 2022;13:825528. doi: 10.3389/fendo.2022.825528
- Kollmann M, Klaritsch P, Martins WP, et al. Maternal and neonatal outcomes in pregnant women with PCOS: comparison of different diagnostic definitions. Hum Reprod. 2015;30(10):2396–2403. doi: 10.1093/humrep/dev187
- Meier RK. Polycystic ovary syndrome. Nurs Clin North Am. 2018;53(3):407–420. doi: 10.1016/j.cnur.2018.04.008
- Huddleston HG, Dokras A. Diagnosis and treatment of polycystic ovary syndrome. JAMA. 2022;327(3):274–275. doi: 10.1001/jama.2021.23769
- Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–47. doi: 10.1093/humrep/deh098
- Teede HJ, Misso ML, Boyle JA, et al. Translation and implementation of the Australian-led PCOS guideline: clinical summary and translation resources from the International evidence-based guideline for the assessment and management of polycystic ovary syndrome. Medical J Australia. 2018;209(S7):S3–S8. doi: 10.5694/mja18.00656
- Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman’s long-term health using data linkage. J Clin Endocrinol Metab. 2015;100(3):911–919. doi: 10.1210/jc.2014-3886
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clinical Endocrinol. 2018;89(6):683–699. doi: 10.1111/cen.13828
- Doherty DA, Newnham JP, Bower C, et al. Implications of polycystic ovary syndrome for pregnancy and for the health of offspring. Obstetrics & Gynecol. 2015;125(6):1397–1406. doi: 10.1097/AOG.0000000000000852
- Yu HF, Chen HS, Rao DP, et al. Association between polycystic ovary syndrome and the risk of pregnancy complications: a PRISMA-compliant systematic review and meta-analysis. Med (Baltimore). 2016;95(51):e4863. doi: 10.1097/MD.0000000000004863
- Barker DJ, Gluckman PD, Godfrey KM, et al. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341(8850):938–941. doi: 10.1016/0140-6736(93)91224-A
- Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS consensus workshop group. Fertil Steril. 2012;97(1):28–38.e25. doi: 10.1016/j.fertnstert.2011.09.024
- Alenezi SA, Khan R, Amer S. The impact of high BMI on pregnancy outcomes and complications in women with PCOS undergoing IVF—A systematic review and meta-analysis. JCM. 2024;13(6):1578. doi: 10.3390/jcm13061578
- Christ JP, Gunning MN, Meun C, et al. Pre-conception characteristics predict obstetrical and neonatal outcomes in women with polycystic ovary syndrome. J Clin Endocrinol Metabol. 2019;104(3):809–818. doi: 10.1210/jc.2018-01787
- De Wilde MA, Lamain-De Ruiter M, Veltman-Verhulst SM, et al. Increased rates of complications in singleton pregnancies of women previously diagnosed with polycystic ovary syndrome predominantly in the hyperandrogenic phenotype. Fertil Steril. 2017;108(2):333–340. doi: 10.1016/j.fertnstert.2017.06.015
- Mumm H, Jensen DM, Sørensen JA, et al. Hyperandrogenism and phenotypes of polycystic ovary syndrome are not associated with differences in obstetric outcomes. Acta Obstet Gynecol Scand. 2015;94(2):204–211. doi: 10.1111/aogs.12545
- Palomba S, Falbo A, Russo T, et al. Pregnancy in women with polycystic ovary syndrome: the effect of different phenotypes and features on obstetric and neonatal outcomes. Fertil Steril. 2010;94(5):1805–1811. doi: 10.1016/j.fertnstert.2009.10.043
- L L, L J, L J. Analysis of different subtypes of polycystic ovary syndrome and pregnancy outcome after in-vitro fertilization and embryo transfer. J Practical Obstet And Gynecol. 2010;26(10):787–790.
- Yang W, Yang R, Lin M, et al. Body mass index and basal androstenedione are independent risk factors for miscarriage in polycystic ovary syndrome. Reprod Biol Endocrinol. 2018;16(1):119. doi: 10.1186/s12958-018-0438-7
- Wei DM, Zhang ZZ, Wang Z, et al. Effect of hyperandrogenism on obstetric complications of singleton pregnancy from in vitro fertilization in women with polycystic ovary syndrome. Zhonghua Fu Chan Ke Za Zhi. 2018;53(1):18–22. doi: 10.3760/cma.j.issn.0529-567X.2018.01.005
- Foroozanfard F, Asemi Z, Bazarganipour F, et al. Comparing pregnancy, childbirth, and neonatal outcomes in women with different phenotypes of polycystic ovary syndrome and healthy women: a prospective cohort study. Gynecol Endocrinol. 2020;36(1):61–65. doi: 10.1080/09513590.2019.1631278
- Dehghani Firoozabadi A, Dehghani Firouzabadi R, Eftekhar M, et al. Maternal and neonatal outcomes among pregnant women with different polycystic ovary syndrome phenotypes: a cross-sectional study. Int J Reprod Biomed. 2020;18(5):339–346. doi: 10.18502/ijrm.v13i5.7154
- Mackens S, Pareyn S, Drakopoulos P, et al. Outcome of in-vitro oocyte maturation in patients with PCOS: does phenotype have an impact? Hum Reprod. 2020;35(10):2272–2279. doi: 10.1093/humrep/deaa190
- Valdimarsdottir R, Wikström AK, Kallak TK, et al. Pregnancy outcome in women with polycystic ovary syndrome in relation to second-trimester testosterone levels. Reprod Biomed Online. 2021;42(1):217–225. doi: 10.1016/j.rbmo.2020.09.019
- Rana S, Lemoine E, Granger JP, et al. Preeclampsia: pathophysiology, challenges, and perspectives. Circulat Res. 2019;124(7):1094–1112. doi: 10.1161/CIRCRESAHA.118.313276
- El-Sayed AAF. Preeclampsia: a review of the pathogenesis and possible management strategies based on its pathophysiological derangements. Taiwan J Obstet Gynecol. 2017;56(5):593–598. doi: 10.1016/j.tjog.2017.08.004
- Hariharan N, Shoemaker A, Wagner S. Pathophysiology of hypertension in preeclampsia. Microvasc Res. 2017;109:34–37. doi: 10.1016/j.mvr.2016.10.002
- Li F, Hagaman JR, Kim HS, et al. eNOS deficiency acts through endothelin to aggravate sFlt-1-induced pre-eclampsia-like phenotype. J Am Soc Nephrol. 2012;23(4):652–660. doi: 10.1681/ASN.2011040369
- Zenclussen AC, Lim E, Knoeller S, et al. Heme oxygenases in pregnancy II: HO-2 is downregulated in human pathologic pregnancies. American J Rep Immunol. 2003;50(1):66–76. doi: 10.1034/j.1600-0897.2003.00047.x
- Lyall F, Barber A, Myatt L, et al. Hemeoxygenase expression in human placenta and placental bed implies a role in regulation of trophoblast invasion and placental function. FASEB J. 2000;14(1):208–219. doi: 10.1096/fasebj.14.1.208
- Belo L, Gaffney D, Caslake M, et al. Apolipoprotein E and cholesteryl ester transfer protein polymorphisms in normal and preeclamptic pregnancies. Eur J Obstet Gynecol Reprod Biol. 2004;112(1):9–15. doi: 10.1016/S0301-2115(03)00240-9
- Kulikova GV, Nizyaeva NV, Nagovitsina MN, et al. Specific features of TLR4 expression in structural elements of placenta in patients with preeclampsia. Bull Exp Biol Med. 2016;160(5):718–721. doi: 10.1007/s10517-016-3259-8
- Xie F, Von Dadelszen P, Nadeau J. CMV infection, TLR-2 and -4 expression, and cytokine profiles in early-onset preeclampsia with HELLP syndrome. Am J Reprod Immunol. 2014;71(4):379–386. doi: 10.1111/aji.12199
- Barrera D, Díaz L, Noyola-Martínez N, et al. Vitamin D and inflammatory cytokines in healthy and preeclamptic pregnancies. Nutri. 2015;7(8):6465–6490. doi: 10.3390/nu7085293
- ACOG practice bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49–e64. doi: 10.1097/AOG.0000000000002501
- Świrska J, Zwolak A, Dudzińska M, et al. Gestational diabetes mellitus - literature review on selected cytokines and hormones of confirmed or possible role in its pathogenesis. Ginekol Pol. 2018;89(9):522–527. doi: 10.5603/GP.a2018.0089
- Yusuf ANM, Amri MF, Ugusman A, et al. Hyperandrogenism and its possible effects on endometrial receptivity: a review. IJMS. 2023;24(15):12026. doi: 10.3390/ijms241512026
- Koster MP, De Wilde MA, Veltman-Verhulst SM, et al. Placental characteristics in women with polycystic ovary syndrome. Hum Reprod. 2015;30(12):2829–2837. doi: 10.1093/humrep/dev265
- Palomba S, Russo T, Falbo A, et al. Decidual endovascular trophoblast invasion in women with polycystic ovary syndrome: an experimental case-control study. J Clin Endocrinol Metab. 2012;97(7):2441–2449. doi: 10.1210/jc.2012-1100
- Sun M, Maliqueo M, Benrick A, et al. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. Am J Physiol Endocrinol Metab. 2012;303(11):E1373–85. doi: 10.1152/ajpendo.00421.2012
- Palomba S, Russo T, Falbo A, et al. Macroscopic and microscopic findings of the placenta in women with polycystic ovary syndrome. Hum Reprod. 2013;28(10):2838–2847. doi: 10.1093/humrep/det250
- Palomba S, Falbo A, Chiossi G, et al. Early trophoblast invasion and placentation in women with different PCOS phenotypes. Reprod Biomed Online. 2014;29(3):370–381. doi: 10.1016/j.rbmo.2014.04.010
- Bahri Khomami M, Boyle JA, Tay CT, et al. Polycystic ovary syndrome and adverse pregnancy outcomes: Current state of knowledge, challenges and potential implications for practice. Clinical Endocrinol. 2018;88(6):761–769. doi: 10.1111/cen.13579
- Puttabyatappa M, Cardoso RC, Padmanabhan V. Effect of maternal PCOS and PCOS-like phenotype on the offspring’s health. Mol Cell Endocrinol. 2016;435:29–39. doi: 10.1016/j.mce.2015.11.030
- Orio F, Palomba JR, Cascella S, et al. The increase of leukocytes as a new putative marker of low-grade chronic inflammation and early cardiovascular risk in polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90(1):2–5. doi: 10.1210/jc.2004-0628
- Seely EW, Solomon CG. Insulin resistance and its potential role in pregnancy-induced hypertension. J Clin Endocrinol Metab. 2003;88(6):2393–2398. doi: 10.1210/jc.2003-030241
- Falbo A, Rocca M, Russo T, et al. Changes in androgens and insulin sensitivity indexes throughout pregnancy in women with polycystic ovary syndrome (PCOS): relationships with adverse outcomes. J Ovarian Res. 2010;3(1):23. doi: 10.1186/1757-2215-3-23