Abstract
Sildenafil is an active substance that has already been approved by FDA for human use. It is known to be an active compound for the treatment of sexual dysfunction in men. Some encouraging results have been published concerning the treatment of infertility with sildenafil in women, but there is no pharmaceutical preparation available. Therefore, various formulations were prepared and the most suitable sildenafil release was found to be with the sildenafil-containing suppositories prepared using Eudragit RS100 and Witepsol H15. The vaginal insert with EVAC 210 polymer containing sildenafil has also provided sildenafil release for a longer period.
INTRODUCTION
The World Health Organization estimates that one in six couples experience a delay in conception (Edi-Osage et al. Citation2004), and the rate is estimated to be as high as 40% for such couples (Wyshak Citation2001). Reports suggest the presence of subtle hormonal and endometrial defects in these conditions, including abnormal endometrial integrin expression and an unfavorable endometrial environment associated with defective biosynthesis and distribution of endometrial glycoconjugates and poor development of the endometrium (Graham et al. Citation1990; Klentzeris et al. Citation1991 and Lessey et al. 1995). Although debate continues about the relative impacts of embryonic versus endometrial factors on implantation (Schwartz et al. Citation1997), little effort is being directed at manipulating the dynamics of endometrial development, specifically the factors influencing its receptivity (Carson et al. Citation2000).
Estrogen-induced endometrial proliferation is in large part dependent upon blood flow to the basal endometrium (Sher et al. Citation2002). It has been shown in animals that nitric oxide (NO) release can lead to relaxation of vascular smooth muscle through a cyclic guanyl monophosphate (cGMP)-mediated pathway (Ballard et al. Citation1998). Phosphodiesterase hydrolyzes cyclic nucleotides such as cGMP (Telfer et al. Citation1997). Sildenafil is a type 5 specific PDE inhibitor and it received FDA approval in 1998 for the treatment of erectile dysfunction (Boyce et al. Citation2001). Sildenafil citrate is a white powder having a molecular weight of 666.7 Da, 3.5 mg/ml aqueous solubility, and a pKa value of 6.5 (Walsh et al. Citation2001). The solubility and the partition of sildenafil depend on the pH of the environment (Liaw et al. Citation2001). Consequently, in vitro release experiments should be performed at pH 4.5 if the sildenafil formulation is designed to be administered through the vaginal route.
Sildenafil is metabolized from the methyl group on the piperasine ring to active metabolites by cytochrome P450 enzyme. These metabolites inhibit phosphodiesterase 5 (PDE 5), resulting in relaxation of smooth muscles by simultaneously increasing nitric oxide (NO) release and thus preventing the degradation of cyclic guanosine mono phosphate (cGMP) (Nevado et al. Citation2002). These mechanisms have been used for the therapeutic stimulation of erectile response (Kloner et al. Citation1999). Sildenafil inhibits PDE type 5 more selectively than other PDE inhibitors (Andersson et al. Citation2000).
Although, sildenafil has been introduced and used with great success to treat male erectile dysfunction, it has been reported that vaginally administered sildenafil could lead to an improvement in uterine blood flow and, in conjunction with controlled ovarian hyper stimulation, led to estrogen induced proliferation of the endometrial lining in patients with in vitro fertilization failure associated with poor endometrial development (Sher et al. Citation2002 and Acartürk et al. Citation2003). The vaginal administration of sildenafil, therefore, is increasing in popularity. According to the literature, patients need to receive vaginal sildenafil administration at a dose of 25 mg 4 times a day for effective treatment (Sher et al. Citation2002 and Acartürk et al. Citation2003). Therefore, a controlled release vaginal sildenafil tablet would reduce administration discomfort and effective therapy can be obtained. This study sought to design and prepare three different types of vaginal dosage forms containing sildenafil, namely vaginal tablets, vaginal suppositories, and vaginal inserts using various polymers and to determine their drug release properties. Different types of vaginal tablet were prepared using poly carbophyl (PC) and chitosan (CH) as bioadhesive polymers. Vaginal suppository formulations were also prepared with Witepsol H15 containing sildenafil in either alginate or Eudragit RS100 beads. Vaginal inserts containing sildenafil ethylene vinyl acetate (EVAC210) were also prepared. The release and swelling properties of the formulations were determined in a pH 4.5 phosphate buffer to mimic vaginal medium.
MATERIALS AND METHODS
Sildenafil was donated by Fako Pharmacuticals (İstanbul-Turkey). Polycarbophil (PC) was obtained from Noveon Inc.-USA. Chitosan (CH)(Kitomer, type CHCN1), Eudragit RS100 and EVAC210 supplied by Marinard Ltd., Canada; Rohm-Pharma, Germany; and Dupond, USA, respectively. Other chemicals and solvents were of analytical grade.
Preparation of Vaginal Tablet Formulations
Vaginal tablets were prepared using various amounts of lactose, CH and PC. The contents of the tablets are shown in . Tablets were compressed using a 10 mm. punch, on a tablet machine (Korsh-Erweka Gmbh). The compression force was 50 barr.
TABLE 1 Contents of vaginal tablet formulations
Determination of Swelling/Water Uptake Properties of the Tablets
The swelling/water uptake properties of the formulations were also determined. Tablets were placed in a small stainless steel cage and immersed into the pH 4.5 phosphate buffer solution at 37°C. Tablets were removed and weighed at predefined time intervals. The swelling/water uptake values as a percentage were calculated as follows:
Determination of Bioadhesion Force of the Tablets
Fresh cow vagina was obtained from a slaughter house soon after slaughter and kept at −4°C until used. The muscles and fatty tissues were removed by dissection. The vagina wall was then cut into 2 × 2 cm samples and kept in isotonic saline solution. The vagina sample and tablet were fixed to the tensile strength meter using acrylate type glue, 50 μ l pH 4.5 phosphate buffer solution was applied to the vaginal mucosa surface, and the mucosa and tablet were brought into contact for a second. The punch was removed at a constant rate and the adhesion force was determined. The tensile strength meter was designed and built in our laboratory. The strength meter consisted of a piezo-electric crystal and an electric circuit connected to the computer. The strength meter was simply calibrated using standard weights.
Preparation of Vaginal Suppositories and Vaginal Insert Containing Sildenafil
Preparation of Vaginal Suppositories with Witepsol H15, PEG4000, or PEG6000 Containing Sildenafil
The vehicle of the suppositories (Witepsol H15 or PEG 4000 or PEG 6000) was melted in a water bath maintained at 60°C and mixed with sildenafil powder and poured into the suppository molds. Each suppository contained 100 mg of sildenafil. Suppositories were wrapped with a thin aluminum sheet and stored at 4°C until used.
Preparation of Vaginal Suppositories with Witepsol H15 Containing Sildenafil in Sodium Alginate Beads
Sodium alginate solutions were prepared in water at various concentrations and mixed with various amounts of sildenafil ().
TABLE 2 Contents of sodium alginate formulations
Sodium alginate solution was transferred slowly (drop by drop) into a 0.5 M CaCl2 solution with a peristaltic pump, while the solution was stirred with a magnetic stirrer at ambient temperature. The beads were hardened in CaCl2 solution and filtered and dried at 35°C.
Not all the formulations provided good bead formation. Only S2 and S3 were considered for further experiments. Witepsol H15 was melted in a water bath maintained at 60°C and mixed with sildenafil beads (S2 or S3) and poured into the suppository molds. Each suppository contained 100 mg of sildenafil. Suppositories were wrapped with a thin aluminum sheet and stored at 4°C until used.
Preparation of Vaginal Suppositories with Witepsol H15 Containing Sildenafil in Eudragit RS100 Beads
Eudragit RS100 (2 g) was dissolved in dichloromethane and mixed with sildenafil (2 g). The organic phase was evaporated at 40°C and dry powder was sieved (128 mesh). Witepsol H15 was melted in a water bath maintained at 60°C and mixed with Eudragit beads containing sildenafil and poured into the suppository molds. Each suppository contained 100 mg of sildenafil. Suppositories were wrapped with a thin aluminum sheet and stored at 4°C until used.
Preparation of Vaginal Insert
Evac-210 (5 g) was dissolved in trichloroethylene (20 ml) and mixed with sildenafil (10 g) solution. The mixture was poured into a horizontally placed petri dish (10 cm radius) and solvent was evaporated at room temperature overnight. The film was then gently cut into a ring shape. The outer and inner diameter and the thickness of the ring were 3 cm, 1 cm, and 0.2 cm, respectively.
Determination of Dissolution and Release Properties of the Vaginal Tablets, Suppositories and Insert
The in vitro dissolution experiments were carried out using the USP paddle method at pH 4.5 phosphate buffer solution at 37°C to mimic the vaginal medium. Samples were taken at predefined time intervals and analyzed spectrophotometrically. The release profiles of the tablets were then obtained and release kinetics compared.
RESULTS AND DISCUSSION
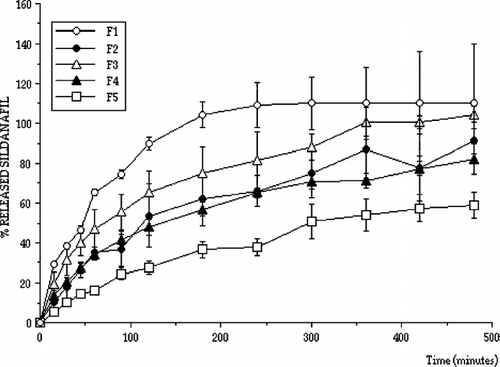
Normal microflora, predominantly lactobacilli, produce sufficient lactic acid to acidify vaginal secretions to pH levels between 3.5 and 4.5. This value is maintained by the lactobacilli, which convert glycogen from exfoliated epithelial cells into lactic acid (Boskey et al. Citation2001; Stewart-Tull Citation1964). Therefore, drug releases from prepared vaginal tablets containing sildenafil were determined at pH 4.5 to mimic vaginal medium conditions and plotted. shows the release characteristics of formulations at pH 4.5 (formulation F1-F5).
The release properties of PC containing tablets were also determined. shows sildenafil releases from various tablet formulations containing various amounts of PC at vaginal pH.
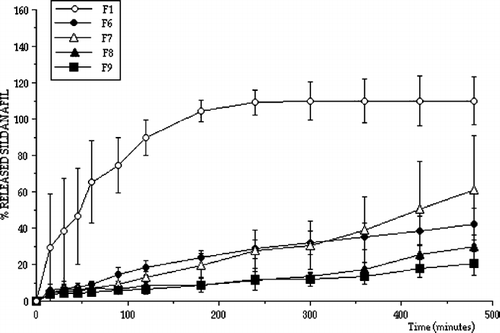
All release profiles were analyzed in terms of release kinetics. shows the results of kinetic analysis. Although the tablets were designed to release sildenafil for a longer time with zero order for effective vaginal therapy, not all tablet formulations showed controlled sildenafil release with zero order kinetics. However, formulations F7, F8, and F9 exhibited controlled release properties, sildenafil was released for a longer period and they also provided zero order release considering r2 values and residuals. Apart from formulations F7, F8, and F9 release kinetics with higher r2 values and residuals fit more closely to Q√t or Hixon-Crowell kinetics indicating that drug release from the matrices by diffusion and swelling creates different diffusion surfaces and pathways. As a result formulations F7, F8, and F9 were found to be suitable for longer drug release with zero order.
TABLE 3 Release kinetics of sildenafil tablets
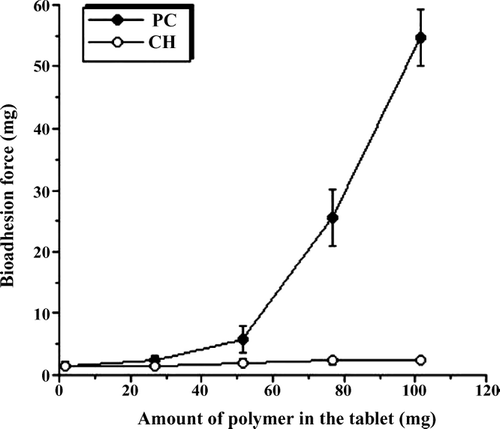
Rational design of vaginal formulations needs to include attention to vehicle properties that optimize vaginal coating and retention. It should be taken into account that formulations interact with the vaginal fluids and cause changes in viscosity. The interactions depend upon the specific macromolecules which are the thickening agents for gels or important auxiliary agents in tablets. Therefore, these macromolecules, such as poly(acrylates), chitosan, and many others, are reported to be very promising excipients for future formulations (Valenta Citation2005). Several bioadhesive polymers have been reported for different mucosal sites such as the buccal cavity, stomach and intestine (Ahuja et al. Citation1997). An increasing interest has been shown for vaginal bioadhesive tablets (Brannon-Peppas Citation1993). With regard to their facilitated and shorter licensing procedure, the polymers that are already in use as pharmaceutical excipients were tested for their mucoadhesive properties. Therefore, the bioadhesion strengths of tablets were also determined and PC was found to produce a higher bioadhesion force ().
El-Kamel and colleagues (Citation2004) have prepared vaginal tablets containing metronidazole, sodiumcarboxymethyl-cellulose (NaCMC), methylcellulose (MC), hydroxypropylmethylcellulose (HPMC) and HPMC/NaCMC mixtures and Carbopol 934, in different ratios, and the dissolution rates of metronidazole from vaginal tablets were compared (El-Kamel et al. Citation2004). Drug dissolution rates in water were obtained in the following order MC > NaCMC > Carbopol > HPMC. Swelling studies indicated an increase in swelling and mucoadhesion and resulted in the same order (El-Kamel et al. Citation2004). Carbopol can be a valuable alternative to cellulose derivatives. In another study bioadhesive acyclovir vaginal tablets were prepared by using poly(acrylic acid), methylcellulose (MC), carboxymethylcellulose (CMC), hydroxypropyl cellulose (HPC) and hydroxypropyl methylcellulose (HPMC) as bioadhesive polymers in different ratios using direct compression and wet granulation techniques. The swelling behavior of these vaginal tablets in distilled water and in lactic acid solution, the acyclovir release rate and bioadhesion to cow vagina were investigated (Genc et al. Citation2000). Swelling of the tablets containing HPC, CMC, and MC was found to be very rapid and caused disintegration of the tablets. The swelling behavior of the tablets containing HPMC lasted 6 h in lactic acid solution (Genc et al. Citation2000). New dosage forms for the antimycotic clotrimazole were developed by including bioadhesive polymers (polycarbophil, HPMC, and sodium hyaluronate) into pessaries made of semisynthetic solid triglycerides. Among the bioadhesive polymers employed, the most positive results were obtained using PC (Ceschel et al. Citation2001).
After these considerations, PC was chosen as a stabile, compatible and non-toxic material for vaginal application (Neves et al. Citation2006 and Fiorilli et al. Citation2005), but it is an anionic polymer and some interactions with the sildenafil molecule may occur. However, our results show that it may be useful to obtain sustained or prolonged drug release.
CH is a polycationic polymer and it has been used to prepare some gel formulations (Degim et al. Citation2005 and Issa et al. Citation2005) and to fabricate transdermal devices for both lipophilic and hydrophilic drugs (Martina et al. Citation2003 and Thacharodi et al. Citation1996). The penetration-enhancing effect of CH through mucosal membrane was also shown and the mechanism was found to be due to opening tight junctions (Artursson et al. Citation1994).
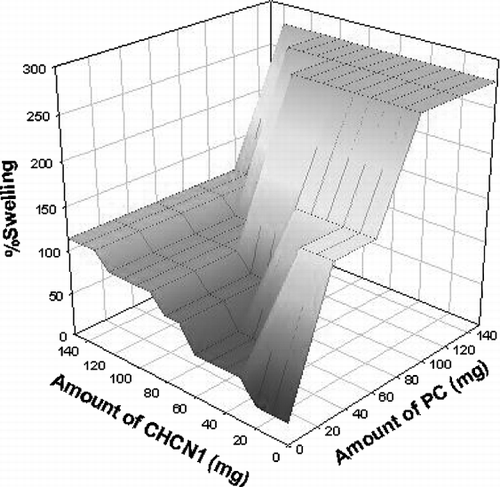
Therefore, CH was also found to be a useful agent for applying tablets containing sildenafil to the vaginal wall or vaginal membranes. Similarly, an augmenting effect on the swelling was observed when a higher amount of PC was used in the formulation in our study. In fact, a higher amount of CH in the formulation increased the swelling, but this effect was found to be higher when PC was used ().
The cationic character of the chitosan and its potential functional groups make it an attractive biopolymer for many biomedical and pharmaceutical applications. Chitosan has been used in various formulations as powders or as an ingredient in tablets, emulsions, and gels. Furthermore, a controlled release of incorporated drugs has been demonstrated (Illum Citation1998 and Dodane et al. Citation1998). Chitosan also has mucoadhesive properties and antimicrobial activity (Kim et al. Citation2003 and Luessen et al. Citation1996). The use of natural polymers is valuable considering proven biocompatibility and safety. Chitosan possesses favorable properties and hence has applications in the pharmaceutical and biomedical fields (Miyazaki et al. Citation1994; Felt et al. Citation1998). It is a promising bioadhesive material at physiological pH and especially in acidic environments such as vaginal pH. This polymer possesses OH and NH2 groups that can increase the hydrogen bonding ability. These properties are reported to be essential for mucoadhesion (Peppas et al. Citation1985; Robinson et al. Citation1995). Moreover, chitosan is suited for longer applications, since it does not become inactivated after the first contact and no decrease on mucoadhesion has been reported after application (Lehr et al. Citation1992).
A general observation is the sustained release behavior of chitosan at a concentration of 50% of tablet weight (Sawayanagi et al. Citation1982; Nigalaye Citation1990). In our studies, the lowest release rate was observed when the highest amount of CH was used in the tablet formulation ( and ). The maximum bioadhesion could result in an increased residence time of a drug at the site of absorption by interacting with the mucosa (Duchene et al. Citation1992; Khanvilkar et al. Citation2001). Chitosan is also known to exhibit antimicrobial activity (Bernkop-Schnurch et al. Citation2003). A common microbial problem in the vulvovaginal tract is the infection with C. albicans (Lanchares et al. Citation2000). Approximately 75% of women will have a vaginal infection with a Candida strain during their life and about 40 to 50% will suffer a second one and a small percentage will show a chronic course (Ferrer et al. Citation2000). Therefore, this may be another advantage of using chitosan in tablet form for vaginal administration.
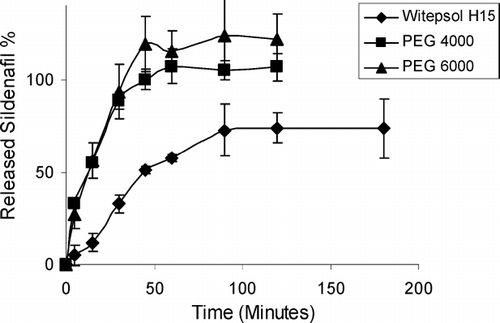
Vaginal drug delivery systems include a large variety of pharmaceutical forms such as semi-solids, tablets, capsules, pessaries, liquid preparations, vaginal films, vaginal rings, foams, and tampons. Most widely used semi-solid preparations for vaginal drug delivery include creams, ointments, gels and suppositories (Vermani et al. Citation2000; Neves et al. Citation2006). Vaginal suppository formulations containing sildenafil were also prepared. Sildenafil releases from prepared vaginal suppositories were also investigated. shows the release profiles of sildenafil from various vaginal suppositories.
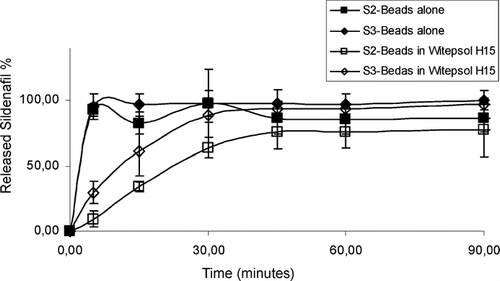
The suppository formulations did not release sildenafil with zero order for a longer period. Therefore, it was decided to use alginate as a release controlling polymer. Alginate beads were prepared containing sildenafil and release characteristics were investigated. The sildenafil release was not found to be slow enough, and alginate beads were mixed with the Witepsol H15 suppository base and molded. The releases from these suppositories are shown in .
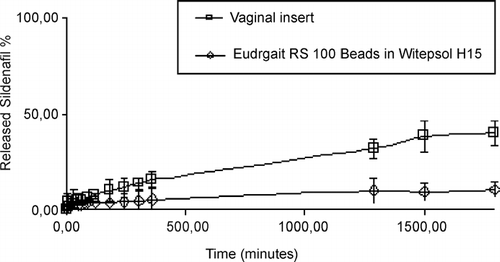
The release of sildenafil was not found to be with the zero order for a longer period. In other words, sildenafil was released fast from these prepared formulations (S2, S3, or other formulations containing S2 or S3). Therefore, sildenafil was mixed with Eudragit RS100 and Eudragit RS100 beads were prepared containing sildenafil to reduce the drug release rate. A vaginal insert containing EVAC 210 polymer and sildenafil was also prepared. The release properties of sildenafil from Eudragit RS100 beads and Witepsol H15 containing suppositories and from the vaginal insert were investigated. shows the in vitro release results of sildenafil containing Eudragit RS100 beads and Witepsol H15 containing suppositories and the vaginal insert.
CONCLUSION
In conclusion, the use of PC and CH for the preparation of controlled release sildenafil tablets for vaginal administration was found to be useful. The most suitable and long-lasting sildenafil release preparation, however, was found to be provided by the sildenafil-containing suppository prepared with Eudragit RS100 beads in Witepsol H15 suppository base. The vaginal insert prepared with EVAC 210 polymer containing sildenafil also provided sildenafil release for a longer period. These proposed formulations may be beneficial for both scientists and patients with infertility problems caused by a poor endometrial development.
REFERENCES
- Acartürk F., Değim T., Tuğcu-Demiröz F., İbasmı S. Vaginal controlled release sildenafil tablets, First EUFEPS conference on Optimising drug delivery and formulation: New challenges in drug delivery. APGI, ParisFrance 2003
- Ahuja A., Khar R. K., Ali J. Mucoadhesive drug delivery systems,. Drug Dev. Ind. Pharm. 1997; 23: 489–515
- Andersson K. E., Stief C. Penile erection and cardiac risk: Pathophysiologic and pharmacologic mechanisms. Am. J. Cardiol. 2000; 86(Suppl)23F–26F
- Artursson P., Lindmark T., Davis S. S., lllum L. Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (CAC O-2). Pharm. Res. 1994; 11: 1358–1361
- Ballard S. A., Gingell C. J., Tang K., Turner L. A., Price M. E., Naylor A. M. Effects of Sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isoenzymes. J. Urol. 1998; 159: 2164–2171
- Bernkop-Schnurch A., Hornof M., Zoidl T. Thiolated polymers—Thiomers: Modification of chitosan with 2-iminothiolane. Int. J. Pharm. 2003; 260: 229–237
- Boskey E. R., Cone R A, Whaley K. J., Moench D. R. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum. Reprod. 2001; 16: 1809–1813
- Boyce E. G., Umland E. M. Sildenafil citrate: A therapeutic update. Clini. Therapeutics. 2001; 23: 2–23
- Brannon-Peppas L. Novel vaginal drug release applications. Adv. Drug Deliv. Rev. 1993; 11: 169–177
- Carson D. D., Bagchi I., Dey S. K., Enders A. C., Fazleabas A. T., Lessey B. A., Bruce A., Yoshinaga L., Yoshinaga K. Embryo implantation. Dev. Biol. 2000; 223: 217–237
- Ceschel G. C., Maffei P., Lombardi B. S., Ronchi C., Rossi S. Development of a mucoadhesive dosage form for vaginal administration. Drug Dev. Ind. Pharm. 2001; 27: 541–554
- Degim Z., Degim T., Acarturk F., Erdogan D., Ozogul C., Koksal M. Rectal and vaginal administration of insulin-chitosan formulations: an experimental study in rabbits. J. Drug Target. 2005; 3: 563–572
- Dodane V., Vilivalam V. D. Pharmaceutical applications of chitosan. Pharm. Sci. Technol. Today 1998; 1: 246–253
- Duchene D., Ponchel G. Principle and investigation of the bioadhesion mechanism of solid dosage forms. Biomaterials 1992; 13: 709–714
- Edi-Osagie E. C. O., Seif M. W., Aplin J. D., Jones C. J. P., Wilson G., Path M. R. C., Lieberman B. A. Characterizing the endometrium in unexplained and tubal factor infertility: a multiparametric investigation. Fertil. Steril. 2004; 82: 1379–1389
- El-Kamel A. H., Sokar M. S., Naggar V. F., Al-Gamal S. Chitosan and sodium alginate-based bioadhesive vaginal tablets. AAPS Pharm Sci. 2004; 4: E44
- Felt O., Buri P., Gurny R. T. Chitosan: a unique polysaccharide for drug delivery. Drug Dev. Ind. Pharm. 1998; 24: 979–1003
- Ferrer J. Vaginal candidosis: epidemiological and etiological factors. Int. J. Gynaecol. Obstet. 2000; 71: S21–S27
- Fiorilli A., Molteni B., Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomised double-blind, placebo-controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005; 120: 202–205
- Genc L., Oguzlar C., Guler E. Studies on vaginal bioadhesive tablets of acyclovir. Pharmazie 2000; 55: 297–299
- Graham R. A., Seif M. W., Aplin J. D., Li T. C., Cooke I. D., Rogers A. W. An endometrial factor in unexplained infertility,. BMJ 1990; 300: 1428–1431
- Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998; 15: 1326–1331
- Issa M. M., Koping-Hoggard M., Artursson P. Chitosan and the mucosal delivery of biotechnology drugs”. Drug Discov. Today 2005; 2: 1–6
- Kim K. W., Thomas R. L., Lee C., Park H. J. Antimicrobial activity of native chitosan, degraded chitosan, and Ocarboxymethylated chitosan. J. Food Prot. 2003; 66: 1495–1498
- Klentzeris L. D., Bulmer J. N., Li T. C., Morrison L., Warren A., Cooke I. D. Lectin binding of endometrium in women with unexplained infertility. Fertil. Steril. 1991; 56: 660–667
- Khanvilkar K., Donovan M. D., Flanagan D. R. Drug transfer through mucus. Adv. Drug Deliv. Rev. 2001; 48: 173–193
- Kloner R. A., Zusman R. M. Cardiovascular effects of sildenafil citrate and recommendations for Its use. Am. J. Cardiol. 1999; 84: 11N–17N
- Lanchares J. L., Hernandez M. L. Recurrent vaginal candidiasis changes in etiopathogenical patterns. Int. J. Gynaecol. Obstet. 2000; 71: S29–S35
- Lehr C. M., Bouwstra J. A., Schacht E. H., Junginger H. E. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int. J. Pharm. 1992; 78: 43–48
- Lessey B. A., Castlebaum A. J., Swain S. W., Sun J. Integrins as markers of uterine receptivity in women with primary unexplained infertility. Fertil. Steril. 1995; 63: 535–542
- Liaw J., Chang T. W. Determination of transdermal sildenafil in nude mouse skin by reversed- phase high- performance liquid chromatography. J. Chromatog. B. 2001; 765: 161–166
- Luessen H. L., Leeuw B. J., Langemeyer M. W., Boer A. B., Verhoef J. C., Junginger H. E. Mucoadhesive polymers in peroral peptide drug delivery: VI. Carbomer and chitosan improve the intestinal absorption of the peptide drug buserelin in vivo. Pharm. Res. 1996; 13: 1668–1672
- Martina L., Wilsona C. G., Kooshab F., Uchegbua I. F. Sustained buccal delivery of the hydrophobic drug denbufylline using physically cross-linked palmitoyl glycol chitosan hydrogels. Eur. J. Pharm. Biopharm. 2003; 55: 35–45
- Miyazaki S., Nakayama A., Oda M., Takad M. Chitosan and sodium alginate based bioadhesive tablets for intra oral drug delivery. Biol. Pharm. Bull. 1994; 17: 745–747
- Nevado J. J. B., Flores J. R., Peńalvo G. C., Farińas N. G. Determination of sildenafil citrate and its main metabolite by sample stacking with polarity switching using micellar electrokinetic chromatography. J. Chromatog. A. 2002; 953: 279–286
- Neves J., Bahia M. F. Gels as vaginal drug delivery systems. Int. J. Pharm. 2006; 318: 1–14
- Nigalaye A. G., Adusumilli P., Bolton S. Investigation of prolonged drug release from matrix formulations of chitosan. Drug Dev. Ind. Pharm. 1990; 16: 449–467
- Peppas N. A., Bury P. A. Surface interfacial and molecular aspects of polymer bioadhesion on soft tissues. J. Control. Release 1985; 2: 257–275
- Robinson J. R., Mlynek G. M. Bioadhesive and phase-change polymers for ocular drug delivery. Adv. Drug Deliv. Rev. 1995; 16: 45–50
- Sawayanagi Y., Nambu N., Nagai T. Use of chitosan for sustained release preparations of water soluble drugs. Chem. Pharm. Bull. 1982; 31: 4213–4215
- Schwartz L. B., Chiu A. S., Courtney M., Krey L., Schmidt-Sarosi C. The embryo versus endometrium controversy revisited as it relates to predicting pregnancy outcome in in-vitro fertilization–embryo transfer cycles. Hum. Reprod. 1997; 12: 45–50
- Sher G., Fisch J. D. Effect of vaginal sildenafil on the outcome of in vitro fertilization (IVF) after multiple IVF failures attributed to poor endometrial development. Fertil. Steril. 2002; 78: 1073–1076
- Stewart-Tull D. E. S. Evidence that vaginal lactobacilli do not ferment glycogen. J. Obstet. Gynaecol. 1964; 88: 676–679
- Telfer J. F., Irvine G. A., Kohnen G., Cambell S., Cameron S., Cameron I. T. Expresion of endothelial and inducible nitric oxide synthase in non-pregnant and decidualized human endometrium. Mol. Hum. Reprod. 1997; 3: 69–75
- Thacharodi D., Panduranga R. K. Collagen-chitosan composite membranes controlled transdermal delivery of nifedipine and propranolol hydrochloride. Int. J. Pharm. 1996; 134: 239–241
- Valenta C. The use of mucoadhesive polymers in vaginal delivery. Advanced Drug Delivery Reviews. 2005; 57: 1692–1712
- Vermani K., Garg S. The scope and potential of vaginal drug delivery. Pharm. Sci. Technol. Today 2000; 3: 359–364
- Walsh P. Physicians' Desk Reference, 55th Edition. Medical Economics Company, Montvale 2001
- Wyshak G. Infertility in American college alumnae. Int. J. Gynecol. Obstet. 2001; 73: 237–242