Abstract
The major indication for testosterone (T) treatment is male hypogonadism that is characterized by low serum T concentrations. Although a recently developed hydroalcoholic gel, Androgel®, containing 1% T addresses many of the problems associated with the more conventional formulations, the bioavailability of T is only 10% requiring 5 to 10 g of gel to be applied daily. The present study was performed to investigate the effect of isopropyl alcohol (IPA) content as a penetration enhancer based on its ability to prevent skin dryness and in turn to increase T permeation from hydroalcoholic gels. Five different hydroalcoholic gel formulations, containing 1% T and carbopol as the gel-forming polymer, were formulated by varying the amount of IPA. The release of T from each gel, including Androgel®, was studied in vitro on Franz diffusion cells using cellulose ester and Celgard® 2400 as synthetic membranes and hairless guinea pig skin as a natural membrane. The amount of drug released from the gels was analyzed using an HPLC-UV method. The results of release/permeation studies on guinea pig skin showed that all the gels were similar to Androgel®, indicating that the addition of IPA does not affect the release of T from hydroalcoholic gels. Although no statistical significant difference was seen, the release profiles of the gels showed a trend of increasing release of T with increasing concentration of IPA. Thus, IPA does have a potential to increase the bioavailability of T from hydroalcoholic gels.
Testosterone (T) is the principle circulating androgen in men, the normal plasma levels being 300–1000 ng/dl (Tripathi Citation2000; Wilson Citation1990). The major indication for T treatment is male hypogonadism characterized by low serum T concentrations caused due to ageing (Morales and Lunenfeld Citation2002; Tenover Citation1998), testicular failure, and/or decreased gonadotropin secretion, resulting in inadequate testosterone production (Jockenhovel Citation2004). Hypogonadism leads to symptoms that include impotence and decreased sexual desire, fatigue and loss of energy, mood depression, regression of secondary sexual characteristics, and osteoporosis (Tripathi Citation2000; Wilson Citation1990).
Over the years many different formulations have been used for T substitution. Oral 17α-substituted T preparations such as methyltestosterone and oxandrolone have been associated with hepatic toxicity and tumors (HIV Hotline Citation1998). Injectable T enanthate and cypionate preparations require injections every 10 to 21 days, causing dramatic peaks and troughs in serum concentrations (Jockenhovel Citation2004; Dobs et al. Citation1999; Mackey, Conway, and Handelsman Citation1995; Schulte-Beerbuhl and Nieschlag Citation1980; Schurmeyer and Nieschlag (Citation1984). Transdermal T patches frequently cause local reactions and tend to fall off during exercise (Jordan Citation1997; Meikle et al Citation1996). Testoderm®, a patch formulation worn on the scrotum, requires preparation of the scrotal skin with hair clipping or shaving to optimize adherence that is unpleasant to most men (Bals-Pratsch et al. Citation1986; Cunningham, Cordero, and Thornby Citation1989).
The disadvantages of the previously existing T formulations finally led to the development of a transdermal hydroalcoholic gel containing 1% T, which has gained considerable attention. At present there are at least three T gels in the market, Androgel® (Unimed Pharmaceuticals, GA, USA), Testim® (Auxillium Pharmaceuticals, PA, USA) and the recently approved generic form (Watson Labs, CA, USA), all hydroalcoholic in nature and containing 1% T. Androgel® was approved by the U.S. Food and Drug Administration in February 2000 making it the first-ever T replacement gel to enter the U.S. market. It is an open transdermal system containing ethanol (67.0%), purified water, sodium hydroxide, carbopol 980, and isopropyl myristate (IPM) as the inactive ingredients (Dudley, Kottayil, and Palatchi 2000). When applied once daily at a dose of 5, 7.5, or 10 g to the shoulders, upper arm, and/or abdomen, the serum T levels are raised to therapeutic levels in 0.5 to 4 hr, and a steady state is achieved within 1 to 3 days of therapy (Swerdloff et al. Citation2000).
However, only 10% of T in the gel applied to the skin is bioavailable (Wang et al. Citation2000) which leaves an immense potential for improving the bioavailability of T from such formulations. Also, being an open transdermal system it offers potential for dermal T transfer following application, on skin-to-skin contact with other subjects. This can cause serious side-effects especially in female partners and prepubertal children. Moreover, subjects on Androgel® therapy are advised to wait up to 6 hr before showering or swimming to ensure that greatest amount of T reaches the system. Therefore, increasing the bioavailability of T in formulations like Androgel® can lead to a smaller dose of gel to be applied and also reduce the frequency of gel application, which were the main concerns of this study. This will also ensure adequate T permeation through the skin in a shorter duration of time such that the skin can be washed sooner preventing any potential T transfer to female partners.
One of the most common approaches to combat the problem of percutaneous absorption of drugs through the skin has been the use of penetration enhancers, chemicals that interact with skin constituents to promote drug flux. Isopropyl alcohol (IPA) is one such penetration enhancer that can work extremely well in a hydroalcoholic environment as it also acts as a good cosolvent, not affecting the consistency of the formulation.
The objective of our study was to prepare gel formulations of T by substituting a portion of ethanol with IPA to reduce the quick drying effects of the solvent on the skin at the site of application and compare the drug release from these formulations, using in vitro release/permeation experiments, to Androgel® The study was directed toward learning the permeation mechanism and release profile of T from Androgel® and thus making an improved T gel formulation.
MATERIALS AND METHODS
Testosterone USP, micronized, was purchased from PCCA (Houston, TX, USA). Carbopol Ultrez 10 was kindly supplied by Noveon, Inc. (Cleveland, OH, USA). Absolute alcohol, 200 proof, was purchased from Pharmaco Products, Inc. (Brookfield, CT, USA) and neutrol was supplied by BASF Corp. (Florham Park, NJ, USA). Cellulose ester membrane, MWCO 10,000, and Celgard® 2400 were purchased from Spectrum Laboratories, Inc. (Rancho Dominguez, CA, USA) and Celgard LLC (Charlotte, NC, USA), respectively. Male hairless guinea pigs, 6 weeks old, were purchased from Charles River Laboratories (Wilmington, MA, USA). Acetonitrile, methanol, IPA, IPM, potassium acid phosphate, disodium phosphate, and sodium chloride were purchased from Fisher Scientific (Fairlawn, NJ, USA). Purified deionized water was prepared using Milli Q50 (Millipore, Bedford, MA, USA) purification system.
Preparation of Testosterone Gels
Carbopol Ultrez 10 (0.9% w/w) was added to distilled water and allowed to hydrate completely while being stirred with a magnetic stirrer. Testosterone (1% w/w) was dissolved in absolute alcohol, IPM (0.5% w/w), and IPA. The T solution was then added to the carbopol dispersion slowly with stirring, until it was completely homogeneous. Agitation was then stopped and the mixture was sonicated for 5–10 sec to minimize the number of air bubbles in the final gel. Gelling was brought about by 5 ml (4.06% w/w) of neutrol solution (prepared as a 10% w/v solution in absolute alcohol), which is, chemically, tetrahydroxypropyl ethylene diamine. Five different gels were prepared by varying the amount of IPA and absolute alcohol ().
TABLE 1 Composition of different T gels
Study Design
Synthetic Membranes
The release experiments with the synthetic membranes were done in replicates of six. The membranes were soaked in the receptor fluid for 24 hr prior to any experiment to ensure equilibration. On each day, an experiment with one membrane and one formulation was performed on 6 diffusion cells until all the gels, including Androgel®, were studied on both cellulose acetate and Celgard® 2400 membranes. Samples of receptor fluid were taken at 0.5, 1, 2, 4, 6, 8, 10, and 12 hr.
Hairless Guinea Pig Skin
The experiment was designed to minimize inter-animal as well as intra-animal variability. On the day of experiment one animal was sacrificed by carbon dioxide asphyxiation. The skin was excised, a total of 4 pieces, 3 cm in diameter, of abdominal skin were taken, soaked in isotonic phosphate buffer, pH 7.4, for 2 hr, and diffusion experiments were carried on such pretreated skin. The skin from each animal was used to study all the gels, including Androgel®, such that there were four replicates for each gel studied. The samples were taken at 3, 6, 12, 18, 24, 30, and 42 hr.
Release Permeation Studies
The release/permeation of T from the T gels was determined using Franz-diffusion cells, having a diameter of 9 mm and a volume of 5.0 ml. In these cells, the membrane was placed between the donor and the receptor compartment. Permeation studies were performed using hairless guinea pig skin as a natural membrane and cellulose ester and Celgard® 2400 as synthetic membranes. A drug-containing formulation was placed in the donor compartment. The receptor compartment was filled with 5.0 ml of degassed receptor fluid containing isotonic phosphate buffer (pH 7.4), distilled water, and methanol in a ratio of 1:1:2, respectively. The cells were maintained at a temperature of 32°C (temperature of skin) throughout the release study with the help of a thermostat. The entire 5.0 ml receptor phase was withdrawn at predetermined intervals and replaced with fresh receptor fluid equilibrated at 32°C. Testosterone concentration within each receptor solution was determined using a validated HPLC-UV method. Similar release experiments were performed with Androgel®. The drug release profiles of the prepared T gels were compared with Androgel®. Gel formula 5 and 10 were only studied on guinea pig skin.
High Performance Liquid Chromatography Assay
The determination of T was performed using an HPLC-UV analytical system (Waters Corporation, Milford, MA, USA) consisting of a Waters automated gradient controller, a Waters 717 autosampler, and two Waters 515 HPLC pumps. Elution of the analyte (T) from a μBondapack C18, 3.9 × 300 mm, 10 μm particle size (Waters Corporation, column, maintained at 37°C using a column oven, was carried out in isocratic elution mode using acetonitrile and deionized water (45:55), previously degassed and filtered using a vacuum pump (GAST, Benton Harbor, MI, USA). The flow rate remained at 1.5 ml/min throughout the assay and detection was performed at a wavelength of 254 nm using a Spectro Monitor 3200 variable wavelength ultraviolet detector (Ko, Needham, and Zia Citation1998). The peaks were integrated using Milleneum32 software, all quantifications having been performed using peak areas.
Data Analysis
Permeation curves were constructed by plotting the cumulative amount of T released versus time for a period of 12 hr and 42 hr for synthetic membranes and guinea pig skin, respectively. The release profiles of the gels were compared with that of Androgel® using Dunnett's test, a multiple comparison ANOVA tool that helps to compare the mean of a test value with that of a control value (Montgomery Citation1991). In this study, Androgel® was taken as the control and all the other gels were compared with it. The gels were considered to be significantly different from Androgel® if the absolute difference between their means was greater than the calculated critical value. The critical value is calculated with the help of Dunnett's tables, based on the degrees of freedom, confidence level, the total number of gels being compared with the control, and the number of replicates. Hence, a Dunnett's test was performed on the cumulative amount released to determine which gel was significantly different from Androgel®.
The rate of permeation and the lag time were also determined by taking the linear regression of cumulative amount released versus time plot. The slope of the linear portion determines the rate and the intercept on the x-axis the lag time. The rate and lag time also were compared using Dunnett's test that was performed at a confidence level of 95% with the aid of MINITAB software.
RESULTS
Cellulose Ester Membrane
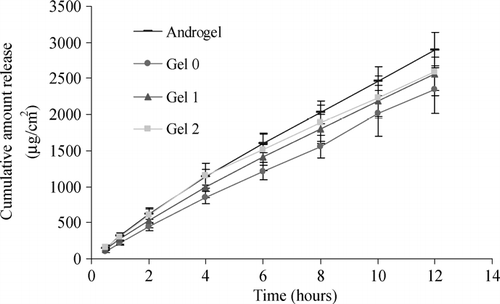
The Dunnett's test results, shown in , indicate that the amount of T released from Gel 0 was significantly less than Androgel®. On the other hand, Gels 1 and 2 were not found to be different with respect to the amount as well as the rate of release of T (), when compared with Androgel®. The release profiles of Gels 0, 1, 2, and Androgel® on cellulose ester membrane are shown in . It is evident from that the permeation of T through cellulose ester membrane, from all the gels tested, is immediate with no lag time. These results indicate that addition of IPA to the gel increases the release of T, although no significant difference was seen between gels containing 1% w/w IPA (Gel 1) and 2% w/w IPA (Gel 2).
TABLE 2 Results of Dunnett's test for comparison of cumulative amount of T permeated different time points from T gels
TABLE 3 Results of Dunnett's test for comparison of rate of permeation of T from T gels
Celgard® 2400 Membrane
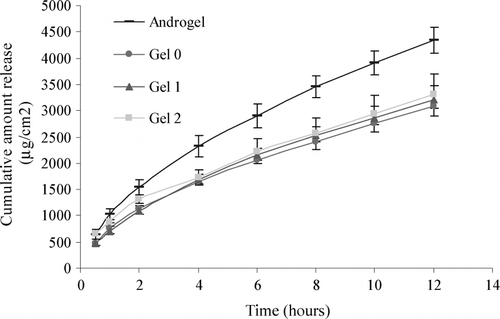
Gel formula 0, 1, and 2 were studied on Celgard® 2400 membrane and compared with Androgel®, the results of which are shown in . We observed that, unlike on cellulose ester membrane, the three gels showed significantly less release of T as compared with Androgel®. The exception was Gel 2 being similar for the first half hour, after which the release from Gel 2 also was significantly less than Androgel®. The rate of release also was found to be different (). The release profiles are shown in . These results indicate that addition of IPA does not increase the release of T, which is in contradiction to the results of cellulose ester membrane.
Hairless Guinea Pig Skin
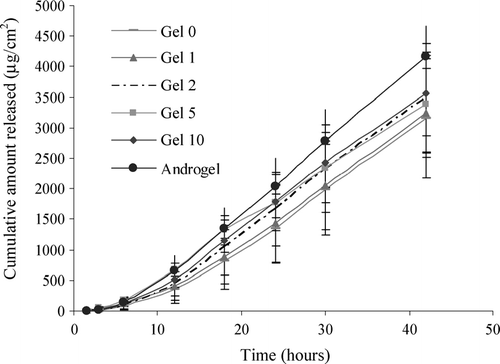
The gels were studied on full thickness guinea pig skin to understand the permeation of T better. The results of Gel formula 0, 1, and 2 are given in . These results show that all the gels were similar to Androgel®. No significant difference was observed in the amount of T released. The rates of release also were observed to be similar (). The release profiles show a lag time unlike the synthetic membranes ( and ). The skin being a multilayered membrane justifies a different profile from the synthetic membranes. The release profiles also show a general trend of increasing drug release with increasing IPA concentration, although a significant difference was not seen. Based on this observation two new gels with higher amount of IPA, 5% and 10% w/w (Gel 5 and Gel 10, respectively), were prepared and compared with Androgel®, on hairless guinea pig skin. The results (), however, show that the release from both Gel 5 and 10 was not significantly different from that of Androgel®. The rates of release and lag times are shown in and . Release from Gel 5 is superimposed on that of Androgel® for approximately the first 20 hr after which it starts decreasing (). Hence, the results of release/permeation studies on guinea pig skin show that all the gels were statistically similar to Androgel® with respect to release of T. This implies that addition of IPA does not affect the release of T from the gel formulation, although a general trend of increase in release of T with increase in IPA was evident from the release profiles, but the difference was not found to be statistically significant.
TABLE 4 Results of Dunnett's test for comparison of lag time in permeation of T from T gels on hairless guinea pig skin
DISCUSSION
Hydroalcoholic gels of T, such as Androgel®, are becoming progressively more accepted in the treatment of male hypogonadism. The introduction of Androgel® in February 2000 increased the market for hypogonadism therapy by ∼35%, but despite that growth, the Endocrine Society estimates that only 5% of 4 million U.S hypogonadal men are being treated (The Medical Letter, Inc 2000). This explains the need for a formulation that is devoid of the major drawbacks associated with the conventional formulations. Androgel® offers all these advantages and provides an easy, effective, and invisible alternative to painful deep intramuscular injections and potentially irritating patches. However, the bioavailability of T from Androgel® is only 10%, requiring large amounts of gel, up to 10 g, covering the area where gel has been applied and waiting for up to 6 hr before washing the area, which ultimately leads to discomfort and also poses the setback of dermal transfer to female partners. Therefore, we speculated that increasing the bioavailability of T would resolve the problems mentioned above. One of the easiest ways to achieve this is through use of penetration enhancers (Chan Citation2005; Williams and Barry Citation2004), isopropyl alcohol being a well-known one.
Results obtained from permeation studies on cellulose ester membrane showed that the release of T from Gel 0 was less than Androgel®, whereas Gel 1 and 2 were similar to Androgel®, implying that addition of IPA does increase the release of T from the gels. However, the same gels when tested on Celgard® 2400 membrane showed contradictory results, as all gels (Gel 0, 1, and 2) revealed significantly less release than Androgel®, indicating IPA does not increase the release of T whatsoever. This discrepancy can be attributed to the differences in the nature and the pore shape and size of the two membranes. Cellulose ester membrane has symmetrical pores with a molecular weight cut off limit of 10,000. On the other hand, Celgard® 2400 is a hydrophobic polypropylene membrane having irregular rectangular pores with an average size of 0.05 × 0.125 μm. The larger pore size of Celgard® 2400 also explains the higher amount of total T permeation in 12 hr from Androgel®; it was 4348.7 ± 243.8 μg/cm2 through Celgard® 2400 whereas it was only 2895.8 ± 245.8 μg/cm2 through cellulose ester. Also, the high amount of alcohol in the gel might be interacting with the membrane, in turn affecting the release of T from the gel.
Hairless guinea pig skin is one of the most reliable models for studying release/permeation of drugs from topical formulations, bearing a close resemblance to human skin. Hence, full-thickness guinea pig skin was chosen to further investigate the release of T from the prepared gels, and we found that all the gels showed similar release to that of Androgel®. Although there was no significant difference between the amounts of T released by different gels, a general trend of increasing release with increasing IPA concentration was evident and based on this observation, two new gels with 5% and 10% w/w IPA (Gel 5 and Gel 10, respectively) were prepared and studied. Even Gel 5 and Gel 10 were found to be similar to Androgel®. This implies that IPA does not increase the release of T, even though an inclination of increasing release with increasing IPA concentration is obvious. The failure to see a statistically significant difference between the gels can be attributed to the high variation observed in the release study data.
The percent coefficient of variation (CV), on hairless guinea pig skin at 42 hr, for Androgel® was 12.399% whereas we found it to be as high as 31.03% for Gel 0. Such high variation can make the method employed less discriminating, which, in turn, makes it difficult to quantify small differences in the release profiles. It was realized during the course of this study that the use of full-thickness skin could be one of the attributes leading to such a high CV. It is a well-known fact that the thickness of skin varies from one part of the body to the other and also from individual to individual (Tan et al. Citation1982). Although care was taken to obtain the skin only from the abdominal region of guinea pigs and to remove the underlying fat tissue carefully and completely, a section of skin with a particular thickness obtained by a dermatome would be more consistent and provide improved results by controlling variability to a great extent.
Carbopol Ultrez 10 offers many advantages over some of the other carbopol grades. It is easier to disperse and mix and is less susceptible to lumping. Moreover, it has much lower viscosity prior to neutralization making handling in mixing tanks and production lines much easier. These superior dispersing properties of Carbopol Ultrez 10 makes it much easier to process and saves production time—the prime reasons for choosing this particular carbopol grade for the formulation of T gels. Although all grades of carbopol contain the same acrylic acid monomer backbone, they differ in the type as well the percentage of cross-linking agents used (Noveon Citation2001; Noveon Citation2002a; Noveon Citation2002b). These rather important structural differences among the carbopols could lead to different drug-releasing characteristics of these polymers, examples of which exist in literature (Mortazavi and Aboofazeli Citation2003; MacLean-McDavitt, Robertson, and Jay 2002). This also could explain the discrepancies observed in the present study. Gel 0 (with 0% IPA) showed significantly less release of T than Androgel®, when the only difference between the two was the type of carbopol. Whereas Androgel® contains Carbopol 980, Carbopol Ultrez 10 was used in gel 0, which suggests a possible drug-polymer interaction leading to lower drug release. Hence, the inability of gels, with increasing amount of IPA, to show a higher release of T than Androgel® might be because of such a possible interaction between T and Carbopol Ultrez 10. Further studies of drug-polymer interactions need to be conducted to rule out such a possibility.
On application of a hydroalcoholic gel, such as Androgel®, to the skin, rapid evaporation of alcohol causes the gel to dry immediately, after which T penetrates through the skin into the systemic circulation. As observed by Williams and Barry (Citation2004) the enhancement effect of ethanol appears to be concentration-dependent. Salicylate ion diffusion across human epidermis was promoted up to an ethanol:water composition of 0.63 whereas higher levels of alcohol decreased permeation (Kurihara-Bergstrom et al. Citation1990). Similar results have been reported for nitrogycerine (Berner et al. Citation1989), estradiol (Pershing, Lambert, and Knutson Citation1990), and zidovudine (Thomas and Panchgnula Citation2003). We thought that the highly dehydrating nature of alcohol causes a quick dryness of the epidermis, which hampers the permeation of drug through the skin. Isopropyl alcohol, on the other hand, being less volatile can prevent the immediate dryness of skin, without hampering the work of alcohol to create a concentration gradient for the drug to permeate. Also, good cosolvent characteristics of IPA are added advantages as it does not affect the consistency and aesthetics of the formulation.
CONCLUSION
Hydroalcoholic gels of T, such as Androgel®, have become extremely popular in the treatment of male hypogonadism because of the many advantages they offer. However, the bioavailability of T from Androgel® is only 10%, leaving a huge potential to increase its systemic availability. In this study we tried to increase the permeation of T across the skin by addition of a penetration enhancer, IPA.
The results of release/permeation studies on guinea pig skin showed that all the gels were similar to Androgel®, that is, none of the gels showed a higher release of T. However, before we could conclude that IPA is unable to, increase the bioavailability of T from hydroalcoholic gels, some of the factors need to be further evaluated, the major one being the differences between carbopol polymers. Carbopol Ultrez 10 was used in the gels formulated whereas Androgel® contains Carbopol 980, which could very well explain the discrepancies. While Androgel® can cause extreme dryness of the skin reducing the permeation on application due to its high ethanol content, addition of IPA can reduce such dryness as it is less volatile than ethanol. Also, IPA acts as a cosolvent and does not affect the formulation with respect to consistency and aesthetic appeal. Because of such possible advantages, we conclude that IPA has potential benefits that can aid in higher release of T from hydroalcoholic gel formulations.
REFERENCES
- Bals-Pratsch M., Knuth U. A., Yoon Y. D., Nieschlad E. Transdermal testosterone substitution therapy for male hypogonadism. Lancet 1986; 2: 943–946
- Berner B., Mazzenga G. C., Otte J. H., Steffens R. J., Juang R. H., Ebert C. D. Ethanol: water mutually enhanced transdermal therapeutic system II: skin permeation of ethanol and nitroglycerin. J. Pharm. Sci. 1989; 78: 402–407
- Chan T. C. Percutaneous penetration enhancers: an update. Biennial Int Conf Perspect Percutaneous Penetration 2005; 9: 18–22
- Cunningham G. R., Cordero E., Thornby J. I. Testosterone replacement with trandermal therapeutic systems. Physiological serum testosterone and elevated dihydrotestosterone levels. J Am. Med. Assoc. 1989; 261: 2525–2530
- Dobs A. S., Meikle A. W., Arver S., Sanders S. W., Caramelli K. E., Mazer N. A. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J. Clin. Endoc. Metab. 1999; 84: 3469–3478
- Dudley R. E., Kottayil S. G., Palatchi O. Pharmaceutical composition and method for treating hypogonadism. U.S. Patent 651777[6,503,894], 2003
- HIV Hotline. Hepatic effects of 17 alpha-alkylated anabolic-androgenic steroids. 1998; 8: 2–5
- Jockenhovel F. Testosterone therapy—what, when and to whom?. Aging Male 2004; 7: 319–324
- Jordan W. P. Allergy and topical irritation associated with transdermal testosterone administration: a comparison of scrotal and nonscrotal transdermal systems. Am. J. Contact Dermatitis 1997; 8: 108–113
- Ko K. T., Needham T. E., Zia H. Emulson formulations of testosterone for nasal administration. J. Microencap. 1998; 15: 197–205
- Kurihara-Bergstrom T., Knutson K., DeNoble L. J., Goates C. Y. Percutaneous absorption enhancement of an ionic molecule by ethanol:water systems in human skin. Pharm. Res. 1990; 7: 762–766
- Mackey M. A., Conway A. J., Handelsman D. J. Tolerability of intramuscular injections of testosterone ester in oil vehicle. Hum. Reprod. 1995; 10: 862–865
- MacLean-McDavitt D. S., Robertson J. D., Jay M. Monitoringthe in vitro delivery of proteins from carbomer hydrogels by x-ray fluorescence. Pharmaceutical Research. 2003; 20: 435–441
- The Medical Letter, Inc. Androgel®. Medical Letter on Drug Therapies 2000; 42(1080)49–52
- Meikle A. W., Arver S., Dobs A. S., Sanders S. W., Rajaram L., Mazer N. A. Pharmacokinetics and metabolism of a permeation-enhanced testosterone transdermal system in hypogonadal men: influence of application site-a clinical research center study. J. Clin. Endocrin. Metab. 1996; 81: 1832–1840
- Montgomery D. C. Design and Analysis of Experiments. John Wiley and Sons, New York 1991
- Morales A., Lunenfeld B. Investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male 2002; 5: 74–86
- Mortazavi S. A., Aboofazeli R. An investigation into the effect of carbopols on the release of propranolol HCI from tablet matrices. Iranian J. Pharm. Res. 2003; 1: 23–27
- Noveon. Polymers in Semisolid Products. Noveon Inc, Cleveland 2001, http://www.pharma.noveon.com/literature/default.asp Retrieved on 05/12/04
- Noveon. Carbopol Ultrez 10 Polymer for Personal Care. Noveon, Inc, Cleveland 2002a, http://www.pharma.noveon.com/literature/default.asp#Tech Retrieved on 05/12/04
- Noveon. Product and Regulatory Guide. Noveon, Inc, Cleveland 2002b, http://www.pharma.noveon.com/literature/default.asp Retrieved on 05/12/04
- Pershing L. K., Lambert L. D., Knutson K. Mechanism of ethanol-enhanced estradiol permeation across human skin in vivo. Pharm. Res. 1990; 7: 170–175
- Schulte-Beerbuhl M., Nieschlag E. Comparison of testosterone, dihydrotestosterone, luteinizing hormone, and follicle-stimulating hormone in serum after injection of testosteron enanthate and testosterone cypionate. Ferti. Steril. 1980; 33: 201–203
- Schurmeyer T., Nieschlag E. Comparative pharmacokinetics of testosterone enanthate and testosterone cyclohexanecarboxylate as assessed by serum and salivary testosterone levels in normal men. Int. J. Androl. 1984; 7: 181–187
- Swerdloff R. S., Wang C., Cunningham G., Dobs A., Iranmanesh A., Matsmoto A. M., Snyder P. J., Weber T., Longstreth J., Berman N. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J. Clin. Endocrin. Metab. 2000; 85: 4500–4510
- Tan C. Y., Statham B., Marks R., Payne P. A. Skin thickness measurement by pulsed ultrasound: its reproducibility, validation and variability. Br. J. Dermatol. 1982; 106: 657–667
- Tenover J. L. Male hormone replaement therapy including “andropause. Endocrin. Metab. Clin. N. Am. 1998; 27: 969–987
- Thomas N. S., Panchagnula R. Transdermal deliery of zidovudine: effect of vehicles on permeation across rat skin and their mechanism of action. Eur. J. Pharm. Sci. 2003; 18: 71–79
- Tripathi K. D. Essentials of Medical Pharmacology. Jaypee Brothers Medical Publishers (P) Ltd., New DelhiIndia 2000; 298–325
- Wang C., Berman N., Longstreth J. A., Chuapoco B., Hull L., Steiner B., Faulkner S., Dudley R. E., Swerdloff R. S. Pharmacokinetics of transdermal testosterone gel in hypogonadal men: application of gel at one site vesus four sites: a general clinical research center study. J. Clin. Endocrin. Metab. 2000; 85: 964–969
- Williams A. C., Barry B. W. Penetration enhancers. Adv. Drug Del Rev. 2004; 56: 603–618
- Wilson J. D. Goodman and Gilman's The Pharmacological Basis of Therapeutics. Pregamon Press, Columbus, Ohio 1990; 1413