ABSTRACT
Background
Fibroblast growth factor 21 (FGF21) has a protective effect against cardiovascular disease. However, the role of FGF21 in hypertension remains elusive.
Methods
Ten-week-old male C57BL/6 mice were randomly divided into normal-salt (NS) group, NS+FGF21 group, deoxycorticosterone acetate-salt (DOCA) group and DOCA+FGF21 group. The mice in NS group underwent uninephrectomy without receiving DOCA and 1% NaCl and the mice in DOCA group were subjected to uninephrectomy and DOCA-salt (DOCA and 1% NaCl) treatment for 6 weeks. At the same time, the mice were infused with vehicle (artificial cerebrospinal fluid, aCSF) or FGF21 (1 mg/kg) into the bilateral paraventricular nucleus (PVN) of mice.
Results
Here, we showed that FGF21 treatment lowered DOCA salt-induced inflammation and oxidative stress in the PVN, which reduced sympathetic nerve activity and hypertension. Mechanistically, FGF21 treatment decreased the expression of HNF4α and inhibited the binding activity of HNF4α to the promoter region of ACE2 in the PVN of DOCA salt-treated mice, which further up-regulated ACE2/Ang (1–7) signals in the PVN. In addition, ACE2 deficiency abolished the protective effect of FGF21 in DOCA salt-treated mice, suggesting that FGF21-mediated antihypertensive effect was dependent on ACE2.
Conclusions
The results demonstrate that FGF21 protects against salt-sensitive hypertension via regulating HNF4α/ACE2/Ang (1–7) axis in the PVN of DOCA salt-treated mice via multi-organ crosstalk between liver, brain and blood vessels.
Fibroblast growth factor 21 (FGF21), a member of the FGF family, is an endocrine factor produced and secreted by the liverCitation1. A large number of studies have suggested that FGF21 not only plays a key role in regulating glucose and lipid metabolism, improving insulin resistance, inhibiting fibrosis, etc., but also participates in the protective effect of cardiovascular diseasesCitation2,Citation3. In animal models and peripheral blood of patients with heart failure, hypertension and atherosclerosis, FGF21 levels are significantly elevated, and the degree of elevation is positively correlated with the severity of the disease, which can be used as one of the effective indicators for disease diagnosis and prognosis evaluationCitation4–6. Furthermore, overexpression of FGF21 or exogenous intervention of FGF21 can play a role in preventing metabolic disorders, organ damage and fibrosis, vascular dysfunction and cardiac hypertrophy caused by hypertensionCitation7–9.
Hypertension is an important risk factor leading to coronary heart disease, stroke and chronic renal insufficiency, especially high salt-induced hypertension, and the prevention and control status is still not optimisticCitation10. In recent years, clinical studies have found that the level of FGF21 in peripheral blood of patients with hypertension is significantly increasedCitation11. Interestingly, animal studies have shown that exogenous FGF21 can improve angiotensin II-induced hypertension, myocardial hypertrophy and cardiac insufficiencyCitation12. Although the metabolic functions of FGF21 are well characterized, the study on the pathogenesis of hypertension needs to be further clarified.
As an important part of the RAS system, angiotensin-converting enzyme 2 (ACE2) plays a negative role in regulating RAS activity, and activation of ACE2/angiotensin (Ang)-(1–7) axis can significantly reduce blood pressure and improve hypertension-related complicationsCitation13. In the present study, we investigated the beneficial effect of FGF21 in reversing deoxycorticosterone acetate (DOCA) salt-induced hypertension. Our results indicated that FGF21 protects against salt-sensitive hypertension via regulating HNF4α/ACE2/Ang (1–7) axis in the hypothalamic paraventricular nucleus of mice.
Materials and methods
Animal and experimental protocol
Experiments were performed on male C57BL/6N and ACE2 knockout (ACE2 KO) mice weighing 20 ~ 25 g (10 weeks), which were obtained from the Jackson laboratory. All animals were housed in temperature and light-controlled room with a 12-h light-dark cycle and had free access to standard rodent chow and drinking water. The mice were randomly divided into normal-salt (NS) group, NS+FGF21 group, DOCA-salt (DOCA) group and DOCA+FGF21 group. Salt-sensitive hypertension was induced as described previouslyCitation14. In brief, the left kidney was removed under pentobarbital (50 mg/kg body wt, intraperitoneally) anesthesia. The DOCA pellets were implanted in the neck area and the mice were given 1% NaCl for 6 weeks. The mice in NS group underwent uninephrectomy without receiving DOCA and NaCl. At the same time, the mice were infused with vehicle (artificial cerebrospinal fluid, aCSF, 0.4 μL/h, Sigma, MO, USA) or FGF21 (1 mg/kg, 0.4 μL/h, Sigma) into the bilateral PVN of mice for 6 weeks. All the experiments conformed to the National Institutes of Health guidelines and the protocols were approved by the Animal Care and Use Committee of Army Medical University.
Blood pressure and heart rate measurement
During the 6-week experimental period, blood pressure and heart rate were measured weekly using a tail-cuff (BP-98A; Softron, Tokyo, Japan). The mice were warmed by placing them on a 37°C heating table. All mice were habituated to the blood pressure measuring system and each mouse was allowed to accommodate to the cuff for 5 min. Blood pressure and heart rate were measured 3 times and the average value was taken.
Biochemical assays
The level of norepinephrine (NE) in plasma and levels of TNF-α, IL-6, GSH and MDA in PVN were quantified using mouse ELISA kits (Invitrogen, Carlsbad, CA).
Immunological assay
FGF21 concentrations were measured by an ultrasensitive mouse-specific ELISA kit (Antibody and Immunoassay Services, HKU). Ang (1–7) concentrations were measured by a commercial EIA kit (Nanjing Jiancheng Bioengineering institute, Nanjing, China).
Real-time qPCR
qRT-PCR was used to quantify mRNA expressions of FGF21in liver and PVN by using specific primers. The primer sequence was as follows, FGF21: Forward primer 5”-CGGAATTCGGGGTGTGCGAGGCATAC, Reverse primer 5‘- CCGCTCGAGCTAAGATGCATAGCTGGGGCTTC-3,’ GAPDH: Forward primer 5‘-AGGTCGGTGTGAACGGATTTG, Reverse primer 5’- TGTAGACCATGTAGTTGAGGTCA-3.” Total mRNA was extracted by the Trizol Reagent method (Invitrogen) and cDNA was synthesized using iScript cDNA synthesis kit (Bio-Rad). The level of target gene expression was normalized against the GAPDH gene.
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation (CHIP) assays were carried out with an EZ-ChIP kit (Millipore, Billerica, MA, USA) per manufacturer protocol. Briefly, Immunoprecipitation was performed overnight at 4°C, using 1 μg of antibody against HNF4α (Abcam) or lgG (CST). Each immunoprecipitated sample was added to protein G agarose beads for 3 hours of incubation at 4°C. The immunocomplexes were extracted and processed by reverse cross-linking, proteinase K digestion, and DNA precipitation. The following primer was used for CHIP-polymerase chain reaction (PCR): ACE2: 5’- ACCCTTCTTACATCAGCCCTACTG-3,’ 5’- TGTCCAAAACCTACCCCACATAT-3.’
Western blot analysis
The protein expressions of related genes (FGF21, HNF4α, ACE2, H3 and GAPDH) in PVN were determined by western-blot. Primary polyclonal antibodies for anti-FGF21 (1:300), anti-HNF4α (1:400), anti-ACE2 (1:300), H3 (1:500) and anti-GAPDH (1:500) were purchased from Abcam. The methods were as described previouslyCitation15. The protein intensity was normalized to that of GAPDH or H3. Band density was analyzed with ImageJ software.
Statistical analysis
The data were expressed as mean ± SD. Statistical analysis was performed using the GraphPad Prism 8.0 software. Data were analyzed by one-way ANOVA followed by Newman-keuls post hoc test whenever appropriate. Value of p < .05 was considered significant.
Results
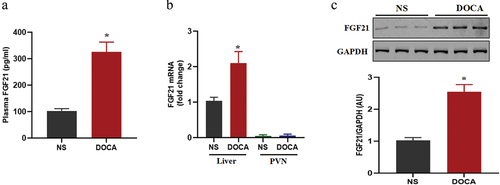
DOCA-salt induced a significant increase in the synthesis and secretion of FGF21
Several studies have found significant increases in FGF21 concentrations in animal models and peripheral blood of patients with hypertensionCitation16. To investigate the pathophysiological role of FGF21 in hypertension, we first examined the change of FGF21 expressional profiles in DOCA salt-treated mice. The results showed that plasma FGF21 levels and hepatic FGF21 mRNA and protein expression were markedly increased in DOCA salt-treated mice (). However, FGF21 levels in PVN were very low, and there was no significant difference between DOCA salt group and DOCA salt with FGF21 treatment group ().
Figure 1. DOCA salt induced a significant increase in the synthesis and secretion of FGF21. The male C57BL/6 mice were subjected to uninephrectomy with or without deoxycorticosterone acetate (DOCA)-salt treatment for 6 weeks. (a) Plasma FGF21 was measured by immunoassay. (b) FGF21 mRNA levels in liver and PVN and (c) FGF21 protein expression levels in liver were examined by real-time qPCR and western-blot, respectively. Data were expressed as the means ± SD (n=5/group). *p<0.05 vs others.
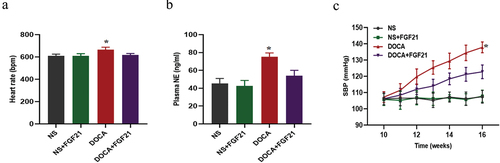
FGF21 intervention reduced DOCA salt-induced sympathetic activation and hypertension
Previous studies have reported that high salt can promote sympathetic nerve activation and increase blood pressureCitation17. In the present study, the result was showed that after DOCA-salt intervention for 6 weeks, indicators of peripheral sympathetic nerve activity including heart rate and NE levels were significantly increased, accompanied by an increase in blood pressure. However, FGF21 treatment significantly reduced the DOCA salt-induced sympathetic nerve activation and increased blood pressure ().
Figure 2. FGF21 intervention reduced DOCA salt-induced sympathetic activation and hypertension. The male C57BL/6 mice were subjected to uninephrectomy with or without deoxycorticosterone acetate (DOCA)-salt treatment for 6 weeks. At the same time, the mice were infused with vehicle (artificial cerebrospinal fluid, aCSF, 0.4 μL/h) or FGF21 (1 mg/kg, 0.4 μL/h) into the bilateral PVN of mice for 6 weeks. (a) Heart rate was measured by tail-cuff plethysmography. (b) NE level in plasma was measured by ELISA kits. (c) Systolic blood pressure (SBP) was measured by tail-cuff plethysmography. Data were expressed as the means ± SD (n=5/group). *p<0.05 vs others.
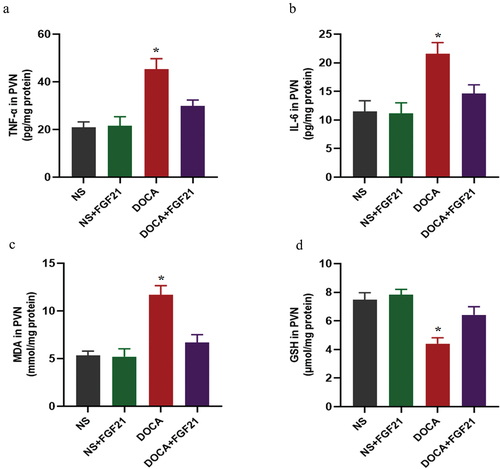
FGF21 intervention lowered DOCA salt-induced inflammation and oxidative stress in the PVN
Inflammation and oxidative stress in the PVN are important factors that promote the activation of peripheral sympathetic nerveCitation18. We found that DOCA salt induced an increase in markers of inflammation including TNF-α and IL-6 in the PVN. In addition, DOCA salt induced an increase in oxidative stress, including increased MDA and decreased GSH. FGF21 treatment significantly reversed DOCA salt-induced increased levels of inflammation and oxidative stress in the PVN ().
Figure 3. FGF21 intervention lowered DOCA salt-induced inflammation and oxidative stress in the PVN. The male C57BL/6 mice were subjected to uninephrectomy with or without deoxycorticosterone acetate (DOCA)-salt treatment for 6 weeks. At the same time, the mice were infused with vehicle (artificial cerebrospinal fluid, aCSF, 0.4 μL/h) or FGF21 (1 mg/kg, 0.4 μL/h) into the bilateral PVN of mice for 6 weeks. (a and b) inflammatory markers including TNF-α and IL-6 in the PVN were measured by ELISA kits. (c and d) oxidative stress markers including MDA and GSH in the PVN were measured by ELISA kits. Data were expressed as the means ± SD (n=5/group). *p<0.05 vs others.
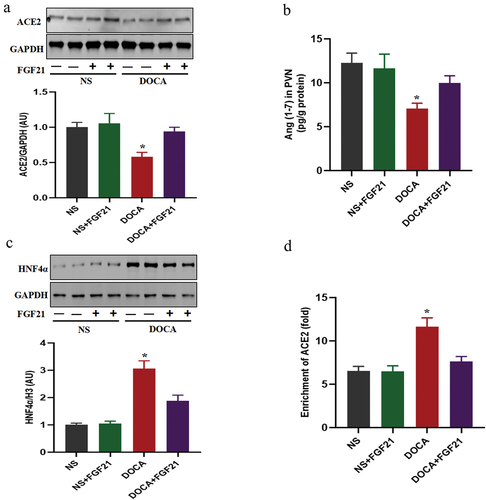
FGF21 intervention up-regulated ACE2/Ang (1–7) signals in the PVN
ACE2/Ang (1–7) signals negatively regulate blood pressureCitation19. Similar to the results of other studiesCitation19, high salt induced significant downregulation of ACE2 expression and Ang (1–7) levels in the PVN. However, FGF21 intervention reversed the abnormalities in ACE2/Ang (1–7) signals (). As a transcription suppressor of ACE2, HNF4α negatively regulates the expression of ACE2. The present results showed that DOCA salt could induce a significant increase in HNF4α level, and enhance the binding activity of HNF4α to the ACE2 promoter region. However, FGF21 treatment decreased the expression of HNF4α and inhibited the binding activity of HNF4α to the promoter region of ACE2 ().
Figure 4. The role of HNF4α/ACE2/Ang (1–7) signaling in antihypertensive function of FGF21. The male C57BL/6 mice were subjected to uninephrectomy with or without deoxycorticosterone acetate (DOCA)-salt treatment for 6 weeks. At the same time, the mice were infused with vehicle (artificial cerebrospinal fluid, aCSF, 0.4 μL/h) or FGF21 (1 mg/kg, 0.4 μL/h) into the bilateral PVN of mice for 6 weeks. (a) ACE2 expression was measured by western-blot. (b) Ang (1–7) levels in the PVN were measured by immunoassay. (c and d) HNF4α expression and the binding activity of HNF4α to the ACE2 promoter region were measured by western-blot and CHIP assay, respectively. Data were expressed as the means ± SD (n=5/group). *p<0.05 vs others.
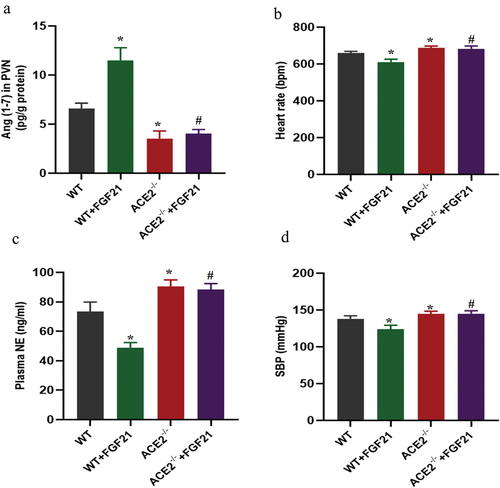
ACE2 deficiency abolished the protective effect of FGF21 in DOCA salt-induced hypertension
Previous data have shown that the antihypertensive effects of FGF21 may be related to up-regulation of ACE2 in the PVN region, we further explored whether ACE2 deficiency would affect the protective effect of FGF21 against DOCA salt-induced hypertension in mice. As expected, consistent with these findings, FGF21 treatment significantly increased Ang (1–7) levels in the PVN of DOCA salt treated mice but not in ACE2 deficiency mice (). In addition, loss of ACE2 accelerated DOCA salt-induced sympathetic activation and hypertension. FGF21 treatment reduced the DOCA salt-induced sympathetic nerve activation and increased blood pressure, but not in ACE2 deficiency mice ().
Figure 5. ACE2 deficiency abolished the protective effect of FGF21 in DOCA salt-induced hypertension. ACE2 deficiency and WT mice were subjected to uninephrectomy with deoxycorticosterone acetate (DOCA)-salt treatment for 6 weeks. At the same time, the mice were infused with vehicle (artificial cerebrospinal fluid, aCSF, 0.4 μL/h) or FGF21 (1 mg/kg, 0.4 μL/h) into the bilateral PVN of mice for 6 weeks. (a) Ang (1–7) levels in the PVN were measured by immunoassay. (b) Heart rate was measured by tail-cuff plethysmography. (c) NE level in plasma was measured by ELISA kits. (d) SBP was measured by tail-cuff plethysmography at the age of 16 weeks. Data were expressed as the means ± SD (n=5/group). *p<0.05 vs WT mice, #p<0.05 vs WT+FGF21 group.
Discussion
Although the regulatory role of FGF21 in metabolic function has been extensively studied, the regulation and mechanism of FGF21 on blood pressure have been less studied. This study revealed that DOCA salt induced increased blood pressure in mice, along with increased production and secretion of FGF21. Exogenous administration of FGF21 could reduce DOCA salt-induced hypertension via regulating the HNF4α/ACE2/Ang(1–7) signaling axis in PVN.
Several experimental and clinical studies have shown that circulating FGF21 levels are significantly elevated in hypertensive animal models and patientsCitation20,Citation21. Our present data also showed that circulating FGF21 is also significantly elevated in a DOCA salt-induced hypertension model, suggesting that upregulated FGF21 could act as a compensatory mechanism to protect against DOCA salt-induced hypertension. Studies have shown that Ang II can induce the reduction of FGF21 level in rats, which is involved in the pathogenesis of hypertensive vascular remodelingCitation22. The reason for the difference of FGF21 level in hypertensive state may be related to different animal models, different species and age. Moreover, increasing evidence has indicated that FGF21 is synthesized and secreted mainly in the liverCitation23. Our data also showed that liver was the major site for the production and maintenance of homeostasis of circulating FGF21 concentrations in response to DOCA salt-induced hypertension. Previous studies have shown that liver-produced FGF21 has a protective effect against cardiovascular diseaseCitation24. Hepatocyte-derived FGF21 controls the expression of cardiomyocyte genes that are involved in DNA repair, oxidative phosphorylation and apoptosis and is involved in IL-22-mediated cardiac repair after acute myocardial infarctionCitation25. Autophagy can promote the degradation of lipid droplets in foam cells, thus inhibiting atherosclerosis. FGF21 can play an anti-atherosclerotic role by inducing autophagy of foam cellsCitation26. In addition, FGF21 attenuates hypertension-induced nephropathy through anti-inflammation and anti-oxidation mechanismCitation27. There are few reports on whether FGF21 plays a direct role in regulating blood pressure. Our present results showed that FGF21 significantly reduced DOCA salt-induced hypertension, and the mechanism may be related to the decreased peripheral sympathetic nerve activity caused by decreased inflammation and oxidative stress in the PVN.
Growing evidence has established that PVN is the central locus that regulates blood pressureCitation28. The change of neuronal activity in this locus can affect the activity of peripheral sympathetic nerve and thus regulate the homeostasis of blood pressureCitation29. ACE2/Ang (1–7) signal in the PVN can antagonize RAS activation and negatively regulate blood pressure. ACE2 overexpression in the PVN results in the down-regulation of AT1R expression, which will have beneficial effects in counteracting Ang II-induced hypertensionCitation30. In addition, upregulation of central ACE2 lowers Ang II-induced hypertension via reducing reactive oxygen species through activation of Nrf2 in the rostral ventrolateral medullaCitation31. These studies suggest that upregulation of ACE2 is an important target for the treatment of hypertension. In our present study, it was also found that DOCA salt induced a significant decrease in the expression level of ACE2 in the PVN of mice, while FGF21 intervention significantly upregulated the expression level of ACE2 and increased Ang (1–7) levels. Since the aforementioned data suggest that the antihypertensive effect of FGF21 may be related to the up-regulation of ACE2, we further observe whether ACE2 knockout would reduce the protective effect of FGF21 against DOCA salt-induced hypertension in mice. As expected, our results found that ACE2 deficiency could exacerbate DOCA salt-induced hypertension, while FGF21-mediated antihypertensive effect is absent, suggesting that FGF21-mediated antihypertensive effect is dependent on ACE2 signaling. However, the pathway through which FGF21 affects ACE2 expression still needs to be further clarified
Previous studies have shown that HNF4α, as a transcription factor, can induce transcriptional regulation of downstream target genes, especially the important components of RAS, including AGT, AGTR1, ACE and ACE2Citation32. Severe acute respiratory syndrome coronavirus 2 enters cells mainly through ACE2 and plays the role of virus transmission. HNF4α is found to negatively regulate ACE2 expression and plays a role in inhibiting viral replication and transmissionCitation33. In order to determine whether HNF4α is involved in the regulation of FGF21 on ACE2, we first detected the expression of HNF4α. The results showed that DOCA salt induced the increase of HNF4α expression in PVN, and FGF21 intervention could significantly down-regulate the expression of HNF4α. More importantly, CHIP results showed that FGF21 intervention enhanced the binding activity of HNF4α and the ACE2 promoter region, suggesting that FGF21 could affect the expression of ACE2 by activating HNF4α. The present study found that in addition to regulating the HNF4α/ACE2 signaling axis, central infusion of FGF21 also reduced inflammation and oxidative stress in the PVN. The decrease of inflammation and oxidative stress will help to reduce ACE/AT1R signaling axis, inhibit excitatory neuron activity, and thus reduce blood pressureCitation34, which may also be one of the possible mechanisms of central FGF21 infusion for lowering blood pressure.
In summary, our study reveals that the protective mechanism of FGF21 against DOCA salt-induced hypertension via regulating HNF4α/ACE2 axis in the PVN of mice. Therefore, FGF21 may be a promising therapeutic agent of salt-sensitive hypertension.
Author contribution
WX conceived and designed the experiments and wrote the manuscript; XG and HL performed the experiments and analyzed the data; QL approved the final version of the manuscript.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Fisher FM, Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol. 2016;78(1):223–7. doi:10.1146/annurev-physiol-021115-105339.
- Geng L, Lam KSL, Xu A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat Rev Endocrinol. 2020;16(11):654–67. doi:10.1038/s41574-020-0386-0.
- Tan H, Yue T, Chen Z, Wu W, Xu S, Weng J. Targeting FGF21 in cardiovascular and metabolic diseases: from mechanism to medicine. Int J Biol Sci. 2023;19(1):66–88. doi:10.7150/ijbs.73936
- Pandhi P, Ter Maaten JM, Anker SD, Ng LL, Metra M, Samani NJ, Lang CC, Dickstein K, de Boer RA, van Veldhuisen DJ, et al. Pathophysiologic processes and novel biomarkers associated with congestion in heart failure. JACC Heart Fail. 2022;10(9):623–32. doi:10.1016/j.jchf.2022.05.013.
- Ferrer-Curriu G, Redondo-Angulo I, Guitart-Mampel M, Ruperez C, Mas-Stachurska A, Sitges M, Garrabou G, Villarroya F, Fernandez-Sola J, Planavila A. Fibroblast growth factor-21 protects against fibrosis in hypertensive heart disease. J Pathol. 2019;248(13):30–40. doi:10.1002/path.5226.
- Yafei S, Elsewy F, Youssef E, Ayman M, El-Shafei M. Fibroblast growth factor 21 association with subclinical atherosclerosis and arterial stiffness in type 2 diabetes. Diabetes Metab Syndr. 2019;13(1):882–88. doi:10.1016/j.dsx.2018.12.007
- Quesada-Lopez T, Cereijo R, Turatsinze JV, Planavila A, Cairo M, Gavalda-Navarro A, Peyrou M, Moure R, Iglesias R, Giralt M, et al. The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat Commun. 2016;7(1):13479. doi:10.1038/ncomms13479.
- Ding P, Yang R, Li C, Fu HL, Ren GL, Wang P, Zheng DY, Chen W, Yang LY, Mao YF, et al. Fibroblast growth factor 21 attenuates ventilator-induced lung injury by inhibiting the NLRP3/caspase-1/GSDMD pyroptotic pathway. Crit Care. 2023;27(1):196. doi:10.1186/s13054-023-04488-5.
- Pan X, Shao Y, Wu F, Wang Y, Xiong R, Zheng J, Tian H, Wang B, Wang Y, Zhang Y, et al. FGF21 prevents angiotensin II-Induced hypertension and vascular dysfunction by activation of ACE2/Angiotensin-(1–7) axis in mice. Cell Metab. 2018;27(6):1323–37.e5. doi:10.1016/j.cmet.2018.04.002.
- Oliveras A, de la Sierra A. Resistant hypertension: patient characteristics, risk factors, co-morbidities and outcomes. J Hum Hypertens. 2014;28(4):213–17. doi:10.1038/jhh.2013.77.
- Greenhill C. Link between FGF21 and blood pressure. Nat Rev Endocrinol. 2018;14(7):380. doi:10.1038/s41574-018-0030-4.
- Li S, Zhu Z, Xue M, Yi X, Liang J, Niu C, Chen G, Shen Y, Zhang H, Zheng J, et al. Fibroblast growth factor 21 protects the heart from angiotensin II-induced cardiac hypertrophy and dysfunction via SIRT1. Biochim Biophys Acta Mol Basis Dis. 2019;1865(6):1241–52. doi:10.1016/j.bbadis.2019.01.019.
- Luo H, Wang X, Chen C, Wang J, Zou X, Li C, Xu Z, Yang X, Shi W, Zeng C. Oxidative stress causes imbalance of renal renin angiotensin system (RAS) components and hypertension in obese Zucker rats. J Am Heart Assoc. 2015;4(2). doi:10.1161/JAHA.114.001559.
- Mathieu NM, Nakagawa P, Grobe CC, Reho JJ, Brozoski DT, Lu KT, Wackman KK, Ritter ML, Segar JL, Grobe JL, et al. ARRB2 (beta-arrestin-2) deficiency alters fluid homeostasis and blood pressure regulation. Hypertens. 2022;79(11):2480–92. doi:10.1161/HYPERTENSIONAHA.122.19863.
- Luo H, Chen C, Guo L, Xu Z, Peng X, Wang X, Wang J, Wang N, Li C, Luo X, et al. Exposure to maternal diabetes mellitus causes renal dopamine D 1 receptor dysfunction and hypertension in adult rat offspring. Hypertens. 2018;72(4):962–70. doi:10.1161/HYPERTENSIONAHA.118.10908.
- Chen K, Zhou M, Wang X, Li S, Yang D. The role of myokines and adipokines in hypertension and hypertension-related complications. Hypertens Res. 2019;42(10):1544–51. doi:10.1038/s41440-019-0266-y.
- Xu C, Zhu J, Gong G, Guo L, Zhang Y, Zhang Z, Ma C. Anthocyanin attenuates high salt-induced hypertension via inhibiting the hyperactivity of the sympathetic nervous system. Clin Exp Hypertens. 2023;45:2233717. doi:10.1080/10641963.2023.2233717.
- Yu XJ, Liu XJ, Guo J, Su YK, Zhang N, Qi J, Li Y, Fu LY, Liu KL, Li Y, et al. Blockade of microglial activation in hypothalamic paraventricular nucleus improves high salt-induced hypertension. Am J Hypertens. 2022;35(9):820–27. doi:10.1093/ajh/hpac052.
- Yu XJ, Miao YW, Li HB, Su Q, Liu KL, Fu LY, Hou YK, Shi XL, Li Y, Mu JJ, et al. Blockade of endogenous angiotensin-(1-7) in hypothalamic paraventricular nucleus attenuates high salt-induced sympathoexcitation and hypertension. Neurosci Bull. 2019;35(1):47–56. doi:10.1007/s12264-018-0297-4.
- Semba RD, Crasto C, Strait J, Sun K, Schaumberg DA, Ferrucci L. Elevated serum fibroblast growth factor 21 is associated with hypertension in community-dwelling adults. J Hum Hypertens. 2013;27(6):397–99. doi:10.1038/jhh.2012.52.
- Chen P, Xu B, Feng Y, Li KX, Liu Z, Sun X, Lu XL, Wang LQ, Chen YW, Fan XX, et al. FGF-21 ameliorates essential hypertension of SHR via baroreflex afferent function. Brain Res Bull. 2020;154:9–20. doi:10.1016/j.brainresbull.2019.10.003.
- Song JJ, Yang M, Liu Y, Song JW, Liu XY, Miao R, Zhang ZZ, Liu Y, Fan YF, Zhang Q, et al. Elabela prevents angiotensin II-induced apoptosis and inflammation in rat aortic adventitial fibroblasts via the activation of FGF21-ACE2 signaling. J Mol Histol. 2021;52(5):905–18. doi:10.1007/s10735-021-10011-3.
- Lewis JE, Ebling FJP, Samms RJ, Tsintzas K. Going back to the biology of FGF21: new insights. Trends Endocrinol Metab. 2019;30(8):491–504. doi:10.1016/j.tem.2019.05.007
- Zhang Y, Liu D, Long XX, Fang QC, Jia WP, Li HT. The role of FGF21 in the pathogenesis of cardiovascular disease. Chin Med J (Engl). 2021;134(24):2931–43. doi:10.1097/CM9.0000000000001890.
- Tang TT, Li YY, Li JJ, Wang K, Han Y, Dong WY, Zhu ZF, Xia N, Nie SF, Zhang M, et al. Liver-heart crosstalk controls IL-22 activity in cardiac protection after myocardial infarction. Theranostic. 2018;8(16):4552–62. doi:10.7150/thno.24723.
- Xiaolong L, Dongmin G, Liu M, Zuo W, Huijun H, Qiufen T, XueMei H, Wensheng L, Yuping P, Jun L, et al. FGF21 induces autophagy-mediated cholesterol efflux to inhibit atherogenesis via RACK1 up-regulation. J Cell Mol Med. 2020;24(9):4992–5006. doi:10.1111/jcmm.15118.
- Weng HC, Lu XY, Xu YP, Wang YH, Wang D, Feng YL, Chi Z, Yan XQ, Lu CS, Wang HW. Fibroblast growth factor 21 attenuates salt-sensitive hypertension-induced nephropathy through anti-inflammation and anti-oxidation mechanism. Mol Med. 2021;27(1):147. doi:10.1186/s10020-021-00408-x.
- Xi H, Li X, Zhou Y, Sun Y. The regulatory effect of the paraventricular nucleus on hypertension. Neuroendocrinol. 2024;114(1):1–13. doi:10.1159/000533691.
- Ye S, Zhong H, Duong VN, Campese VM. Losartan reduces central and peripheral sympathetic nerve activity in a rat model of neurogenic hypertension. Hypertens. 2002;39(6):1101–06. doi:10.1161/01.hyp.0000018590.26853.c7.
- Sriramula S, Cardinale JP, Lazartigues E, Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res. 2011;92(3):401–08. doi:10.1093/cvr/cvr242
- Ma A, Gao L, Wafi AM, Yu L, Rudebush T, Zhou W, Zucker IH. Overexpression of central ACE2 (angiotensin-converting enzyme 2) attenuates the pressor response to chronic central infusion of Ang II (Angiotensin II): a potential role for Nrf2 (nuclear factor [Erythroid-Derived 2]-Like 2). Hypertens. 2020;76(5):1514–25. doi:10.1161/HYPERTENSIONAHA.120.15681.
- Niehof M, Borlak J, Bonini M. HNF4alpha dysfunction as a molecular rational for cyclosporine induced hypertension. PLoS One. 2011;6(1):e16319. doi:10.1371/journal.pone.0016319
- Han H, Luo RH, Long XY, Wang LQ, Zhu Q, Tang XY, Zhu R, Ma YC, Zheng YT, Zou CG. Transcriptional regulation of SARS-CoV-2 receptor ACE2 by SP1. Elife. 2024;13. doi:10.7554/eLife.85985.
- Gao HL, Yu XJ, Liu KL, Zuo YY, Fu LY, Chen YM, Zhang DD, Shi XL, Qi J, Li Y, et al. Chronic infusion of astaxanthin into hypothalamic paraventricular nucleus modulates cytokines and attenuates the renin-angiotensin system in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2021;77(2):170–81. doi:10.1097/FJC.0000000000000953.
