Abstract
This review critically examines the long-term management of per- and polyfluoroalkyl substances (PFAS) using constructed floating wetlands (CFW) as a sustainable approach for contaminated water treatment. PFAS, which are persistent and widely distributed environmental contaminants, pose significant challenges due to their resistance to natural degradation. CFW, featuring buoyant platforms that support vegetation, show promise in mitigating PFAS contamination through a range of natural processes such as plant uptake, sorption to growth media, and accumulation in biofilms. The review explores CFW opportunities, emphasizing their suitability for installation in existing urban environments with minimal earthwork and drainage adjustments to address PFAS contamination. It also assesses CFW limitations, such as potential PFAS trophic transfer, highlighting the need for comprehensive studies on PFAS fate and transport within these systems. To ensure CFW efficiency in long-term PFAS management, the review highlights the importance of site-specific assessments, including plant species selection, hydrodynamics, and PFAS-microbial community interactions. The potential of synergistic removal approaches, combining wetland plants’ phytoremediation capabilities with removable sorptive materials or other substrates are also discussed. Over-all, the study provides valuable insights into CFW, covering opportunities, limitations, and essential implementation considerations for achieving sustainable, long-term PFAS management in contaminated water treatment.
Handling Editor:
1. Introduction
Per- and polyfluoroalkyl substances (PFAS) represent a wide range of synthetic chemicals known for their distinctive performance characteristics, making them widely utilized across the globe in a variety of industrial and consumer applications (Buck et al., Citation2021; Glüge et al., Citation2020). These substances have become a major concern for human health and the environment due to their high mobility, persistence, and bioaccumulative properties (HEPA, Citation2020; HEPA, Citation2022). PFAS have been detected in various environmental media including air, soil, sediment, groundwater, surface water, and biota (Kurwadkar et al., Citation2022). These occurrences stem from direct releases (e.g., aqueous film forming foam, manufacturing chemicals, household use products, etc.), exposure to impacted materials, and/or deposition from the atmosphere (ITRC, Citation2022). As environmental authorities worldwide release guidelines for PFAS and evidence of associated risks continues to emerge (Brennan et al., Citation2021; HEPA, Citation2020; HEPA, Citation2022; US EPA, Citation2023), there is a pressing need for short- and long-term remediation technologies that can remove these contaminants from the environment.
Research on strategies for PFAS remediation have generally prioritized highly contaminated source areas (ITRC, Citation2022; Wanninayake, Citation2021). This is not surprising given their higher risk profile to human and environmental health, and the potential for transport of contamination from these source areas to off-site locations. It is important to consider the high mobility and persistence of PFAS in the environment could result in extensive and ongoing contamination of surface and groundwater across broad areas/regions. Low-level (ng/L-µg/L) PFAS are often detected in stormwater ponds and lagoons (Chen et al., Citation2023; Griffin et al., Citation2022) as well as treated wastewater effluents (Lenka et al., Citation2021; Thompson et al., Citation2022) in areas near and far from PFAS contaminated areas/sources. Several PFAS compounds, including perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) have been frequently detected in both urban and rural stormwater (PFOS: 38.7–44.1 ng/L; PFOA: 22.5–28.4 ng/L (Saifur & Gardner, Citation2021)). In some cases, up to 30 µg/L PFOS concentration in surface water and stormwater has been reported (Arcadis U.S. Inc, Citation2022; Casson & Chiang, Citation2018; Department of Defence, Citation2019).
Although PFAS concentrations in stormwater and surface water may be low relative to groundwater, the persistence and mobility of many PFAS suggests they can travel extended distances, potentially bioaccumulate within exposed organisms and enter the food chain, with potential adverse consequences for both flora and fauna with time (Arslan & Gamal El-Din, Citation2021). In addition, the effect of long-term exposure to low concentrations of many PFAS and/or mixtures is still not well understood.
In addition to the direct impact, it has been reported that contamination-impacted community residents may face many stressors, including pervasive uncertainty, future health worries, long-term impacts on day-to-day activities, financial uncertainty, and complex chronic social stressors (Banwell et al., Citation2021; Calloway et al., Citation2020). Hence, low-level PFAS often present in high volumes in surface water, groundwaters, and associated discharges will need to be removed and/or managed if they ultimately pose a long-term risk of human and ecosystem health. Calloway et al. (Citation2020) reported that taking visible and transparent action to address the PFAS contamination is recommended to reestablish and build trust with affected communities, consequently assists in reducing the psychosocial stressors in communities impacted by PFAS contamination.
Current strategies for treating PFAS-contaminated water mainly utilize large-scale and energy-intensive treatment systems that involve separation techniques (e.g. sorbents) (Merino et al., Citation2016). However, the performance of these treatment systems is still variable and mostly limited by the sorbents used, matrix composition (e.g., pH, electrical conductivity, dissolved organic carbon), PFAS chemicals, and concentration (Merino et al., Citation2016). Furthermore, installation of active treatment systems may be considered impractical for treating high volumes of low-level PFAS contamination in waters, particularly in regional areas that may not be able to afford highly technical solutions and associated maintenance. Conversely, passive remediation technologies for other contaminant classes have used natural processes, such as natural treatment media, to gradually remove contaminants from the environment through physical, chemical, and biological mechanisms. Examples include wetlands (natural and constructed), permeable reactive barriers, and natural attenuation (e.g., indigenous microorganisms) (Daraz et al., Citation2023). They often find application in situations with diffuse and wide-spread contamination problems (e.g., acid mine drainage (Acharya & Kharel, Citation2020)) requiring long-term, cost-efficient and low operational maintenance solutions. As challenges pertaining to PFAS contamination persist (i.e., achieving low regulatory guidelines for increasing the number of fluorinated compounds), exploring the viability of passive approaches for the long-term management of PFAS becomes imperative. The application of plants for the restoration of contaminated environments has been proposed as a promising green alternative to traditional physical and chemical methods (Suman et al., Citation2018).
Constructed floating wetlands (CFW, ) are one such technology which has gained increasing attention for its ability to remove contaminants from waters through natural processes to improve water quality, wildlife, and habitat (Ayres et al., Citation2022). CFW mimic the functions of a natural ecosystem by employing a nature-based approach to water treatment system design (Rigotti et al., Citation2020). CFW promote the growth of plant species in buoyant structures. Similar to hydroponic systems, the vegetation is not rooted in soil and this allows roots to grow freely in the water column (Ayres et al., Citation2022; Awad, Brunetti, et al., Citation2022).
Unlike traditional wetland system (having a soil bottom), CFW can be readily installed into existing urban environments (e.g. detention basins) without impacting flood storage capacity (Ayres et al., Citation2022), and with minimal requirements for earthworks and drainage adjustments (MacDonald et al., Citation2016; Schwammberger et al., Citation2019) that could potentially result in savings in civil drainage infrastructure costs. For example, MacDonald et al. (Citation2016) reported that the design for a traditional wetland required significant flow bypass infrastructure (for an urban development in Queensland, Australia) that was eliminated with the adoption of the CFW, resulting in approximately USD$1.3 million in savings for civil drainage infrastructure costs. Further, plants roots and shoots in a CFW system can be easily harvested and replaced, unlike traditional wetland system, offering a potential mechanism for sustainable removal of contaminants from the water. In a traditional wetland (a rooted vegetation system bedded in static soil media) system, plant uptake of long-chain perfluorosulfonic acids (PFSAs) is generally limited (He et al., Citation2023) as these compounds have greater molecular size and hydrophobicity, and consequently tend to be retained by soil particles (Mejia-Avendaño et al., Citation2020) and are less available for root uptake. However, a CFW uses floating structures to support the growth of plants in water, which allows the plant roots to absorb and/or adsorb contaminants from the water column, while also capturing contaminated-suspended particles (Schwammberger et al., Citation2019).
A crucial aspect of CFW design involves an approach to secure plant species for optimal growth and surface water contact. For instance, the Beemat® system employs biodegradable plastic aerator pots to anchor juvenile plants, which are subsequently placed into precut holes (White, Citation2021). Alternatively, other systems adopt a different approach, employing removable baskets filled with various media such as gravel, rock wool, scoria, and biochar (e.g., Clarity Aquatic system). Plants are positioned within the media and secured by additional substrate around the base or through pinning in place (Huth et al., Citation2021). The addition of sorptive substrates and the presence of biofilms () growing on the roots and water column could further enhance these removal processes of pollutants. In addition to contaminant removal, CFWs improve landscape amenity and can increase the socio-economic value of existing urban environments (MacDonald et al., Citation2016; Takavakoglou et al., Citation2021).
With the growing body of research on the use of plants for PFAS removal (Mayakaduwage et al., Citation2022), and interest in CFWs as a strategy to remove PFAS from contaminated water (Awad, Brunetti, et al., Citation2022), this review aims to assess the potential of CFW as a passive treatment technology for PFAS-contaminated water bodies and identify important considerations for their implementation. Whilst there are no field studies published on CFWs for PFAS, this paper specifically: (1) explores the current understanding of PFAS removal by wetland plants, microorganisms, and substrates, which are all integral components of CFW; (2) identifies key factors that may affect field implementation; (3) evaluates the strengths, weaknesses, opportunities associated with CFW for PFAS remediation; and (4) provides recommendations for future research to increase the application of CFW for PFAS water remediation to ensure the long-term protection of human and ecosystem health.
2. Constructed floating wetlands (CFW) for removal of pollutants
Over the past two decades, there has been a significant increase in the adoption of CFW as a novel nature-based water treatment technology (Colares et al., Citation2020). Also known as floating treatment wetlands (FTW) or artificial floating islands (AFI), CFW embody the principles of naturally occurring floating islands (Yeh et al., Citation2015). Unlike natural floating islands, which primarily serve ancillary purposes, CFW are intentionally designed to replicate the biological and biogeochemical processes found in traditional constructed wetlands (Shahid et al., Citation2018). These man-made structures are buoyant and rely on water column sustenance rather than soil for plant growth (). Supporting media (e.g., gravel) are used to anchor plants in place within CFW structures. CFW are designed with the ability to adapt to changes in flowrate and water level, which enables the systems to rise and fall accordingly, compensating for water level fluctuations. CFW have been used for the management of surface water, primarily for nutrient removal from different effluent types including stormwater (Awad et al., Citation2022), domestic wastewater (Huth et al., Citation2021), and runoff (Borne et al., Citation2014). CFW have also been reported to remove metal(loid)s (Afzal et al., Citation2019), pharmaceutical and personal care product compounds (Hwang et al., Citation2020) and pesticides (Hwang et al., Citation2021) from contaminated waters. To date, most studies using CFWs have been conducted at mesocosm and pilot scales, with limited demonstrations at field scale (Huth et al., Citation2021). An overview of the current knowledge on CFW technology has recently been reported (Ayres et al., Citation2022).
A significant advantage of CFW is their ability to continuously improve water quality without negatively affecting the surrounding environment or the landscape. CFW are designed with the ability to adapt to changes in flowrate and water level, can be retrofitted into existing waterbodies without impacting flood storage capacity and provide esthetic benefits makes them a suitable choice for sustainable water management. In the case of CFW, the continuous removal of contaminants is not a limitation since plant roots and shoots can be harvested and plants can be regularly replaced, allowing for an ongoing removal process (Awad, Brunetti, et al., Citation2022). Moreover, CFW exhibit versatility and adaptability as a treatment technology since they can effectively operate in systems characterized by fluctuating water levels (Ayres et al., Citation2022) unlike constructed wetlands and can be installed into existing water bodies without any impact on the waterbody storage capacity (MacDonald et al., Citation2016).
Further, using CFW could be also seen as a simple management approach to reduce greenhouse gas emissions from man-made water bodies. In freshwater systems, high nutrient levels can create ideal conditions for microbes to produce greenhouse gases through methanogenesis (which produces CH4), nitrification, and denitrification (both of which produce N2O) (Li et al., Citation2021). It has been reported that reducing dissolved nitrogen and phosphorus concentrations by 25% can decrease CH4 emissions from man-made water bodies on average by 50% (Ollivier et al., Citation2019). CFW are extensively reported to remove nitrogen (up to 90% removal of TN) and phosphorous (up to 98% removal of TP) from various water types (Ayres et al., Citation2022).
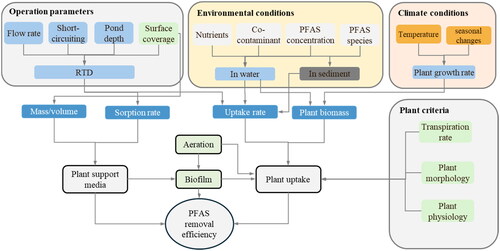
For a full CFW treatment system, contaminant removal depends on the fraction of water in the system that passes through a root zone, and the residence time distribution (RTD) within those root zones. As such, understanding the hydraulics of the entire pond and CFW system is crucial in order to assess and enhance contaminant removal efficiency (Colares et al., Citation2020; Lucke et al., Citation2019). Ponds and CFW are typically evaluated or designed based on a nominal hydraulic retention time (HRT), calculated as the volume divided by rate of discharge. However, a more comprehensive understanding of the hydraulics is provided by an RTD in the root zone which illustrates the probability of a water molecule remaining within the pond and in contact with the roots for a specific duration. RTD reflects the mixing that occurs within the CFW and can reveal phenomena such as short-circuiting, preferential flow paths and recirculation (Lucke et al., Citation2019). Short-circuiting of flow around and/or underneath the CFW decrease contact time with the plant roots and thus minimize treatment efficiency. Therefore, limiting short-circuiting of flows is crucial and can be achieved by better positioning the CFW or with flow diversion devices such as baffle curtains (Ayres et al., Citation2022). Water depth is another key aspect to be considered when designing CFW systems. Deep water allows for the formation of a free water zone beneath floating modules which minimizes the contact of roots, water and microorganisms. However, shallow water depths may lead to the attachment of roots to sedimented solids. Based on previous CFW studies on nutrient and metal removal, it is recommended to maintain a minimum water depth of 0.8–1.0 m to prevent plants from becoming rooted or anchored to the bottom whilst preventing the formation of a free water zone (Colares et al., Citation2020). Further, the water level also directly affects oxygen diffusion from the atmosphere as well as the HRT (Chen et al., Citation2016). Other factors that can influence the overall removal efficiency of the system such as temperature and plant age are further discussed in Supplementary information (SI) and are previously reported (Ayres et al., Citation2022).
Additional limitations for the CFW application exist. CFW generally require more time compared to alternative active treatment systems to achieve substantial contaminant removal. In general, longer HRT can enhance the efficiency of treatment by allowing more contact with contaminants. However, longer HRTs might not be compatible with site-specific conditions. For instance, if the treatment site has a limited available detention basin, extended HRTs may not be practical. Therefore, when designing CFW, it is essential to assess the benefits of longer HRT in terms of enhanced treatment efficiency against the associated costs and constraints, such as detention basin availability. As such, CFW may be useful for controlled flows in ornamental water bodies or community wastewater treatment schemes. However, their feasibility for scaling up to large-scale stormwater management systems remains uncertain. Moreover, certain climate conditions can potentially impact plant growth, further influencing the effectiveness of the treatment (Al-Baldawi et al., Citation2021). Variation in transpiration rates due to seasonal changes can also alter contaminant uptake rates by wetland plants (Williams, Citation2002).
To date, published research on CFW for PFAS is in its infancy. Most studies have focussed on laboratory-controlled plant uptake experiments, with few reports of ongoing field studies. Nevertheless, insights into factors that may influence its implementation including how a CFW system is designed could be informed by learnings from applications of CFW for other contaminants. In CFW, PFAS can be potentially removed from water through several mechanisms such as plant uptake, sorption to growth media, as well as accumulation in biofilms. However, as observed in nutrient or organic compound removal applications (Ayres et al., Citation2022; Colares et al., Citation2020; Lucke et al., Citation2019), various factors including plant species, PFAS type and concentration, environmental/solution conditions (e.g., presence of co-contaminants, organics, salinity, turbidity), and exposure time will influence plant removal efficacy. Nutrient concentration, climate, and vegetation coverage ratio will influence the available biomass for uptake (Ayres et al., Citation2022; Lucke et al., Citation2019) while the type, mass/volume and sorption capacity of plant growth media will influence PFAS removal via sorptive processes (plant growth media) (Awad, Hewa, et al., Citation2022; Awad, Brunetti, Juhasz, et al., Citation2022). Additionally, biofilm growth on plant roots and growth media can impact the removal of PFAS (Ma et al., Citation2023).
In the following sections, a critical examination of existing knowledge on PFAS removal pathways (via plants, growth media and biofilms) are detailed based on factors highlighted in , with particular emphasis on plant uptake as this is central to PFAS removal in CFW systems. Insights from various studies will provide a solid foundation for future research and practical implementation of CFW in PFAS remediation efforts.
3. PFAS removal via plants
Removal of PFAS from impacted water by plants is predominantly through phyto-uptake (passive via the apoplastic route or active through the symplastic route (Sima & Jaffé, Citation2021)) including bioaccumulation, and translocation mechanisms (Dalahmeh et al., Citation2018). A previous investigation demonstrated a strong correlation between PFAS concentration in plant roots and that in the water, implying that the uptake of PFAS by plants involves passive diffusion to some extent (Zhang et al., Citation2021). This is due to the considerable solubility of PFAS ions in water. Besides these passive mechanisms, various transporter proteins and ion channels are integrated into root cell membranes, enabling the active transportation of essential nutrients as well as PFAS from the environment into plant root cells (Wang et al., Citation2020). The contribution of active and passive processes to PFAS uptake varies depending on the concentration of PFAS and the length of its carbon chain. For example, Zhang et al. (Citation2019) indicates a higher percentage of active uptake at lower PFAS concentrations and shorter carbon chain lengths. However, Wang et al. (Citation2020) demonstrated that the uptake of non-ionized PFAS in plant roots is predominantly passive.
Although some recent research has reported the capacity of bacteria to transform/degrade selected PFAS (Grgas et al., Citation2023), limited reports of plant-facilitated PFAS degradation are available. However, the extent of plant-PFAS removal from the environment is directly influenced by factors including plant species, environmental conditions, PFAS physicochemical properties, concentration and exposure time (i.e., hydraulic retention time) (Arslan & Gamal El-Din, Citation2021; Zhang & Liang, Citation2020); these factors will be discussed in subsequent sections.
3.1. Effect of plant species on PFAS bioaccumulation
A comprehensive literature review was performed using Google Scholar, Scopus, and Web of Science databases, focusing on data from 2012 to 2023 using the keywords “Floating Treatment Wetlands”, “PFAS”, “wetland species” and “plant uptake”. Information gathered from twenty-four plant uptake studies utilizing wetland plants for the treatment of PFAS from wastewater, stormwater, halogenated solutions, and synthetic waters is summarized in Table S1, SI for the hydroponic studies, and Table S2, SI for the soil-based studies.
To date, studies on the phytoremediation of PFAS in wetland systems (includes constructed wetlands) have primarily focused on laboratory investigations of fourteen wetland plant families (Figure S1a), covering thirty-seven species. Among these, Poaceae (grasses), Cyperaceae (sedges), and Juncaceae (rushes) families have been studied more extensively likely due to their prevalence in wetland ecosystems. Further, Poaceae and Juncaceae have been reported to have the ability to thrive in challenging environments, generate significant biomass, exhibit rapid growth, and accumulate toxic metals in shoots (Patra et al., Citation2021). Some species of sedges (e.g., Cyperus) are fast-growing aquatic wetland weeds and can tolerate environmental stress factors (i.e., hot and cold weather), tolerate salt stress, and bioaccumulate toxic heavy metals (Mishra et al., Citation2016).
Grasses (Arundo donax, Phragmites australis, Bromus diandrus, Cynodon dactylon, Festuca rubra, and Schedonorus arundinaceus), which are dominant in dry open habitats, have been the subject of most studies, followed by sedges (Baumea articulata, Carex comosa, Cyperus alternifollus, Cyperus congestus, Cyperus papyrus, Eleocharis dulcis, and Schoenoplectus. corymbosus) and rushes (Juncus effusus, Juncus krausii, and Juncus sarophorus) that thrive in wetter regions.
Indeed, these plants are good candidates for uptake of PFAS due to their fast growth, high biomass production, and fibrous root systems—with the latter providing a large surface area for contact with contaminants and hence can potentially facilitate greater contaminant uptake (Surriya et al., Citation2015; Schwab, Citation1998). The high number of studies on grasses is not surprising given their wide distribution and abundance compared to sedges and rushes (Christenhusz & Byng, Citation2016), and their use in pioneering phytoremediation research (Rabêlo et al., Citation2021). However, it is important to note that the biomass production of grasses can significantly reduce or even stop due to low temperatures (Rabêlo et al., Citation2021).
Comparing the efficiency of PFAS removal across different plant species in existing literature is challenging due to differences in exposure conditions (such as matrix type, media, exposure time, and scale) and the concentration and type of PFAS tested (Tables S1 and S2, SI—tables of different studies with removal efficiencies, translocation factors and concentration in roots and shoots by wetland species in hydroponic-based and soil-based conditions, respectively). Uptake rates of various PFAS species by roots and shoots from hydroponic studies are shown in . From these studies, root and shoot PFAS bioconcentration factors (BCF) (as reported for PFOA and PFOS) were calculated for grasses, rushes, and sedges from hydroponic studies (Figure S2) to illustrate the variability in PFAS-specific plant uptake
Table 1. Uptake rate (µg/g of plant tissue) of PFASs by wetland species in hydroponic studies.
Table 2. Correlation between PFAS bioaccumulation and translocation factors and plant physiological characteristics.
Among the limited number of studies assessing multiple plant families (Awad, Brunetti, et al., Citation2022; Qiao et al., Citation2021; García-Valcárcel et al., Citation2014), PFAS removal efficiency (as reported for PFOA and PFOS) was consistently higher in grasses (mean: 53% and 42%), followed by sedges (29% and 24%), with poor PFAS removal performance by rushes (5% and 5%) relative to grasses and sedges in one study (Awad, Brunetti, et al., Citation2022). In hydroponic studies, PFOS BCF in grasses and sedges (roots and shoots) were ∼6–9 and ∼6–10 fold higher compared to rushes. Similarly, for PFOA, BCF in grasses and sedges (roots and shoots) were ∼10 and ∼3 fold higher compared to rushes as shown in Figure S2.
This could be attributed to the enhanced phytoextraction capabilities of grasses and their resilience in harsh environments (Arslan & Gamal El-Din, Citation2021; Khan et al., Citation2013). Helophytic grasses (such as Phragmites australis), in particular, can release atmospheric oxygen into their root zone, creating a cone-shaped oxic zone around root tips that enhances rhizosphere redox reactions which potentially improves PFAS uptake (Syranidou et al., Citation2017). It has been previously reported that many PFAS species have high surface activity, consequently, PFAS could be absorbed onto the molecular oxygen, which are then separated from the solution (Lee et al., Citation2017) and become more available to the plant. Further, this could increase aerobic microbial actions that leads to the transformation of PFAS precursors in the presence of molecular oxygen (Arslan & Gamal El-Din, Citation2021).
It is important to note that performance could also be species specific and that different species within the same plant family may exhibit different behaviors. For example, in a hydroponic study, Qian, et al. (Qian et al., Citation2023) reported significant variation in PFAS removal efficiency (0.9–19.3% of 200–300 µg PFAS−1) by five fern species. Translocation factors also varied between the plants (0.5–10) as shown in Table S1. Similar findings have been reported for edible crops (Ghisi et al., Citation2019; Lewis et al., Citation2022) whereby root and shoot biomass (He et al., Citation2023), transpiration rate (Wang et al., Citation2020), protein content (Wen et al., Citation2016), and lipid content (Armitage et al., Citation2012; He et al., Citation2023; Shi et al., Citation2018) appear to influence the PFAS accumulation in plant tissue. Although this is yet to be determined in wetland plant families/species, it is expected that these parameters will also affect plant-specific PFAS uptake in wetland varieties. Consequently, when designing CFW, site-specific environmental conditions need to be considered to support maximum PFAS plant uptake.
3.2. Effect of PFAS concentration, and exposure time on plant uptake
PFAS uptake by plants will be influenced by the physicochemical properties of the PFAS– its perfluorocarbon chain length and head group functionality (He et al., Citation2023; Qian et al., Citation2023). PFAS uptake by roots involves two main processes i.e., the equilibration stage where the concentration of PFAS in the aqueous phase of the plant roots reaches a balance with the concentration in the surrounding solution and the sorption stage where the PFAS is absorbed onto lipophilic roots. PFAS uptake by the root system is associated with the hydrophobicity and molecular weight size of the compound. PFAS possess a polar head, enabling them to enter plant cell membranes after sorption onto the root surface (Bolan et al., Citation2021). Perfluorocarbon (CF2) chain length plays a significant role, with short-chain compounds capable of passive diffusion through the lipid bilayer of plant cells (Arslan & Gamal El-Din, Citation2021) while long-chain PFAS require proteins to serve as transport systems due to the natural barrier posed by membranes (Zhang et al., Citation2019).
To date, most studies on wetland plants have been focused on legacy PFOA, PFOS, and PFHxS which were the first compounds to be regulated in many jurisdictions and listed under the Stockholm Convention (UNEP, Citation2021). More recently, other studies have investigated plant-PFAS interactions with short and long chain perfluoroalkyl acids (PFAAs), and ether-PFAS that are legacy PFAS replacement compounds (see and Fig. S3).
Some comparisons can be made for PFOA and PFOS that have been assessed in the majority of studies (24 wetland species in hydroponic studies) as shown in Fig. S4. In hydroponic experiments, PFOS has been found to predominantly accumulate in the roots (Fig. S4a), while PFOA may translocate to the shoots (Fig. S4b). Although these PFAS differ in their CF2-chain length (7 for PFOA and 8 for PFOS), the trend where perfluorocarboxylic acids (PFCAs) are taken up and translocated to the shoots to a greater extent than perfluorosulfonic acids (PFSAs) is consistent with other reports for different plants (Krippner et al., Citation2015). These findings are consistent with the n-octanol-water partition coefficient (log Kow) of these compounds (PFOA 5.3, PFOS 6.3 (Webster et al., Citation2010), showing that the more hydrophobic PFAS tend to concentrate in the roots. In addition to differences in PFAS root to shoot translocation, some differences in partitioning within sub-cellular components have been observed. Wang et al. (Citation2020) reported that for Alisma orientale, PFOA was found in the water-soluble fractions of roots, stems, and leaves, while PFOS was primarily found in the cell walls of roots and leaves.
Considering plant families highlighted above, PFOA and PFOS concentration in plant shoots follow the same trend of grasses > sedges > rushes (mean values of 3.5, 0.6, and 0.06 µg/kg for PFOA and 3.4, 0.8, and 0.05 µg/kg for PFOS respectively). PFOA and PFOS translocation factors (TFs) were also found to follow the same trend of grasses > sedges > rushes (mean values of 7.8, 3.4, and 2.7 for PFOA and 3.6, 0.9, and 0.8 for PFOS). Reported PFOS BCF and PFOA BCF values in hydroponically grown grasses and sedges were higher compared to soil-based wetland grasses and sedges (more details are presented in SI).
Trends in PFAS accumulation in different plant compartments are less clear when considering multiple PFAS with different functional head groups (i.e., PFCA, PFSA, fluorotelomers-FtS, and perfluoroalkyl ether acids-PFEA) as seen from limited studies that investigated the uptake of a wider range of PFAS. In a study on 13 PFAS using five fern species, Qian et al. (Citation2023) reported that bioconcentration factors of PFCAs in roots and shoots/leaves exhibited a U-shaped dependency with increasing CF2-chain length (minima at CF2 = 5). PFSA and FtS bioconcentration factors increased in roots as CF2-chain length increased, although it decreased in shoots with increasing CF2-chain length. In contrast, in the study by Zhi et al. (Citation2022), the U-shaped dependency between bioconcentration factor of PFCAs and CF2 chain length was only observed in the roots of four common urban weeds. The distribution of PFAS (i.e., PFCAs, PFSAs, FtS, and PFEA) in shoots was also evenly spread without any discernible trend for most groups. The only exception was observed in one plant species (i.e., Aster indicus) and pertained solely to PFCAs where bioconcentration factors decreased with increasing CF2-chain length. Over-all, as highlighted by Qian et al. (Citation2023) the uptake of PFAS by the root system correlated with PFAS molecular structure. Regardless of the PFAS class, uptake in the roots tended to increase with increasing PFAS molecular weight, logarithmic n-octanol/water partition coefficient and van der Waals surface area, while PFAS translocation was negatively correlated with these characteristics. Consequently, in order to effectively treat long-chain PFAS, root harvesting is essential. In the case of CFW, the continuous removal of long-chain PFAS species is not a limitation since plant roots and shoots can be harvested and plants can be regularly replaced, to address the limitation of long-chain PFAS translocation.
In a study by Felizeter et al. (Citation2014) on hydroponically-grown crops, it was suggested that PFAS branched isomers occupy a smaller molecular volume than linear forms which resulted in decreased sorption onto root surfaces and subsequently decreased uptake. However, this phenomenon is yet to be investigated in wetland plants. A recent study (Zhang et al., Citation2021) comparing the uptake of different PFAS replacement compounds by one sedge species (Carex comosa) showed higher uptake of short-chain compounds compared to their long-chain homologues with uptake greater for PFCAs compared to PFSAs. Some exceptions to this were observed, specifically for PFMOPrA, which accumulated at lower concentrations than PFMOBA, Gen, and ADONA, despite having the shortest chain lengths and highest water solubility. The authors suggested that factors such as interactions with phospholipids (phospholipid partitioning) and PFAS structure (i.e., linear vs. branched) may also contribute to the observed results (Zhang et al., Citation2021). Previous soil-based studies have also observed a preference for the uptake of short-chain PFAS by plants (Blaine et al., Citation2014; Lan et al., Citation2018). These results indicate that the accumulation and translocation of specific PFAS in plants was influenced by their molecular size and hydrophobicity. PFAS with longer hydrophobic chains and larger molecular sizes have a tendency to be adsorbed and sequestered on the root epidermis or within the rhizosphere. On the other hand, PFAS with shorter hydrophobic chains and smaller molecular sizes are readily absorbed by roots, rapidly transported upwards, and stored in shoots. Note that in soil, plant uptake of long-chain PFSAs (e.g., PFOS) is generally limited (He et al., Citation2023) as these compounds have greater molecular size and hydrophobicity, and consequently tend to be retained by soil particles (Mejia-Avendaño et al., Citation2020) and are less available for root uptake.
Generally, increasing PFAS exposure concentration results in an increase in PFAS-tissue accumulation although the response is not linear. For hydroponically grown Juncus krausii, Awad, Brunetti, et al. (Citation2022), showed that a 10-fold increase in PFOS solution concentration (between 0.2 and 30 µg/L) resulted in a 5-fold and ∼2-fold increase in root and shoot accumulation respectively, while the overall removal efficacy by the plant decreased from ∼19% to ∼5%. A similar trend was also observed for hydroponically grown Juncus effusus (Zhang et al., Citation2019) where a 10-fold increase in PFOS solution concentration (4,300 vs 43,000 µg/L) led to a 5-fold and 8-fold increase in concentration accumulated in root and shoot tissue respectively and decrease in the overall efficacy removal by the plant from 12% to 7%. Note that these values are typically lower than those reported for wetland plants grown in soil (e.g., for Typha latifolia: 1.5-fold and 1.2-fold increases for a 10-fold increase in PFOS exposure concentration (Zhang et al., Citation2020)) which was likely due to PFAS sorption to soil decreasing the phytoavailable fraction.
A similar trend was also found for PFOA where increasing PFOA solution concentration increased PFOA accumulation in hydroponically grown plants (Juncus krausii: 10-fold and 2-fold increase in PFOA in roots and shoots respectively (Awad, Brunetti, et al., Citation2022)) compared to wetland plants grown in soil (for Typha latifolia: 1.1-fold and 1.2-fold increase in roots and shoots respectively for a 10-fold increase in PFOA exposure concentration (Zhang et al., Citation2020)). The overall removal efficacy was also decreased (∼11%–5% (Awad, Brunetti, et al., Citation2022)). Consequently, higher HRT or larger surface coverage by plants are necessary to achieve similar removal efficiency for high-level PFAS contaminated waters compared to water containing less elevated concentrations.
However, PFAS exposure may induce phytotoxic effects at the biochemical level including enhanced generation of reactive oxygen species (ROS) leading to oxidative damage (lipid peroxidation, DNA damage and changes in enzymatic and non-enzymatic antioxidant activity/content) (Lin et al., Citation2020; Li et al., Citation2022). Although low PFAS concentrations (<100 μg/L) are unlikely to cause inhibitory impacts on plant physiology, threshold limits for PFAS-related toxic impacts on wetland plant functionality are yet to be determined. However, an advantage of CFWs is that if physiological or biochemical effects occur, plants can be removed and replaced to facilitate continued PFAS removal.
3.3. Effect of plant physiology on PFAS bioaccumulation
Plant root characteristics may influence the accumulation and translocation of PFAS as uptake begins with adsorption on root surfaces. This is followed by PFAS transport to root epidermal cells, and subsequent radial movement to the cortex, where vascular bundles transport some PFAS throughout the plant (Arslan & Gamal El-Din, Citation2021). In a hydroponic study using five fern species, Qian et al. (Citation2023) reported that the macroscopic morphology of the root system impacted PFAS uptake. Root length, projected area, surface area, and surface area per unit length showed a significant positive correlation with root PFAS concentration and bioaccumulation factors (see ). Conversely, the average root diameter showed a negative correlation with translocation and bioaccumulation factors. This was supported by results from the study of He et al. (Citation2023) where a positive correlation between root length and bioaccumulation factor was observed in seven weed species in addition to a negative correlation between average root diameter and translocation factor (). However, the authors found no correlation between root microstructure (i.e., the thickness of the epidermis, endothelial layer, xylem or vascular column) and PFAS bioaccumulation (He et al., Citation2023). The relationship between root diameter and PFAS root bioaccumulation was also observed by Zhi et al. (Citation2022) in a hydroponic study using four urban plant species, where plants with finer roots had higher root bioaccumulation factors. These results indicate that plant species characterized by extensive root systems with thinner, more fibrous roots providing large root surface area and a greater contact area with the water are likely to be more suitable for PFAS phytoextraction.
Plant growth rate is another factor that affects PFAS uptake by plants. Fast-growing plant species with high-biomass production are likely to be more suitable for phytoextraction (Suman et al., Citation2018). In the study by He et al. (Citation2023) weed species with the highest plant biomass also exhibited the highest PFAS uptake. It was also identified that characteristics of the recovered biomass, particularly the specific leaf area, was negatively correlated with PFAS concentration and bioaccumulation factors in roots and shoots, a trend also reported for urban weed plants by Zhi et al. (Citation2022). Indeed, plant species characterized by small leaf areas have been reported to exhibit shorter leaf lifespans and higher relative growth rates (Wright & Cannon, Citation2001). Therefore, these plants may experience a faster and more significant accumulation of PFAS through transportation within their vascular system and be more favorable for PFAS uptake and storage.
Several studies reported that plant physiological properties, such as lipid and protein content, appear to influence plant uptake and bioaccumulation of PFAS (García-Valcárcel et al., Citation2014; Wen et al., Citation2016). In seven agriculture plants (i.e., maize, soybean, radish, mung bean, lettuce, alfalfa and Italian ryegrass), Wen et al. (Citation2016) observed a positive correlation between root protein content and PFAS bioaccumulation, whereas root lipid content showed a negative correlation. Further, PFAS translocation was found to correlate positively with the shoot to root protein content ratio. This is particularly apparent for long-chain PFAS, indicating that these species likely rely on proteins as transport systems. According to Zhang et al. (Citation2019), an energy-dependent active transport facilitated by protein carriers is the primary mechanism for the uptake of perfluoroalkyl acids (PFAA) in wheat (Triticum acstivnm L.).
In other studies, the relationship between plant physiological properties and the transport and bioaccumulation of PFAS was not observed. He et al. (Citation2023) reported that both root protein and lipid content were significantly positively correlated with bioaccumulation factors in roots and shoots while Qian et al. (Citation2023) observed no correlation between these parameters. Ng and Hungerbühler (Citation2014) suggested that PFAS transport and bioaccumulation may be dependent on the specific type of protein (e.g., albumin and structural proteins) or lipid (e.g., neutral triglycerides and carbohydrates and polar phospholipids) and not the overall lipid/protein content. These inconsistent results indicate that additional studies are required to better understand the function and role of specific protein and lipid types on PFAS plant uptake (He et al., Citation2023).
To better understand which families and/or species are most effective at taking up PFAS, future studies should consider a wider range of plant species from diverse families exposed under the same conditions. Currently, considering studies under the same exposure matrix (Hoagland’s solution), the evidence suggests that the plant families that have been considered in these studies can remove PFAS by 1.2–35% (median removal of 15%) under hydroponic conditions.
4. PFAS removal via microorganisms
Microorganisms are well recognized as having critical roles in biogeochemical cycles (e.g., carbon, nitrogen, phosphorus) and degradation of pollutants in wetlands (De Mandal et al., Citation2020; Overton et al., Citation2023; Ravikumar et al., Citation2022; Wang et al., Citation2022). Waterlogging or intermediate stages of flooding and the presence of plant roots can create oxic and anoxic conditions in wetlands suitable for the growth of both aerobic and anaerobic microbial communities. In CFW, aerobic and anaerobic conditions may be present in root zones dependent on the media type and characteristics (e.g., small pores can promote increase surface area and adsorption, and microbial mediated oxic - anoxic zones) and its configuration (e.g., depth, circulation, aeration). The physiological properties of wetlands (e.g., pH, electron activity, salinity, nutrients) will influence the abundance and diversity of microorganisms/communities present in the water column, sediment, and associated with biofilms in water and roots (De Mandal et al., Citation2020; Wang et al., Citation2022).
In a review by Wang et al. (Citation2022) on constructed wetlands the authors identified the phylum Proteobacteria, Bacteroidetes, Actinobacteria, and Firmicutes as important functional microorganisms for the removal of pollutants likely by catalyzing chemical reactions, biodegradation, and biosorption processes. In CFW, microbial communities may be manipulated in the system thereby influencing remediation efficacy (e.g., manipulating communities and biofilms) (Sheth et al., Citation2016; Shi et al., Citation2022). This could be achieved through the addition of substrates (e.g., compost and porous substrates (Meng et al., Citation2014)) to stimulate microbial growth, the addition of sorptive phases to facilitate biofilm formation or bioaugmentation of specific microorganisms for nutrient cycling/degradation of organic constituents.
Although naturally occurring or inoculated microorganisms have been shown to be successful in the bioremediation of different types of organic pollutants (e.g., polycyclic aromatic hydrocarbons, organic solvents) (Gupta & Pathak, Citation2020; Megharaj et al., Citation2011), their role in PFAS bioremediation/defluorination remains a complex and debated topic (e.g. (Liu et al., Citation2023; Liu & Mejia Avendaño, Citation2013)). Microbial biotransformation or defluorination of PFAS has been reported in the literature mainly for polyfluorinated compounds (dependent on head group/unsaturation) under aerobic and anerobic conditions (Grgas et al., Citation2023; LaFond et al., Citation2023; Liu & Mejia Avendaño, Citation2013; Yu et al., Citation2022; Zhang et al., Citation2022). Until recently there has been little reported defluorination of saturated perfluorinated structures (i.e., PFOA and PFOS) (Yu et al., Citation2020). Yu et al. (Citation2020) reported C − F bond cleavage in unsaturated perfluorinated structures (e.g., perfluoro-3,7-dimethyloctanoic acid (PFdiMeOA) and (E)-perfluoro (4-methylpent-2-enoic acid) (PFMeUPA)). However, further research is needed to confirm these findings (e.g., fluorine mass balance, detection of intermediates and final products in proposed transformation pathway(s)) and elucidate the relevance/importance of specific microorganisms (i.e., Acidimicrobiaceae bacteria) in PFAS defluorination pathway(s) in nature. However it is also important to note that microorganisms present in CFW may be able to transform precursor compounds to persistent terminal PFAS end-products (LaFond et al., Citation2023).
The defluorination of polyfluorinated compounds has been suggested to be associated with HF elimination at the α and β positions for polyfluorocarboxylic acids (e.g., fluorotelomer alcohols (FTOHs)) and desulfonation for polyflurosulfonic acids (e.g., fluorotelomer sulfonic acids (FTS)) (Yang et al., Citation2022; Wang et al., Citation2005; Wang et al., Citation2011). Yang et al. (Citation2022) reported under anaerobic conditions α, β-unsaturation was critical for the biotransformation (via reductive defluorination and/or hydrogenation) of fluorinated carboxylated acids. These authors also highlighted the enhanced degradability and defluorination capacity of unsaturated fluorinated carboxylated acid structures (i.e., trimethyl branches at α, β-carbon). A correlation has been reported between the amount of PFAS defluorination (branched PFAS), the local C-F bonding environment and calculated bond dissociation energy (Liu et al., Citation2018). Liu et al. (Citation2018) found calculated bond dissociation energies increased from tertiary C-F bonds < secondary C-F bonds < primary C-F bonds. Yu et al. (Citation2020) suggested unsaturated fluorinated substances are more bioavailable to microorganisms with the first cleavage of the C-F bond at the sp2 position critical that could lead to further defluorination in the environment.
Wetland plant roots (hanging down into the water column or in sediments) can act as a natural filter for contaminant removal and/or provide surface area for enhanced growth of microorganisms and biofilms formation (Overton et al., Citation2023; Ravikumar et al., Citation2022). Microorganisms in biofilms may enhance the biotransformation or defluorination of PFAS (as described above) and/or increase the accumulation/retention of PFAS (or transformation by-products) on the root zone for potential uptake by plants. Depending on the specific PFAS and plant species, there is a possibility that PFAS sorbed to biofilms could be taken up by plant roots. However, physical disturbances or changes in environmental conditions may lead to the disruption of biofilms resulting in the release of sorbed PFAS back into the water. Research focused specifically on the accumulation of PFAS in biofilm and biofilm-plant relationships is required to elucidate the consequence of changes in water quality on the dynamics of biofilm communities that could lead to the mobilization of PFAS.
Fu et al. (Citation2023) found increased retention of PFOA in biofilm-coated sand columns compared to uncoated sand columns. This was likely because the biofilm promoted hydrophobic interactions and reduced repulsion between the negatively-charged PFAS and uncoated sand due to decreased surface charge. In a study by Zhang et al. (Citation2022), the authors reported colonized biofilms on rocks effectively bioaccumulated PFAS from water and biomagnified PFAS to freshwater snails that highlighted the important role biofilm can play in PFAS trophic transfer in aquatic environments.
Microbial biotransformation or defluorination of PFAS may be enhanced through coupling with innovative microbial electrochemical technologies in wetlands (Ji et al., Citation2020; Ruiz-Urigüen et al., Citation2022; Wang et al., Citation2020). In a recent study by Ji et al. (Citation2023), the authors demonstrated the potential for a constructed wetland-microbial fuel cell system to efficiently (>96%) remove PFOA and PFOS from wastewaters through adsorption to electrode materials (PFOA: ∼350 ng/g of Anode GAC; PFOS: 484 ng/g of Anode GAC) and uptake by plants (PFOA: 49 ng/g of plant leaf & 33 ng/g of plant stem; PFOS: 33 ng/g of plant leaf & 16 ng/g plant steam). Furthermore, microbial electrochemical systems have the potential to enhance the removal of PFAS (e.g., enhanced biodegradation or mobilization into water column) from contaminated sediments in wetlands (Cao et al., Citation2023; Li et al., Citation2017), predominantly though sorption to the electrode material. However, scaling up microbial electrochemical systems for large surface areas may present challenges. Achieving uniform distribution of electric fields and electrodes across extensive surfaces can be logistically complex and may require substantial resources.
5. PFAS removal via plant growth media
CFW technologies with removable plant baskets (as detailed in the introduction section), can be preestablished with removable sorptive materials such as granular activated carbon (GAC) or biochar in combination with gravel to secure plants in place. Additional measures such as the inclusion of removable sorptive materials in CFW could also be a means to further remove PFAS from the solution (Yin et al., Citation2017). Although long-chain PFAS species can accumulate in the roots and shoots of plants as summarized above, it has been reported that these compounds are preferentially removed by sorptive processes (Arslan & Gamal El-Din, Citation2021). Considering the propensity of plants to take up short chain PFAS (as summarized above) and the demonstrated ability of sorbents such as GAC and biochar to sorb long chain PFAS, a combined approach with plants and sorbents may be an efficient approach to enable the removal of a wider range of PFAS.
Currently, there is a variety of sorbents available in the market that have been used for PFAS and could be incorporated in CFW systems (Lei et al., Citation2023). Considering the range of sorbents available, methylene blue surface area (mesopores) has been identified as a reliable indicator of their effectiveness for removal of PFAS in water and could assist in selecting appropriate sorbents for use in CFW, i.e., sorbents with higher surface area would imply more available sites for sorption (Kabiri et al., Citation2023). Introducing sorbents to CFW may affect plant performance in that sorbents can reduce the availability of long chain PFAS, that may reduce the PFAS load on the plant and promote the uptake of species that are less sorbed to activated carbon and biochars. The sorbents may also decrease the nutrient load of the CFW, potentially impacting plant growth, although some studies have shown either positive effects or no discernible impact on wetland plants (Olsen et al., Citation2018). Hence, the choice of sorbent should also consider its capacity to support plant growth and provide sufficient sorption sites for PFAS.
The porous nature structure of biochar and activated carbon not only serves as an adsorptive matrix for PFAS (Kabiri et al., Citation2023) but also provide physical support for microbial colonization and biofilm formation, creating a bioactive zone around plant roots (Qi et al., Citation2022). These biofilms can act as a biological interface, facilitating the binding and uptake of waterborne contaminants, including PFAS (Ji & Zhao, Citation2024), through multiple mechanisms, including electrostatic, cation exchange, complexation, hydrophobic and micropore filling interactions (Li et al., Citation2021). Over time, PFAS may accumulate within the root zone, enhancing its uptake by plants. As biofilms form and mature, removal of PFAS in CFW may improve due to the synergistic interaction between plant roots, biochar/activated carbon, and biofilm. While this process is complex and requires further investigation regarding PFAS, it underscores the potential of hydroponic systems in promoting passive water remediation by improving contaminant uptake. More detailed discussion on the role of biofilms in phytoremediation and the plant-biofilm-sorbent approach for remediation, are previously provided (Chojnacka et al., Citation2023; Sharma, Citation2022). An example from a constructed wetland system is presented in the SI.
6. Conclusions and recommendations for future work
CFW are a potential green, passive remediation strategy for removing PFAS from contaminated waters. This treatment approach lies in the adaptability to remove PFAS via biological (plant) and physicochemical (sorptive media—sorbents and biofilms) mechanisms. In light of the preceding discussion, the selection of plants emerges as critical for maximizing remediation efficiency, given that plant uptake serves as the primary mechanism for PFAS removal. In practice, plants could be chosen based on the properties that generally show high PFAS uptake (e.g., high protein and lipid content, high root surface area, see ) and what is available/local to a particular region. The integration of sorbents may be important at sites with elevated concentrations of long-chain PFAS to improve over-all PFAS removal, given these are generally less taken up by plants. Sorbent selection should prioritize effective PFAS removal while ensuring that sufficient nutrients remain available to support plant growth. At this stage, the impacts of microbes at the field-scale are unclear, as these will be influenced by plant/sorbent choices and could vary from site to site. In the field, hydraulic retention time, and surface coverage are two critical design considerations that will play integral roles in determining the overall efficiency of CFW for PFAS removal. These factors should be tailored to the specific installation site and remediation objectives to optimize the system’s performance. Unlike traditional wetland systems, plant roots and shoots in CFW systems can be readily harvested and replaced, offering a mechanism for the sustainable removal of contaminants from water.
Despite the potential of CFW for PFAS removal, several challenges persist which need to be addressed to transition from successful laboratory-oriented results to full-scale treatment implementation (summarized in Table S3, SI). These knowledge gaps are detailed below:
Environmentally relevant exposure: To date, the majority of hydroponic studies investigating the PFAS uptake by wetland plants have utilized synthetic nutrient solution (e.g., Hoagland’s solution, synthetic irrigation water, and synthetic wastewater; see Table S1, SI). However, studies conducted under environmentally relevant matrices, such as effluents, surface waters, and groundwaters, are less common. Whilst studies utilizing synthetic solutions provide better control over environmental exposure conditions, they may not accurately reflect conditions that wetland plants encounter. Further, water matrices consist of a diverse array of constituents, incorporating both organic and inorganic components, that collectively shape the chemical composition of water. In addition to PFAS, CFW can remove other co-contaminants (e.g., pharmaceutical and personal care product compounds, heavy metals and nutrients) from the water via plant uptake (Afzal et al., Citation2019; Hwang et al., Citation2020; Hwang et al., Citation2021) or sorptive processes. However, the effect of co-contaminants on the efficiency of CFW to remove PFAS from contaminated waters is unclear. Understanding the efficiency of CFW using field-impacted media containing PFAS mixtures and common environmental treatment variable (e.g., water quality parameters, co-occurring chemicals of concern, etc.) is a critical data gap. Further, the uptake of emerging fluorinated alternatives, such as GenX, F-53B, and ADONA, by floating wetland species is not well studied, emphasizing the need for further research in this area.
Pilot/Field demonstration: To date, all studies using CFW for PFAS removal have been conducted at mesocosm and laboratory scales, with no demonstrations at field scale. From a field perspective, and as we discussed above, key design and operation parameters for CFW include RTD, plant surface coverage and water depth. These parameters and its effect on PFAS removal efficiency by CFW are not yet fully understood. Validating the performance of CFW systems is crucial for establishing confidence in the technology’s ability to achieve the desired clean up criterium. The validation process entails demonstrating that CFW can consistently produce water of the required quality across a defined range of environmental and operational conditions. Field-scale assessments are necessary to demonstrate that CFW can consistently treat water to the required standards under differing conditions.
Combined approaches: To reduce treatment time and to improve PFAS removal efficacy, the addition of sorbents or substrates that enhance PFAS sorption and plant/microbial growth in CFW systems should be considered. Combined approaches may provide a more effective strategy for removing a wider array of contaminants, including short- and long-chain PFAS, overcoming the limitations of each strategy if applied singularly. Furthermore, CFW coupling with other treatment approaches (e.g., aeration, floatation, bioelectric, and electrochemical) could further improve the efficiency of CFW systems.
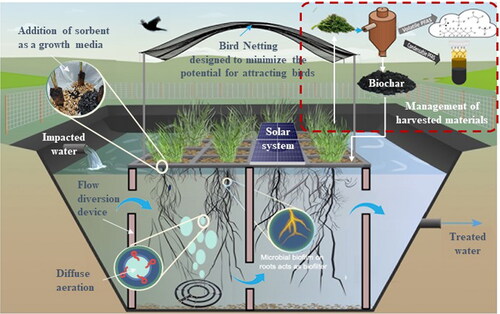
In hydroponic systems, the roots of plants typically concentrate in the upper layer of the water body, often leaving the lower layer unoccupied. This can result in untreated portions of the water since the treatment performance relies on the proportion of flow that traverses through the root zone, impacting the overall efficiency of the system. To concentrate PFAS in the root zone and remove water depth as a potential limitation, a diffuse aeration system could be used to push PFAS to the water surface where they can be rapidly adsorbed on biochar or GAC in support media or taken up by plants via roots (). Air bubbles act as carriers for surfactant PFAS, lifting them to the water’s surface (Morrison et al., Citation2023) alongside the root zone and plant media, thereby increasing the availability of PFAS for plant uptake. Aeration of the water could also increase the growth rate of the plants and support aerobic microorganisms and fungi in support media, which can increase the uptake of PFAS. To lower the energy burden of operating an aeration system, a solar-powered aeration system can be installed above the buoyant structures of CFW ().
However, additional investigations are required to determine how combined approaches can effectively improve contaminant removal, especially from the perspective of sorption capacity and plant compatibility under field conditions. Further, more research is required to identify and characterize plant and bacterial species, as well as substrates that offer effective plant-microbe interplay in CFW. Similarly, plant richness and various species growth forms could also be used to improve the efficiency of CFW. However, studies are yet to report on the effect of these factors for PFAS uptake by wetland plants.
Management of harvested PFAS-contaminated materials: To date, incineration is currently the only commercial full-scale technology available to destroy PFAS in solid and liquid waste streams. Thermal treatment by oxygen-deficient pyrolysis processes is emerging as a potential technology for PFAS destruction from biosolids (Navarro et al., Citation2023). Factors influencing the efficiency of PFAS destruction by pyrolysis include temperature, residence time, and the specific PFAS present. Pyrolysis processes could be also seen as a promising method for destroying PFAS from CFW plant biomass, which could produce PFAS-free biochar materials. However, more research is required to understand and optimize operational parameters to enhance the efficiency of pyrolysis in producing biochars with superior PFAS sorption capabilities. Further, critical knowledge gaps remain concerning the fate and transport of PFAS/fluorine in potential pyrolysis configurations and treatment conditions in order to demonstrate the readiness, viability, and level of safety of the process (Navarro et al., Citation2023). The resulting biochar may have the potential to be used as a sorbent within CFW () or elsewhere instead of entering waste streams. However, further studies are needed to assess the long-term stability and environmental impact of these biochars.
Minimize exposure pathways: Although the concept of CFW has been suggested as a means to provide habitats that support biota, including amphibians, birds, fish and macro-invertebrates (Colares et al., Citation2020), it may not be beneficial as transfer of PFAS to the food chain from contaminated plants could threaten ecosystem and human health. When contaminants migrate to above-ground plant tissue, there is a potential for their entry into the terrestrial food web through consumption by land-dwelling herbivores (Hammill et al., Citation2022). Subsequently, the risk of bioaccumulation or biomagnification arises as predators consume prey impacted with these substances through consumption. Hence, ensuring restricted access to CFW for birds and aquatic biota is an essential design consideration. Employing commonly used netting (e.g., 7 × 1.5mm cross woven aperture crop protection net) could be a viable solution to address this concern and minimize potential impacts (Ayres et al., Citation2022). However, to date, studies are yet to be conducted to evaluate the effectiveness of CFW in mitigating water contaminants while minimizing adverse impacts on surrounding ecosystems. Understanding trophic transfer pathways in CFW is essential for assessing potential risks associated with PFAS exposure to higher trophic levels, including predators that inhabit the CFW plant communities (e.g., damselflies, dragonflies, and spiders).
Acknowledgements
The authors gratefully acknowledge the financial support provided by the Commonwealth Scientific and Industrial Research Organization (Strategic project: OD227455) and Salisbury Water, South Australia (project ID: C035229).
| List of Acronyms | ||
| 4:2 FtS | = | 4:2 Fluorotelomer sulfonic acid |
| 6:2 FtS | = | 6:2 fluorotelomer sulfonate acid |
| AFI | = | Artificial floating islands |
| BCF | = | Bioaccumulation factor |
| CFWs | = | Constructed floating wetlands |
| FTW | = | Floating treatment wetlands |
| FtS | = | Fluorotelomers |
| GAC | = | Granular activated carbon |
| HRT | = | Hydraulic retention time |
| PFAS | = | Per- and polyfluoroalkyl substances |
| PFCs | = | Perfluorinated compounds |
| PFMOPrA | = | Perfluoro-3-methoxypropanoic acid |
| PFMOBA | = | Perfluoro-4-methoxybutanoic acid |
| PFAA | = | Perfluoroalkyl acids |
| PFEA | = | Perfluoroalkyl ether acids |
| PFBS | = | Perfluorobutane sulfonate |
| PFBA | = | Perfluorobutanoic acid |
| CF2 | = | Perfluorocarbon |
| PFDA | = | Perfluorodecanoic acid |
| PFHpA | = | Perfluoroheptanoic acid |
| PFHpA | = | Perfluoroheptanoic acid |
| PFHxA | = | Perfluorohexanoic acid |
| PFOS | = | Perfluorooctane sulfonate |
| PFOA | = | Perfluorooctanoic acid |
| PFPeA | = | Perfluoropentanoic acid |
| PFSAs | = | Perfluorosulfonic acids |
| PFTeDA | = | Perfluorotetradecanoic Acid |
| RTD | = | Residence time distribution |
| ROS | = | Reactive oxygen species |
| TF | = | Translocation factor |
Revised_Supplementary information.docx
Download MS Word (118.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Acharya, B. S., & Kharel, G. (2020). Acid mine drainage from coal mining in the United States – An overview. Journal of Hydrology, 588, 125061. https://doi.org/10.1016/j.jhydrol.2020.125061
- Afzal, M., Rehman, K., Shabir, G., Tahseen, R., Ijaz, A., Hashmat, A. J., & Brix, H. (2019). Large-scale remediation of oil-contaminated water using floating treatment wetlands. Npj Clean Water, 2(1), 3. https://doi.org/10.1038/s41545-018-0025-7
- Al-Baldawi, I. A., Mohammed, A. A., Mutar, Z. H., Abdullah, S. R. S., Jasim, S. S., Almansoory, A. F., & Ismail, N. I. (2021). Application of phytotechnology in alleviating pharmaceuticals and personal care products (PPCPs) in wastewater: Source, impacts, treatment, mechanisms, fate, and SWOT analysis. Journal of Cleaner Production, 319, 128584. https://doi.org/10.1016/j.jclepro.2021.128584
- Arcadis U.S. Inc. (2022). Summary of characterization activities for Pond 1 at Ellsworth air force base (EAFB). U.S. Army Corps of Engineers.
- Armitage, J. M., Arnot, J. A., & Wania, F. (2012). Potential role of phospholipids in determining the internal tissue distribution of perfluoroalkyl acids in biota. Environmental Science & Technology, 46(22), 12285–12286. https://doi.org/10.1021/es304430r
- Arslan, M., & Gamal El-Din, M. (2021). Removal of per- and poly-fluoroalkyl substances (PFASs) by wetlands: Prospects on plants, microbes and the interplay. Science of the Total Environment, 800, 149570. https://doi.org/10.1016/j.scitotenv.2021.149570
- Awad, J., Brunetti, G., Juhasz, A., Navarro, D., Vanderzalm, J., Walker, C., & Beecham, S. (2022). Investigating phytoremediation coupled with sorption for remediation of PFAS-contaminated surface water. International Cleanup - 9th International Contaminated Site Remediation Conference, Adelaide, South Australia (pp. 345–346).
- Awad, J., Brunetti, G., Juhasz, A., Williams, M., Navarro, D., Drigo, B., Bougoure, J., Vanderzalm, J., & Beecham, S. (2022). Application of native plants in constructed floating wetlands as a passive remediation approach for PFAS-impacted surface water. Journal of Hazardous Materials, 429, 128326. https://doi.org/10.1016/j.jhazmat.2022.128326
- Awad, J., Hewa, G., Myers, B. R., Walker, C., Lucke, T., Akyol, B., & Duan, X. (2022). Investigation of the potential of native wetland plants for removal of nutrients from synthetic stormwater and domestic wastewater. Ecological Engineering, 179, 106642. https://doi.org/10.1016/j.ecoleng.2022.106642
- Ayres, J., Awad, J., Walker, C., Page, D., van Leeuwen, J., & Beecham, S. (2022). Constructed floating wetlands for the treatment of surface waters and industrial wastewaters. In N. Pachova, P. Velasco, A. Torrens, V. Jegatheesan (Eds.), Regional perspectives of nature-based solutions for water: Benefits and challenges (pp. 35–66). Springer International Publishing. https://doi.org/10.1007/978-3-031-18412-3_3
- Banwell, C., Housen, T., Smurthwaite, K., Trevenar, S., Walker, L., Todd, K., Rosas, M., & Kirk, M. (2021). Health and social concerns about living in three communities affected by per-and polyfluoroalkyl substances (PFAS): A qualitative study in Australia. PLOS One, 16(1), e0245141. https://doi.org/10.1371/journal.pone.0245141
- Blaine, A. C., Rich, C. D., Sedlacko, E. M., Hyland, K. C., Stushnoff, C., Dickenson, E. R. V., & Higgins, C. P. (2014). Perfluoroalkyl acid uptake in lettuce (Lactuca sativa) and strawberry (Fragaria ananassa) irrigated with reclaimed water. Environmental Science & Technology, 48(24), 14361–14368. https://doi.org/10.1021/es504150h
- Bolan, N., Sarkar, B., Yan, Y., Li, Q., Wijesekara, H., Kannan, K., Tsang, D. C. W., Schauerte, M., Bosch, J., Noll, H., Ok, Y. S., Scheckel, K., Kumpiene, J., Gobindlal, K., Kah, M., Sperry, J., Kirkham, M. B., Wang, H., Tsang, Y. F., Hou, D., & Rinklebe, J. (2021). Remediation of poly- and perfluoroalkyl substances (PFAS) contaminated soils – To mobilize or to immobilize or to degrade? Journal of Hazardous Materials, 401, 123892. https://doi.org/10.1016/j.jhazmat.2020.123892
- Borne, K. E., Fassman-Beck, E. A., & Tanner, C. C. (2014). Floating treatment wetland influences on the fate of metals in road runoff retention ponds. Water Research, 48, 430–442. https://doi.org/10.1016/j.watres.2013.09.056
- Brennan, N. M., Evans, A. T., Fritz, M. K., Peak, S. A., & von Holst, H. E. (2021). Trends in the regulation of per- and polyfluoroalkyl substances (PFAS): A scoping review. International Journal of Environmental Research and Public Health, 18(20), 10900. https://doi.org/10.3390/ijerph182010900
- Buck, R. C., Korzeniowski, S. H., Laganis, E., & Adamsky, F. (2021). Identification and classification of commercially relevant per- and poly-fluoroalkyl substances (PFAS). Integrated Environmental Assessment and Management, 17(5), 1045–1055. https://doi.org/10.1002/ieam.4450
- Calloway, E. E., Chiappone, A. L., Schmitt, H. J., Sullivan, D., Gerhardstein, B., Tucker, P. G., Rayman, J., & Yaroch, A. L. (2020). Exploring community psychosocial stress related to per- and poly-fluoroalkyl substances (PFAS) contamination: Lessons learned from a qualitative study. International Journal of Environmental Research and Public Health, 17(23), 8706. https://doi.org/10.3390/ijerph17238706
- Cao, X., Zhang, C., Zhang, S., Sakamaki, T., Wang, H., & Li, X.-N. (2023). Simultaneous removal of sediment and water contaminants in a microbial electrochemical system with embedded active electrode by in-situ utilization of electrons. Journal of Hazardous Materials, 443(Pt A), 130172. https://doi.org/10.1016/j.jhazmat.2022.130172
- Casson, R., & Chiang, S.-Y. (2018). Integrating total oxidizable precursor assay data to evaluate fate and transport of PFASs. Remediation Journal, 28(2), 71–87. https://doi.org/10.1002/rem.21551
- Chen, Y., Zhang, H., Liu, Y., Bowden, J. A., Tolaymat, T. M., Townsend, T. G., & Solo-Gabriele, H. M. (2023). Evaluation of per- and polyfluoroalkyl substances (PFAS) in leachate, gas condensate, stormwater and groundwater at landfills. Chemosphere, 318, 137903. https://doi.org/10.1016/j.chemosphere.2023.137903
- Chen, Z., Cuervo, D. P., Müller, J. A., Wiessner, A., Köser, H., Vymazal, J., Kästner, M., & Kuschk, P. (2016). Hydroponic root mats for wastewater treatment—A review. Environmental Science and Pollution Research, 23(16), 15911–15928. https://doi.org/10.1007/s11356-016-6801-3
- Chojnacka, K., Moustakas, K., & Mikulewicz, M. (2023). The combined rhizoremediation by a triad: Plant-microorganism-functional materials. Environmental Science and Pollution Research, 30(39), 90500–90521. https://doi.org/10.1007/s11356-023-28755-8
- Christenhusz, M. J., & Byng, J. W. (2016). The number of known plants species in the world and its annual increase. Phytotaxa, 261(3), 201. https://doi.org/10.11646/phytotaxa.261.3.1
- Colares, G. S., Dell’Osbel, N., Wiesel, P. G., Oliveira, G. A., Lemos, P. H. Z., da Silva, F. P., Lutterbeck, C. A., Kist, L. T., & Machado, Ê. L. (2020). Floating treatment wetlands: A review and bibliometric analysis. Science of the Total Environment, 714, 136776. https://doi.org/10.1016/j.scitotenv.2020.136776
- Dalahmeh, S., Tirgani, S., Komakech, A. J., Niwagaba, C. B., & Ahrens, L. (2018). Per- and polyfluoroalkyl substances (PFASs) in water, soil and plants in wetlands and agricultural areas in Kampala, Uganda. Science of the Total Environment, 631-632, 660–667. https://doi.org/10.1016/j.scitotenv.2018.03.024
- Daraz, U., Li, Y., Ahmad, I., Iqbal, R., & Ditta, A. (2023). Remediation technologies for acid mine drainage: Recent trends and future perspectives. Chemosphere, 311(Pt 2), 137089. https://doi.org/10.1016/j.chemosphere.2022.137089
- De Mandal, S., Laskar, F., Panda, A. K., & Mishra, R. (2020). Chapter 12 – Microbial diversity and functional potential in wetland ecosystems. In S. De Mandal, P. Bhatt (Eds.), Recent advancements in microbial diversity (pp. 289–314). Academic Press. https://doi.org/10.1016/B978-0-12-821265-3.00012-8
- Department of Defence. (2019). RAAF Base Edinburgh Environmental Investigation of PFAS.
- Felizeter, S., McLachlan, M. S., & De Voogt, P. (2014). Root uptake and translocation of perfluorinated alkyl acids by three hydroponically grown crops. Journal of Agricultural and Food Chemistry, 62(15), 3334–3342. https://doi.org/10.1021/jf500674j
- Fu, J., Gao, B., Xu, H., Hao, S., Ren, J., Wu, J., & Sun, Y. (2023). Effects of biofilms on the retention and transport of PFOA in saturated porous media. Journal of Hazardous Materials, 443(Pt B), 130392. https://doi.org/10.1016/j.jhazmat.2022.130392
- García-Valcárcel, A. I., Molero, E., Escorial, M. C., Chueca, M. C., & Tadeo, J. L. (2014). Uptake of perfluorinated compounds by plants grown in nutrient solution. Science of the Total Environment, 472, 20–26. https://doi.org/10.1016/j.scitotenv.2013.10.054
- Ghisi, R., Vamerali, T., & Manzetti, S. (2019). Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review. Environmental Research, 169, 326–341. https://doi.org/10.1016/j.envres.2018.10.023
- Glüge, J., Scheringer, M., Cousins, I. T., Dewitt, J. C., Goldenman, G., Herzke, D., Lohmann, R., Ng, C. A., Trier, X., & Wang, Z. (2020). An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environmental Science: Processes & Impacts, 22(12), 2345–2373. https://doi.org/10.1039/D0EM00291G
- Grgas, D., Petrina, A., Štefanac, T., Bešlo, D., & Landeka Dragičević, T. (2023). A review: Per- and polyfluoroalkyl substances-biological degradation. Toxics, 11(5), 446. https://doi.org/10.3390/toxics11050446
- Griffin, E. K., Aristizabal-Henao, J., Timshina, A., Ditz, H. L., Camacho, C. G., da Silva, B. F., Coker, E. S., Deliz Quiñones, K. Y., Aufmuth, J., & Bowden, J. A. (2022). Assessment of per- and polyfluoroalkyl substances (PFAS) in the Indian River Lagoon and Atlantic coast of Brevard County, FL, reveals distinct spatial clusters. Chemosphere, 301, 134478. https://doi.org/10.1016/j.chemosphere.2022.134478
- Gupta, S., & Pathak, B. (2020). Chapter 4. Bioremediation of polycyclic aromatic hydrocarbons (PAHs): An overview. In M. H. Fulekar & B. Pathak (Eds.), Bioremediation Technology (1st ed., 63–90). CRC Press. https://www.taylorfrancis.com/chapters/edit/10.1201/9780429296031-4/bioremediation-polycyclic-aromatic-hydrocarbons-pahs-overview-shalini-gupta-bhawana-pathak
- Hammill, E., Pendleton, M., Brahney, J., Kettenring, K. M., & Atwood, T. B. (2022). Metal concentrations in wetland plant tissues influences transfer to terrestrial food webs. Ecotoxicology, 31(5), 836–845. https://doi.org/10.1007/s10646-022-02550-6
- He, Q., Yan, Z., Qian, S., Xiong, T., Grieger, K. D., Wang, X., Liu, C., & Zhi, Y. (2023). Phytoextraction of per- and polyfluoroalkyl substances (PFAS) by weeds: Effect of PFAS physicochemical properties and plant physiological traits. Journal of Hazardous Materials, 454, 131492. https://doi.org/10.1016/j.jhazmat.2023.131492
- HEPA. (2020). PFAS national environmental management plan: Version 2.0. The National Chemicals Working Group of the Heads of EPAs Australia and New Zealand. https://www.dcceew.gov.au/environment/protection/publications/pfas-nemp-2
- HEPA. (2022). Draft PFAS National Environmental Management Plan: Version 3.0. The National Chemicals Working Group of the Heads of EPAs Australia and New Zealand. https://consult.dcceew.gov.au/nemp-pfas
- Huth, I., Walker, C., Kulkarni, R., & Lucke, T. (2021). Using constructed floating wetlands to remove nutrients from a waste stabilization pond. Water, 13(13), 1746. https://doi.org/10.3390/w13131746
- Hwang, J. I., Li, Z., Andreacchio, N., Ordonez Hinz, F., & Wilson, P. C. (2020). Potential use of floating treatment wetlands established with Canna flaccida for removing organic contaminants from surface water. International Journal of Phytoremediation, 22(12), 1304–1312. https://doi.org/10.1080/15226514.2020.1768511
- Hwang, J.-I., Hinz, F. O., Albano, J. P., & Wilson, P. C. (2021). Enhanced dissipation of trace level organic contaminants by floating treatment wetlands established with two macrophyte species: A mesocosm study. Chemosphere, 267, 129159. https://doi.org/10.1016/j.chemosphere.2020.129159
- ITRC. (2022). PFAS technical and regulatory guidance document and fact sheets PFAS-1. ITRC.
- Ji, B., & Zhao, Y. (2024). Interactions between biofilms and PFASs in aquatic ecosystems: Literature exploration. Science of the Total Environment, 906, 167469. https://doi.org/10.1016/j.scitotenv.2023.167469
- Ji, B., Kang, P., Wei, T., & Zhao, Y. (2020). Challenges of aqueous per- and polyfluoroalkyl substances (PFASs) and their foreseeable removal strategies. Chemosphere, 250, 126316. https://doi.org/10.1016/j.chemosphere.2020.126316
- Ji, B., Zhao, Y., Yang, Y., Li, Q., Man, Y., Dai, Y., Fu, J., Wei, T., Tai, Y., & Zhang, X. (2023). Curbing per- and polyfluoroalkyl substances (PFASs): First investigation in a constructed wetland-microbial fuel cell system. Water Research, 230, 119530. https://doi.org/10.1016/j.watres.2022.119530
- Kabiri, S., Navarro, D. A., Hamad, S. A., Grimison, C., Higgins, C. P., Mueller, J. F., Kookana, R. S., & McLaughlin, M. J. (2023). Physical and chemical properties of carbon-based sorbents that affect the removal of per- and polyfluoroalkyl substances from solution and soil. Science of the Total Environment, 875, 162653. https://doi.org/10.1016/j.scitotenv.2023.162653
- Khan, S., Afzal, M., Iqbal, S., & Khan, Q. M. (2013). Plant–bacteria partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere, 90(4), 1317–1332. https://doi.org/10.1016/j.chemosphere.2012.09.045
- Krippner, J., Falk, S., Brunn, H., Georgii, S., Schubert, S., & Stahl, T. (2015). Accumulation potentials of perfluoroalkyl carboxylic acids (PFCAs) and perfluoroalkyl sulfonic acids (PFSAs) in maize (Zea mays). Journal of Agricultural and Food Chemistry, 63(14), 3646–3653. https://doi.org/10.1021/acs.jafc.5b00012
- Kurwadkar, S., Dane, J., Kanel, S. R., Nadagouda, M. N., Cawdrey, R. W., Ambade, B., Struckhoff, G. C., & Wilkin, R. (2022). Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Science of the Total Environment, 809, 151003. https://doi.org/10.1016/j.scitotenv.2021.151003
- LaFond, J. A., Hatzinger, P. B., Guelfo, J. L., Millerick, K., & Jackson, W. A. (2023). Bacterial transformation of per- and poly-fluoroalkyl substances: A review for the field of bioremediation. Environmental Science: Advances, 2(8), 1019–1041. https://doi.org/10.1039/D3VA00031A
- Lan, Z., Zhou, M., Yao, Y., & Sun, H. (2018). Plant uptake and translocation of perfluoroalkyl acids in a wheat–soil system. Environmental Science and Pollution Research, 25(31), 30907–30916. https://doi.org/10.1007/s11356-018-3070-3
- Lee, Y.-C., Wang, P.-Y., Lo, S.-L., & Huang, C. P. (2017). Recovery of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) from dilute water solution by foam flotation. Separation and Purification Technology, 173, 280–285. https://doi.org/10.1016/j.seppur.2016.09.012
- Lei, X., Lian, Q., Zhang, X., Karsili, T. K., Holmes, W., Chen, Y., Zappi, M. E., & Gang, D. D. (2023). A review of PFAS adsorption from aqueous solutions: Current approaches, engineering applications, challenges, and opportunities. Environmental Pollution, 321, 121138. https://doi.org/10.1016/j.envpol.2023.121138
- Lenka, S. P., Kah, M., & Padhye, L. P. (2021). A review of the occurrence, transformation, and removal of poly- and perfluoroalkyl substances (PFAS) in wastewater treatment plants. Water Research, 199, 117187. https://doi.org/10.1016/j.watres.2021.117187
- Lewis, A. J., Yun, X., Spooner, D. E., Kurz, M. J., McKenzie, E. R., & Sales, C. M. (2022). Exposure pathways and bioaccumulation of per- and polyfluoroalkyl substances in freshwater aquatic ecosystems: Key considerations. Science of the Total Environment, 822, 153561. https://doi.org/10.1016/j.scitotenv.2022.153561
- Li, H., Tian, Y., Qu, Y., Qiu, Y., Liu, J., & Feng, Y. (2017). A pilot-scale benthic microbial electrochemical system (BMES) for enhanced organic removal in sediment restoration. Scientific Reports, 7(1), 39802. https://doi.org/10.1038/srep39802
- Li, J., Sun, J., & Li, P. (2022). Exposure routes, bioaccumulation and toxic effects of per- and polyfluoroalkyl substances (PFASs) on plants: A critical review. Environment International, 158, 106891. https://doi.org/10.1016/j.envint.2021.106891
- Li, X-Q., Hua, Z-L., Wu, J-y., & Gu, L. (2021). Removal of perfluoroalkyl acids (PFAAs) in constructed wetlands: Considerable contributions of submerged macrophytes and the microbial community. Water Research, 197, 117080. https://doi.org/10.1016/j.watres.2021.117080
- Li, Y., Shang, J., Zhang, C., Zhang, W., Niu, L., Wang, L., & Zhang, H. (2021). The role of freshwater eutrophication in greenhouse gas emissions: A review. Science of the Total Environment, 768, 144582. https://doi.org/10.1016/j.scitotenv.2020.144582
- Lin, Q., Zhou, C., Chen, L., Li, Y., Huang, X., Wang, S., Qiu, R., & Tang, C. (2020). Accumulation and associated phytotoxicity of novel chlorinated polyfluorinated ether sulfonate in wheat seedlings. Chemosphere, 249, 126447. https://doi.org/10.1016/j.chemosphere.2020.126447
- Liu, J., & Mejia Avendaño, S. (2013). Microbial degradation of polyfluoroalkyl chemicals in the environment: A review. Environment International, 61, 98–114. https://doi.org/10.1016/j.envint.2013.08.022
- Liu, J., Edwards, E., Van Hamme, J., Manefield, M., Higgins, C. P., Blotevogel, J., Liu, J., & Lee, L. S. (2023). Correspondence on “defluorination of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) by Acidimicrobium sp. Strain A6. Environmental Science & Technology, 57(48), 20440–20442. https://doi.org/10.1021/acs.est.3c06681
- Liu, J., Van Hoomissen, D. J., Liu, T., Maizel, A., Huo, X., Fernández, S. R., Ren, C., Xiao, X., Fang, Y., Schaefer, C. E., Higgins, C. P., Vyas, S., & Strathmann, T. J. (2018). Reductive defluorination of branched per- and polyfluoroalkyl substances with cobalt complex catalysts. Environmental Science & Technology Letters, 5(5), 289–294. https://doi.org/10.1021/acs.estlett.8b00122
- Lucke, T., Walker, C., & Beecham, S. (2019). Experimental designs of field-based constructed floating wetland studies: A review. Science of the Total Environment, 660, 199–208. https://doi.org/10.1016/j.scitotenv.2019.01.018
- Ma, H., Kang, Y., Li, M., Dong, J., Wang, Y., Xiao, J., & Guo, Z. (2023). Enhancement of perfluorooctanoic acid and perfluorooctane sulphonic acid removal in constructed wetland using iron mineral: Performance and mechanisms. Journal of Hazardous Materials, 447, 130819. https://doi.org/10.1016/j.jhazmat.2023.130819
- MacDonald, D., Walker, C., Lucke, T., Flipp, R., Covey, K., & Shadforth, P. (2016). Floating wetland treatment systems in residential development: Assessing the benefits for residents, local authorities, and developers. Novatech 2016-9ème Conférence Internationale Sur Les Techniques et Stratégies Pour la Gestion Durable de lEau Dans la Ville/9th International Conference on Planning and Technologies for Sustainable Management of Water in the City, GRAIE, Lyon, France.
- Mayakaduwage, S., Ekanayake, A., Kurwadkar, S., Rajapaksha, A. U., & Vithanage, M. (2022). Phytoremediation prospects of per- and polyfluoroalkyl substances: A review. Environmental Research, 212(Pt B), 113311. https://doi.org/10.1016/j.envres.2022.113311
- Megharaj, M., Ramakrishnan, B., Venkateswarlu, K., Sethunathan, N., & Naidu, R. (2011). Bioremediation approaches for organic pollutants: A critical perspective. Environment International, 37(8), 1362–1375. https://doi.org/10.1016/j.envint.2011.06.003
- Mejia-Avendaño, S., Zhi, Y., Yan, B., & Liu, J. (2020). Sorption of polyfluoroalkyl surfactants on surface soils: Effect of molecular structures, soil properties, and solution chemistry. Environmental Science & Technology, 54(3), 1513–1521. https://doi.org/10.1021/acs.est.9b04989
- Meng, P., Pei, H., Hu, W., Shao, Y., & Li, Z. (2014). How to increase microbial degradation in constructed wetlands: Influencing factors and improvement measures. Bioresource Technology, 157, 316–326. https://doi.org/10.1016/j.biortech.2014.01.095
- Merino, N., Qu, Y., Deeb, R. A., Hawley, E. L., Hoffmann, M. R., & Mahendra, S. (2016). Degradation and removal methods for perfluoroalkyl and polyfluoroalkyl substances in water. Environmental Engineering Science, 33(9), 615–649. https://doi.org/10.1089/ees.2016.0233
- Mishra, S., Tripathi, A., Tripathi, D. K., & Chauhan, D. K. (2016). Role of sedges (Cyperaceae) in wetlands, environmental cleaning and as food material. In M. M. Azooz & P. Ahmad (Eds.), Plant-environment interaction: Responses and approaches to mitigate stress (pp. 327–338). John Wiley & Sons, Ltd. https://doi.org/10.1002/9781119081005.ch18
- Morrison, A. L., Strezov, V., Niven, R. K., Taylor, M. P., Wilson, S. P., Wang, J., Burns, D. J., & Murphy, P. J. C. (2023). Impact of salinity and temperature on removal of PFAS species from water by aeration in the absence of additional surfactants: A novel application of green chemistry using adsorptive bubble fractionation. Industrial & Engineering Chemistry Research, 62(13), 5635–5645. https://doi.org/10.1021/acs.iecr.3c00150
- Navarro, D. A., Kabiri, S., Ho, J., Bowles, K. C., Davis, G., McLaughlin, M. J., & Kookana, R. S. (2023). Stabilisation of PFAS in soils: Long-term effectiveness of carbon-based soil amendments. Environmental Pollution, 323, 121249. https://doi.org/10.1016/j.envpol.2023.121249
- Ng, C. A., & Hungerbühler, K. (2014). Bioaccumulation of perfluorinated alkyl acids: Observations and models. Environmental Science & Technology, 48(9), 4637–4648. https://doi.org/10.1021/es404008g
- Ollivier, Q. R., Maher, D. T., Pitfield, C., & Macreadie, P. I. (2019). Punching above their weight: Large release of greenhouse gases from small agricultural dams. Global Change Biology, 25(2), 721–732. https://doi.org/10.1111/gcb.14477
- Olsen, M., Moy, F. E., Mjelde, M., & Lydersen, E. (2018). An in situ experimental study of effects on submerged vegetation after activated carbon amendment of legacy contaminated sediments. Water, Air, & Soil Pollution, 229(8), 263. https://doi.org/10.1007/s11270-018-3918-7
- Overton, O. C., Olson, L. H., Majumder, S. D., Shwiyyat, H., Foltz, M. E., & Nairn, R. W. (2023). Wetland removal mechanisms for emerging contaminants. Land, 12(2), 472. https://doi.org/10.3390/land12020472
- Patra, D. K., Acharya, S., Pradhan, C., & Patra, H. K. (2021). Poaceae plants as potential phytoremediators of heavy metals and eco-restoration in contaminated mining sites. Environmental Technology & Innovation, 21, 101293. https://doi.org/10.1016/j.eti.2020.101293
- Pi, N., Ng, J. Z., & Kelly, B. C. (2017). Uptake and elimination kinetics of perfluoroalkyl substances in submerged and free-floating aquatic macrophytes: Results of mesocosm experiments with Echinodorus horemanii and Eichhornia crassipes. Water Research, 117, 167–174. https://doi.org/10.1016/j.watres.2017.04.003
- Qi, X., Yin, H., Zhu, M., Shao, P., & Dang, Z. (2022). Understanding the role of biochar in affecting BDE-47 biodegradation by Pseudomonas plecoglossicida: An integrated analysis using chemical, biological, and metabolomic approaches. Water Research, 220, 118679. https://doi.org/10.1016/j.watres.2022.118679
- Qian, S., Lu, H., Xiong, T., Zhi, Y., Munoz, G., Zhang, C., Li, Z., Liu, C., Li, W., Wang, X., & He, Q. (2023). Bioaccumulation of per- and polyfluoroalkyl substances (PFAS) in ferns: Effect of PFAS molecular structure and plant root characteristics. Environmental Science & Technology, 57(11), 4443–4453. https://doi.org/10.1021/acs.est.2c06883
- Qiao, W., Li, R., Tang, T., & Zuh, A. A. (2021). Removal, distribution and plant uptake of perfluorooctane sulfonate (PFOS) in a simulated constructed wetland system. Frontiers of Environmental Science & Engineering, 15(2), 1–11. https://doi.org/10.1007/s11783-020-1312-3
- Rabêlo, F. H. S., Vangronsveld, J., Baker, A. J. M., van der Ent, A., & Alleoni, L. R. F. (2021). Are grasses really useful for the phytoremediation of potentially toxic trace elements? A review. Frontiers in Plant Science, 12, 778275. https://doi.org/10.3389/fpls.2021.778275
- Ravikumar, Y., Yun, J., Zhang, G., Zabed, H. M., & Qi, X. (2022). A review on constructed wetlands-based removal of pharmaceutical contaminants derived from non-point source pollution. Environmental Technology & Innovation, 26, 102504. https://doi.org/10.1016/j.eti.2022.102504
- Rigotti, J. A., Postal Pasqualini, J., & Ribeiro Rodrigues, L. (2020). Nature-based solutions for managing the urban surface runoff: An application of a constructed floating wetland. Limnetica, 39(1), 441–454. https://doi.org/10.23818/limn.39.28
- Ruiz-Urigüen, M., Shuai, W., Huang, S., & Jaffé, P. R. (2022). Biodegradation of PFOA in microbial electrolysis cells by Acidimicrobiaceae sp. strain A6. Chemosphere, 292, 133506. https://doi.org/10.1016/j.chemosphere.2021.133506
- Saifur, S., & Gardner, C. M. (2021). Loading, transport, and treatment of emerging chemical and biological contaminants of concern in stormwater. Water Science and Technology, 83(12), 2863–2885. https://doi.org/10.2166/wst.2021.187
- Schwab, A. (1998). Phytoremediation of soils contaminated with PAHs and other petroleum compounds, Beneficial effects of vegetation in contaminated soils workshop. Kansas State University.
- Schwammberger, P. F., Lucke, T., Walker, C., & Trueman, S. J. (2019). Nutrient uptake by constructed floating wetland plants during the construction phase of an urban residential development. Science of the Total Environment, 677, 390–403. https://doi.org/10.1016/j.scitotenv.2019.04.341
- Shahid, M. J., Arslan, M., Ali, S., Siddique, M., & Afzal, M. (2018). Floating wetlands: A sustainable tool for wastewater treatment. CLEAN – Soil, Air, Water, 46(10), 1800120. https://doi.org/10.1002/clen.201800120
- Sharma, P. (2022). Role and significance of biofilm-forming microbes in phytoremediation -A review. Environmental Technology & Innovation, 25, 102182. https://doi.org/10.1016/j.eti.2021.102182
- Sheth, R. U., Cabral, V., Chen, S. P., & Wang, H. H. (2016). Manipulating bacterial communities by in situ microbiome engineering. Trends in Genetics, 32(4), 189–200. https://doi.org/10.1016/j.tig.2016.01.005
- Shi, Y., Chen, T., Shaw, P., & Wang, P.-Y. (2022). Manipulating bacterial biofilms using materiobiology and synthetic biology approaches. Frontiers in Microbiology, 13, 844997. https://doi.org/10.3389/fmicb.2022.844997
- Shi, Y., Vestergren, R., Nost, T. H., Zhou, Z., & Cai, Y. (2018). Probing the differential tissue distribution and bioaccumulation behavior of per- and polyfluoroalkyl substances of varying chain-lengths, isomeric structures and functional groups in crucian carp. Environmental Science & Technology, 52(8), 4592–4600. https://doi.org/10.1021/acs.est.7b06128
- Sima, M. W., & Jaffé, P. R. (2021). A critical review of modeling poly- and perfluoroalkyl substances (PFAS) in the soil-water environment. Science of the Total Environment, 757, 143793. https://doi.org/10.1016/j.scitotenv.2020.143793
- Suman, J., Uhlik, O., Viktorova, J., & Macek, T. (2018). Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Frontiers in Plant Science, 9, 1476. https://doi.org/10.3389/fpls.2018.01476
- Surriya, O., Sarah Saleem, S., Waqar, K., & Gul Kazi, A. (2015). Chapter 1 – Phytoremediation of soils: Prospects and challenges. In K. R. Hakeem, M. Sabir, M. Öztürk, A. R. Mermut (Eds.), Soil remediation and plants (pp. 1–36). Academic Press. https://doi.org/10.1016/B978-0-12-799937-1.00001-2
- Syranidou, E., Christofilopoulos, S., & Kalogerakis, N. (2017). Juncus spp.—The helophyte for all (phyto)remediation purposes? New Biotechnology, 38(Pt B), 43–55. https://doi.org/10.1016/j.nbt.2016.12.005
- Takavakoglou, V., Georgiadis, A., Pana, E., Georgiou, P. E., Karpouzos, D. K., & Plakas, K. V. (2021). Screening life cycle environmental impacts and assessing economic performance of floating wetlands for marine water pollution control. Journal of Marine Science and Engineering, 9(12), 1345. https://doi.org/10.3390/jmse9121345
- Thompson, K. A., Mortazavian, S., Gonzalez, D. J., Bott, C., Hooper, J., Schaefer, C. E., & Dickenson, E. R. V. (2022). Poly- and perfluoroalkyl substances in municipal wastewater treatment plants in the United States: seasonal patterns and meta-analysis of long-term trends and average concentrations. ACS ES&T Water, 2(5), 690–700. https://doi.org/10.1021/acsestwater.1c00377
- UNEP. (2021). Proposal to list long-chain perfluorocarboxylic acids, their salts and related compounds in annexes A, B And/or C to the Stockholm convention on persistent organic pollutants. Retrieved August 27, 2023 from https://www.pops.int/TheConvention/POPsReviewCommittee/Meetings/POPRC17/MeetingDocuments/tabid/8918/Default.aspx
- US EPA. (2023). Per- and polyfluoroalkyl substances (PFAS): Proposed PFAS national primary drinking water regulation. Retrieved August 23, 2023 from https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas
- Wang, J., Long, Y., Yu, G., Wang, G., Zhou, Z., Li, P., Zhang, Y., Yang, K., & Wang, S. (2022). A review on microorganisms in constructed wetlands for typical pollutant removal: Species, function, and diversity. Frontiers in Microbiology, 13, 845725. https://doi.org/10.3389/fmicb.2022.845725
- Wang, N., Liu, J., Buck, R. C., Korzeniowski, S. H., Wolstenholme, B. W., Folsom, P. W., & Sulecki, L. M. (2011). 6:2 Fluorotelomer sulfonate aerobic biotransformation in activated sludge of waste water treatment plants. Chemosphere, 82(6), 853–858. https://doi.org/10.1016/j.chemosphere.2010.11.003
- Wang, N., Szostek, B., Buck, R. C., Folsom, P. W., Sulecki, L. M., Capka, V., Berti, W. R., & Gannon, J. T. (2005). Fluorotelomer alcohol biodegradation-direct evidence that perfluorinated carbon chains breakdown. Environmental Science & Technology, 39(19), 7516–7528. https://doi.org/10.1021/es0506760
- Wang, T.-T., Ying, G.-G., He, L.-Y., Liu, Y.-S., & Zhao, J.-L. (2020). Uptake mechanism, subcellular distribution, and uptake process of perfluorooctanoic acid and perfluorooctane sulfonic acid by wetland plant Alisma orientale. Science of the Total Environment, 733, 139383. https://doi.org/10.1016/j.scitotenv.2020.139383
- Wang, T.-T., Ying, G.-G., Shi, W.-J., Zhao, J.-L., Liu, Y.-S., Chen, J., Ma, D.-D., & Xiong, Q. (2020). Uptake and translocation of perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) by wetland plants: Tissue- and cell-level distribution visualization with desorption electrospray ionization mass spectrometry (DESI-MS) and transmission electron microscopy equipped with energy-dispersive spectroscopy (TEM-EDS). Environmental Science & Technology, 54(10), 6009–6020. https://doi.org/10.1021/acs.est.9b05160
- Wanninayake, D. M. (2021). Comparison of currently available PFAS remediation technologies in water: A review. Journal of Environmental Management, 283, 111977. https://doi.org/10.1016/j.jenvman.2021.111977
- Webster, E., Ellis, D. A., & Reid, L. K. (2010). Modeling the environmental fate of perfluorooctanoic acid and perfluorooctanoate: An investigation of the role of individual species partitioning. Environmental Toxicology and Chemistry, 29(7), 1466–1475. https://doi.org/10.1002/etc.181
- Wen, B., Wu, Y., Zhang, H., Liu, Y., Hu, X., Huang, H., & Zhang, S. (2016). The roles of protein and lipid in the accumulation and distribution of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in plants grown in biosolids-amended soils. Environmental Pollution, 216, 682–688. https://doi.org/10.1016/j.envpol.2016.06.032
- White, S. A. (2021). Plant nutrient uptake in full-scale floating treatment wetlands in a Florida stormwater pond: 2016–2020. Water, 13(4), 569. https://doi.org/10.3390/w13040569
- Williams, J. B. (2002). Phytoremediation in Wetland ecosystems: Progress, problems, and potential. Critical Reviews in Plant Sciences, 21(6), 607–635. https://doi.org/10.1080/0735-260291044386
- Wright, I. J., & Cannon, K. (2001). Relationships between leaf lifespan and structural defences in a low-nutrient, sclerophyll flora. Functional Ecology, 15(3), 351–359. https://doi.org/10.1046/j.1365-2435.2001.00522.x
- Yang, S.-H., Shi, Y., Strynar, M., & Chu, K.-H. (2022). Desulfonation and defluorination of 6:2 fluorotelomer sulfonic acid (6:2 FTSA) by Rhodococcus jostii RHA1: Carbon and sulfur sources, enzymes, and pathways. Journal of Hazardous Materials, 423(Pt A), 127052. https://doi.org/10.1016/j.jhazmat.2021.127052
- Yeh, N., Yeh, P., & Chang, Y.-H. (2015). Artificial floating islands for environmental improvement. Renewable and Sustainable Energy Reviews, 47, 616–622. https://doi.org/10.1016/j.rser.2015.03.090
- Yin, T., Chen, H., Reinhard, M., Yi, X., He, Y., & Gin, K. Y.-H. (2017). Perfluoroalkyl and polyfluoroalkyl substances removal in a full-scale tropical constructed wetland system treating landfill leachate. Water Research, 125, 418–426. https://doi.org/10.1016/j.watres.2017.08.071
- Yu, Y., Che, S., Ren, C., Jin, B., Tian, Z., Liu, J., & Men, Y. (2022). Microbial defluorination of unsaturated per- and polyfluorinated carboxylic acids under anaerobic and aerobic conditions: A structure specificity study. Environmental Science & Technology, 56(8), 4894–4904. https://doi.org/10.1021/acs.est.1c05509
- Yu, Y., Zhang, K., Li, Z., Ren, C., Chen, J., Lin, Y.-H., Liu, J., & Men, Y. (2020). Microbial cleavage of C–F bonds in Two C6 per- and polyfluorinated compounds via reductive defluorination. Environmental Science & Technology, 54(22), 14393–14402. https://doi.org/10.1021/acs.est.0c04483
- Zhang, D. Q., Wang, M., He, Q., Niu, X., & Liang, Y. (2020). Distribution of perfluoroalkyl substances (PFASs) in aquatic plant-based systems: From soil adsorption and plant uptake to effects on microbial community. Environmental Pollution, 257, 113575. https://doi.org/10.1016/j.envpol.2019.113575
- Zhang, L., Sun, H., Wang, Q., Chen, H., Yao, Y., Zhao, Z., & Alder, A. C. (2019). Uptake mechanisms of perfluoroalkyl acids with different carbon chain lengths (C2-C8) by wheat (Triticum acstivnm L.). Science of the Total Environment, 654, 19–27. https://doi.org/10.1016/j.scitotenv.2018.10.443
- Zhang, W., & Liang, Y. (2020). Removal of eight perfluoroalkyl acids from aqueous solutions by aeration and duckweed. Science of the Total Environment, 724, 138357. https://doi.org/10.1016/j.scitotenv.2020.138357
- Zhang, W., Cao, H., & Liang, Y. (2021). Plant uptake and soil fractionation of five ether-PFAS in plant-soil systems. Science of the Total Environment, 771, 144805. https://doi.org/10.1016/j.scitotenv.2020.144805
- Zhang, W., Pang, S., Lin, Z., Mishra, S., Bhatt, P., & Chen, S. (2021). Biotransformation of perfluoroalkyl acid precursors from various environmental systems: Advances and perspectives. Environmental Pollution, 272, 115908. https://doi.org/10.1016/j.envpol.2020.115908
- Zhang, W., Zhang, D., Zagorevski, D. V., & Liang, Y. (2019). Exposure of Juncus effusus to seven perfluoroalkyl acids: Uptake, accumulation and phytotoxicity. Chemosphere, 233, 300–308. https://doi.org/10.1016/j.chemosphere.2019.05.258
- Zhang, Y., Qv, Z., Wang, J., Yang, Y., Chen, X., Wang, J., Zhang, Y., & Zhu, L. (2022). Natural biofilm as a potential integrative sample for evaluating the contamination and impacts of PFAS on aquatic ecosystems. Water Research, 215, 118233. https://doi.org/10.1016/j.watres.2022.118233
- Zhang, Z., Sarkar, D., Biswas, J. K., & Datta, R. (2022). Biodegradation of per- and polyfluoroalkyl substances (PFAS): A review. Bioresource Technology, 344(Pt B), 126223. https://doi.org/10.1016/j.biortech.2021.126223
- Zhi, Y., Lu, H., Grieger, K. D., Munoz, G., Li, W., Wang, X., He, Q., & Qian, S. (2022). Bioaccumulation and translocation of 6:2 fluorotelomer sulfonate, GenX, and perfluoroalkyl acids by urban spontaneous plants. ACS ES&T Engineering, 2(7), 1169–1178. https://doi.org/10.1021/acsestengg.1c00423