Abstract
Dexamethasone sodium phosphate (Dex-SP) is the most commonly used drug administered via intratympanic injection for the treatment of acute hearing loss, but its penetration efficiency into the inner ear is very low. To address this problem, we evaluated the possibility of administering dexamethasone nanosuspensions via intratympanic injection because hydrophobic drugs might be more effective in penetrating the inner ear. Three types of dexamethasone nanosuspensions were prepared; the dexamethasone nanoparticles in the three nanosuspensions were between approximately 250 and 350 nm in size. To compare the efficiency of Dex-SP and dexamethasone nanosuspension in delivering dexamethasone to the inner ear, the concentrations of dexamethasone in perilymph and cochlear tissues were compared by liquid chromatography–mass spectrometry. The dexamethasone nanosuspensions resulted in significantly higher drug concentrations in perilymph and cochlear tissues than Dex-SP at 6 h; interestingly, animals treated with nanosuspensions showed a 26-fold higher dexamethasone concentrations in their cochlear tissues than animals treated with Dex-SP. In addition, dexamethasone nanosuspension caused better glucocorticoid receptor phosphorylation than Dex-SP both in vitro and in vivo, and in the ototoxic animal model, the nanosuspension showed a significantly better hearing-protective effect against ototoxic drugs than Dex-SP. In the in vivo safety evaluation, the nanosuspension showed no toxicity at concentrations up to 20 mg/mL. In conclusion, a nanosuspension of dexamethasone was able to deliver dexamethasone to the cochlea very safely and efficiently and showed potential as a formula for intratympanic injection.
Introduction
Dexamethasone is widely used to treat acute hearing loss (Stachler et al., Citation2012). However, since dexamethasone is not very soluble in water, dexamethasone sodium phosphate (Dex-SP), which is approved for intravenous use, is used off label for intratympanic injection in patients with acute hearing loss. Among the several pathways by which drugs enter the cochlea via the middle ear, the round window membrane is the most important route (King et al., Citation2011). Unfortunately, little is known about the mechanism by which drugs pass through the round window membrane or what properties of drugs affect their passage through that barrier. Recently, Salt et al. (Citation2018) reported that the lipophilicity of drugs affects their permeation of the round window membrane and that hydrophobic drugs pass through the membrane more effectively than hydrophilic drugs. The mechanism by which polyp nanoparticles (PLGA, polyp-lactic-co-glycolic acid) translocate across the round window membrane was previously reported by Zhang et al. The penetration of PLGA nanoparticles across the round window membrane via the transcellular pathway was observed by confocal laser scanning microscopy (Zhang et al., Citation2018). A hydrophobic drug would be advantageous, as these drugs penetrate the cell membrane when they pass through the transcellular pathway.
Since most of the steroids and antioxidants used in acute hearing loss are hydrophobic, it is possible to develop a new type of intratympanic injection for the treatment of acute hearing loss if these properties of the round window membrane are exploited. Several methods, including nanoparticles, have been tested as ways to formulate hydrophobic drugs into intratympanic injections (Sun et al., Citation2015, Citation2016; Wang et al., Citation2018; Yang et al., Citation2018; Rousset et al., Citation2019; Jung et al., Citation2021). We wondered whether dexamethasone, a hydrophobic drug, could be made into a nanosuspension and injected into the middle ear cavity; furthermore, we wondered whether this dexamethasone nanosuspension solution would be more efficient than the hydrophilic compound Dex-SP in delivering the drug to the cochlea.
Nanosuspensions are nanosized colloidal dispersion systems that are stabilized by surfactants and/or polymers; they are efficient and intelligent drug delivery systems for water-insoluble drugs because these platforms increase the saturation solubility and the surface area available for dissolution (Wang et al., Citation2013). Reduction of the drug particle size to the nanometer level increases the total effective surface area, thereby increasing the dissolution rate. Additionally, a reduction in particle size leads to a reduction in the thickness of the diffusion layer surrounding the drug particles, resulting in an increase in the concentration gradient. Each of these properties leads to improved bioavailability of the drug (Dizaj et al., Citation2015). The active pharmaceutical ingredient (API) particles in a nanosuspension consist entirely of the drug that is to be delivered; no carriers or vehicles are included in the particles. Thus, high drug loading (100%) of the nanosuspensions could result in highly efficient transportation of the drug into cells, achieving adequately high therapeutic concentrations and maximizing the pharmacological effects (Wang et al., Citation2017).
Due to the above advantages, nanosuspensions are used for drug delivery to various organs of the body through the oral, ocular, pulmonary, and transdermal routes (Piao et al., Citation2008; Gupta et al., Citation2010; Lemke et al., Citation2010; Wang et al., Citation2010; Yang et al., Citation2012; Rossi et al., Citation2018; Park et al., Citation2019; Jacob et al., Citation2020). Delivery of drugs to the inner ear through the middle ear cavity is one of the best applications of nanosuspensions for several reasons. Steroids and antioxidants, which are important drugs in the treatment of acute hearing loss, are mostly hydrophobic, and hydrophobic drugs in nanosuspensions would permeate the round window membrane more effectively than the currently used hydrophilic drugs. The middle ear is an air-filled cavity, and there is little concern that a drug injected into the middle ear will mix with other body fluids, including blood; thus, the injected drug remains in suspension more readily there than in most other organs of the human body.
In this experiment, we investigated the difference in the concentration of dexamethasone absorbed in the cochlea between a dexamethasone nanosuspension and the currently used Dex-SP, and we also investigated the safety and efficacy of dexamethasone nanosuspension in the ear.
Materials and methods
Preparation of dexamethasone nanosuspension
In this study, nanoparticles were prepared using fat and supercritical fluid (NUFS™) for nanoparticle fabrication, which is a novel technique for nanoparticle preparation (Park et al., Citation2019). The preparation of dexamethasone nanoparticles consisted of the following two steps: polyvinylpyrrolidone (PVP), poloxamer, polyethylene glycol (PEG)-40 stearate and a saccharide were dissolved in deionized water at a ratio determined by the design of the experiment. The polymer solution was then mixed with the active ingredient, a fatty alcohol. The mixture was milled with a roller compactor, then dried under reduced pressure at room temperature. This solid dispersion was placed inside a pressure-resistant reactor (Bio-Synectics, Inc., BS-SF-1, Seoul, Republic of Korea), and a continuous flow of liquid carbon dioxide was used to remove the fatty alcohol from the solid dispersion.
Characterization of dexamethasone nanoparticles
Particle size measurement
The hydrodynamic particle size of various nanoparticle formulations was measured using dynamic light scattering (DLS) (ELSX-1000, Otsuka Electronics Co., Ltd, Osaka, Japan) (Bondi et al., Citation2015). After dispersion by sonication, dexamethasone suspensions were collected at predetermined time points (0, 1, 2, 6, and 8 h), and particle size was measured with DLS. For visual observation of the nanoparticles, a 120 kV bio-transmission electron microscope (JEM-1400 plus, JEOL, Tokyo, Japan) was employed according to the method used in previous studies (Yoon et al., Citation2016).
In vitro dissolution studies
In vitro dissolution tests of the formulations were conducted with USP Dissolution Apparatus 2 (Vision Elite 8, Hanson, Chatsworth, CA) at 75 rpm. For each measurement, powder (100 mg as dexamethasone) was placed in 900 mL deionized water at 37 ± 0.5 °C. Samples of 3 mL were collected at predetermined time points (3, 7, 15, 30, 60, and 120 min) and were replaced with an equal volume of fresh dissolution medium. The aliquot was filtered through a 0.2 µm membrane filter. The drug content was analyzed using an Alliance series high-performance liquid chromatography (HPLC) system (Waters Corporation, Milford, MA) with ultraviolet (UV) detection at 249 nm. Twenty microliters of each sample was injected into an Epic C18 column (5 μm particle size, 4.6 × 250 mm) adjusted to 25 °C at a flow rate of 0.8 mL/min. The mobile phase consisted of 0.01 M KH2PO4 buffer and acetonitrile (5:4% v/v).
In vitro safety and drug efficacy evaluation of dexamethasone nanosuspensions in HEI-OC1 cells
Culture of HEI-OC1 cells
The immortalized mouse organ of Corti cell line HEI-OC1 was used for in vitro tests (Kalinec et al., Citation2003). HEI-OC1 cells were grown and passaged in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 50 U/mL recombinant mouse interferon-γ, and 10 ng/mL ampicillin and then cultured in a humidified 10% CO2 environment at 33 °C.
Cell counting kit (CCK) assay
The safety of the three dexamethasone nanosuspensions in HEI-OC1 cells was tested using a CCK-8 (Dojindo Molecular Technologies, Rockville, MD) assay. The cells were incubated with various concentrations of dexamethasone nanosuspensions (0.1–100 µg/mL) for 24 h. Cell viability was determined using a CCK-8 assay in accordance with the manufacturer’s protocol; optical densities were determined at 450 nm using a microplate reader (Bio-Rad, Hercules, CA).
Western blotting
HEI-OC1 cells were treated with Dex-SP and dexamethasone nanosuspensions for 1 h and 6 h. Cells were lysed in RIPA buffer containing protease and phosphatase inhibitors (50 mM Tris; 150 mM NaCl; 1 mM EDTA; 1% sodium deoxycholate (DOC); 1% Triton X-100; 0.1% SDS; and 1× protease, phosphatase-1, and phosphatase-2 inhibitor cocktails (Sigma Aldrich, Darmstadt, Germany)). The cell lysates were centrifuged at 13,200 rpm for 15 min at 4 °C, and the supernatant was subjected to western blot analysis. Equal amounts of protein for each sample were electrophoresed and transferred onto nitrocellulose membranes. These membranes were incubated for 1 h with blocking solution (Translab, Daejeon, Republic of Korea) and then incubated overnight with primary antibodies against glucocorticoid receptor (Cell Signaling, Danvers, MA) and phospho-glucocorticoid receptor (Cell Signaling, Danvers, MA). Protein expression was detected by using a ChemiDoc XRS Image system (Bio-Rad, Hercules, CA).
In vivo evaluation of inner ear drug delivery efficiency and safety of dexamethasone nanosuspensions
Three forms of dexamethasone nanosuspensions or control drugs were injected into the middle ear cavity of animals, and the concentration of dexamethasone in the perilymph was investigated for up to 24 h. After comparing the drug concentration in the perilymph between the three nanosuspensions, we selected one dexamethasone suspension and further investigated its safety and efficacy in vivo.
Middle ear administration of drugs
Eight-week-old male BALB/c mice (Orient Bio, Seoul, Republic of Korea; weight 20–23 g) were used in the in vivo experiments. Drugs were injected into the middle ear using a surgical method described previously (Jung et al., Citation2021). Before surgery, the mice were anesthetized using a mixture of 30 mg/kg tiletamine/zolazepam (Zoletil®, Virbac, Carros, France) and 10 mg/kg xylazine (Rompun®, Bayer, Leverkusen, Germany) via intramuscular injection. Then, we placed the mice on a thermoregulated heating pad in the supine position; a midline incision was made, and the left bulla was exposed. A hole was created in the bulla using fine forceps, and a drug solution was injected into the bulla using an insulin syringe. To reflect clinical practice, we did not use gel foam to retain the drug in the middle ear. Next, rimadyl (1.0 mg/kg; Pfizer, Walton Oaks, UK) was injected to relieve pain. Baytril (10 mg/kg; Orion, Hamburg, Germany) was intraperitoneally injected once daily as prophylaxis against middle ear infection.
Perilymph sampling and measurement of dexamethasone concentration
Perilymph was sampled from the lateral semicircular canal (SCC) under the same anesthetic regimen. During perilymph sampling, an incision was made behind the left ear of the animal to prevent contamination with perilymph, and the drug was injected into the middle ear cavity. Then, the animal's pinna was retracted anteriorly, and the muscle of the temporal bone was bluntly dissected to expose the lateral and posterior SCC. A small hole was made in the middle portion of the lateral SCC with a 26 G needle. After we observed perilymph leakage through the hole, approximately 3 µL of perilymph was sampled into a calibrated capillary tube. Each perilymph sample was transferred to an Eppendorf tube and centrifuged for 5 min at 13,200 rpm. Then, 2 µL of the supernatant was collected and diluted 25-fold in 50% methanol to a volume of 50 µL, and liquid chromatography–mass spectrometry (LC/MS; 1290 Infinity II/Qtrap 6500; Sciex, Washington, DC) was performed on the diluted perilymph sample (Wang et al., Citation2011).
Preparation of cochlear homogenates and measurement of dexamethasone concentrations
In order to determine the concentration of dexamethasone absorbed into the tissues of the cochlea, the concentration of dexamethasone in cochlear homogenate was analyzed by LC/MS (Liebau et al., Citation2019). The cochlea was harvested from animals, then immersed in isotonic phosphate-buffered saline (PBS) and washed to remove any drug residue from the outside of the cochlea. Additionally, the round window membrane was opened, and the inside of the cochlea was washed with PBS to remove the perilymph. Then, the cochlea was homogenized using Tissue Lyser II (Qiagen, Hilden, Germany) with RIPA buffer. The lysates were centrifuged at 13,200 rpm for 15 min at 4 °C, and the supernatants were collected.
To compare the concentration of dexamethasone in the cochlear tissue of each individual animal, the protein concentration of the supernatants was adjusted to an equal value, as in western blotting. A dilution of 30 µL of supernatant containing approximately 25 µg of protein was prepared and then combined with 30 µL of methanol to make a sample of 60 µL, and the concentration of dexamethasone in the sample was examined by LC/MS. The results were expressed as the amount of dexamethasone per 25 µg of protein in the cochlear tissue.
Western blotting
To compare the efficacy of dexamethasone nanosuspensions and Dex-SP in the cochlea, we compared the quantities of phosphorylated glucocorticoid receptors (P-GRs) in cochlear tissue. Western blotting was performed in cochlear homogenates using the same method described for the in vitro experiments.
Evaluation of the efficacy of dexamethasone nanosuspensions in a mouse model of ototoxicity
Eight-week-old male BALB/c mice (Orient Bio, Seoul, Republic of Korea; weight 20–23 g) were divided into three groups. The animals in three groups received middle ear drugs 2 h prior to the induction of ototoxicity, as described above; these animals then received dexamethasone nanosuspension, Dex-SP, or distilled water (deaf-sham group). Ototoxicity was induced by the intraperitoneal injection of kanamycin (1000 mg/kg; Sigma-Aldrich, St. Louis, MO) and intraperitoneal injection of furosemide (180 mg/kg; Sigma-Aldrich, St. Louis, MO) 30 min later. The mice were subjected to ABR testing and whole-mount staining of the organ of Corti 2 weeks later.
Histological evaluation
Two weeks after injection of the drug into the middle ear cavity, the animals' hearing was evaluated by using the auditory brainstem response (ABR); afterward, the cochlea and bulla were collected, and histological damage was observed with an optical microscope. Cochleae and bullae were fixed in 4% paraformaldehyde (Merck, Darmstadt, Germany) and decalcified in 5% ethylenediaminetetraacetic acid (EDTA, 0.3 M). Cochlea sections on slides were stained with hematoxylin (YD Diagnostics Corp., Yongin-si, Republic of Korea) and eosin (BBC Biochemical, McKinney, TX). Images were examined using a light microscope system (Nikon Eclipse E400, Tokyo, Japan).
Immunofluorescence staining for dexamethasone was performed to examine the absorption of dexamethasone in the cochlear tissue (Yang et al., Citation2018). Anti-dexamethasone antibody (Abcam, Cambridge, UK) was used as the primary antibody, and the tissue was stained using the Vectastain Elite ABC HRP Kit and the Vector NovaRED Peroxidase Substrate (Vector Laboratories, Burlingame, CA). Images were captured using a light microscope system (Nikon Eclipse E400, Tokyo, Japan).
ABR test in vivo
ABRs were recorded 2 weeks after the operation. The animals were anesthetized with a mixture of 30 mg/kg tiletamine/zolazepam (Zoletil®, Virbac, Carros, France) and 10 mg/kg xylazine (Rompun®, Bayer, Leverkusen, Germany). The active electrode was placed approximately at the vertex of the skull, the reference electrode was inserted under the pinna of the left ear, and the ground electrode was inserted under the contralateral ear. ABR thresholds were measured in response to frequencies from 4 to 32 kHz as well as clicks; only the operated ear was tested. TDT System-3 (Tucker Davies Technologies, Gainesville, FL) hardware and software were used to record the ABRs. A computer-generated tone pip was used for stimulation. Tone bursts at frequencies of 4, 8, 16, and 32 kHz with a duration of 4 ms and a rise-fall time of 1 ms were used, along with clicks. The sound intensity was varied in 5-dB intervals for the clicks near the threshold and in 10-dB intervals for the tone bursts. The ABRs were analyzed using a custom program (BioSig RP, ver. 4.4.1). Threshold differences among mouse groups were statistically compared.
Statistical analysis
The data are presented as the means ± standard errors of triplicate measurements. Statistical significance was identified by one-way analysis of variance (ANOVA) for ABR, the Kruskal–Wallis test for dexamethasone concentration comparison, and Student’s t-test for all other results. A p value of <.05 was taken to indicate statistical significance.
Study approval
All procedures were performed in accordance with national ethics guidelines. This study was approved by the Institutional Review Board of Clinical Research Institute, Daejeon St. Mary's Hospital, College of Medicine, The Catholic University of Korea (approval no. CMCDJ-AP-2020-009).
Results
Preparation and characterization of dexamethasone nanosuspension
Three kinds of dexamethasone nanoparticles (NUFS A, B, C) were prepared by combining a polymer, a surfactant, and a saccharide. PVP and poloxamer were used as the basic composition, and the effects of lactose and dextrose were compared by NUFS A and NUFS B (). The ratio of polymer and the effect of adding PEG-40 stearate were compared by NUFS B and NUFS C (). The particle size of nanosuspensions is estimated for eight hours to evaluate the size and stability of the nanosuspension ().
Table 1. Composition of the formulations of dexamethasone nanoparticles.
Table 2. Changes in nanoparticle sizes over time.
The dissolution behaviors of dexamethasone nanoparticles and micronized dexamethasone powder are compared in and . The dexamethasone nanoparticles were dispersed in the media within a short period of time and reached their highest dissolution rate within 10 min from the start of dissolution. The dissolution rates of the nanoparticles were proportional to the particle size and consistently exceeded 70%. NUFS C, despite containing more polymers and surfactants than the other two formulations, also showed a lower dissolution rate. Thus, the particle size seems to play an important role in the dissolution rate. On the other hand, micronized dexamethasone had poor dispersibility in the media and reached its maximum dissolution rate of approximately 50% only 2 h after the start of dissolution.
Figure 1. (A) Dispersion of dexamethasone nanoparticles and micronized dexamethasone powder in water over time. (B) Transmission electron microscopy images of micronized dexamethasone powder and dexamethasone nanoparticles. Scale bar, 500 nm
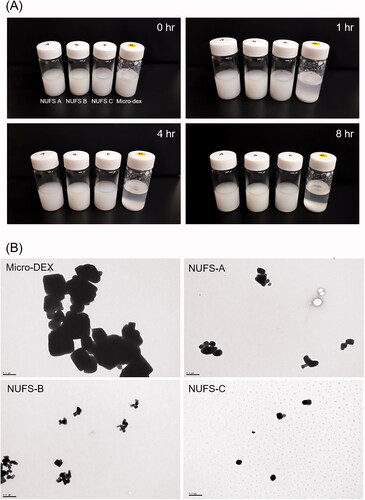
Figure 2. Dissolution profiles were drawn as the percentage of drug dissolved versus time. Dexamethasone nanoparticles (NUFS A, B, C) and micronized dexamethasone powder were dispersed in distilled water, collected over time (3, 7, 15, 30, 60, and 120 min) and detected by a high-performance liquid chromatography system. Compared to micronized dexamethasone powder, nanoparticles dissolved faster and maintained a high dissolution rate over time.
In vitro safety evaluation
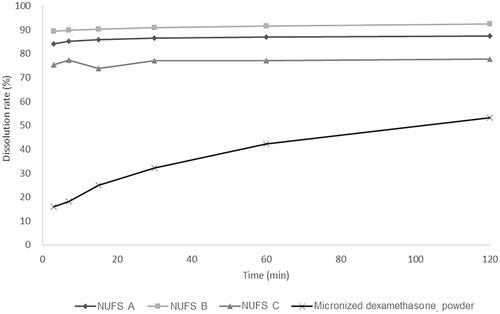
Neither micronized dexamethasone powder nor the three nanosuspensions elicited toxic effects in HEI-OC 1 at concentrations of up to 100 µg/mL; assuming that the drug was absorbed into the cochlea, none of the four formulations had any particular toxicity ().
Figure 3. The cytotoxicity of the three dexamethasone nanosuspensions (NUFS) and micronized dexamethasone (micro-dex) in HEI-OC1 cells. HEI-OC1 cells were treated with NUFS A, B, C (mean particle size 382 nm, 239.3 nm, and 287.3 nm, respectively) and micro-dex (mean particle size 2357.4 nm) at various concentrations (0–100 µg/mL) for 24 hours. Cell viability was detected by a CCK-8 assay. At concentrations up to of 100 µg/mL, NUFS and micro-dex treatment for 24 h did not significantly affect cell viability. Data are the means ± SDs from three independent experiments performed in duplicate.
Comparison of inner ear dexamethasone delivery efficiency and drug efficacy
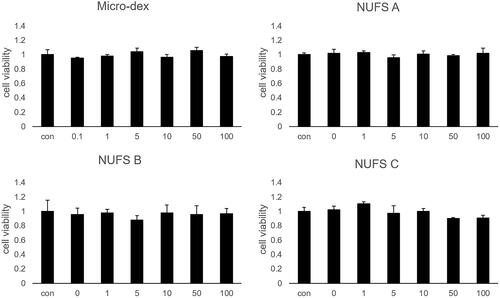
The same 5 mg/mL solutions of the three dexamethasone nanosuspensions, micronized dexamethasone powder, and Dex-SP were injected into the middle ear, and then the concentrations of dexamethasone in the perilymph were compared over time (). As calculated by the Kruskal–Wallis test, the mean concentrations of dexamethasone at 1 h and 6 h were significantly different between groups. The perilymph dexamethasone concentrations of the three dexamethasone nanosuspensions were similar to that of Dex-SP at 1 h but higher than that of Dex-SP at 6 h. However, there was no significant difference in concentration among the three nanosuspensions in either the 1-h or 6-h results. Since the results of the three nanosuspensions are similar, we chose NUFS B, which had the smallest particle size, and compared it with Dex-SP to determine more about the drug delivery efficiency and efficacy of dexamethasone nanosuspensions.
Figure 4. Comparison of dexamethasone concentrations in the perilymph after middle ear drug administration. (A) The administered concentrations of NUFS particles, micronized dexamethasone (micro-dex) particles, and dexamethasone sodium phosphate (Dex-SP) were fixed at 5 mg, and the concentrations of dexamethasone in the perilymph were compared 1, 6, and 24 h after injection into the middle ear cavity. In the test, the concentrations of each group at 1 h and 6 h were significantly different. (B) In a comparison between NUFS B and Dex-SP at different concentrations 6 h after injection, the NUFS B group showed significantly higher dexamethasone concentrations in the perilymph at all three concentrations. *p<.05 and **p<.01.
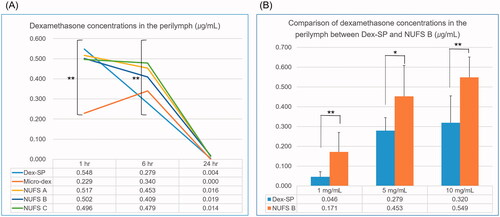
First, to confirm whether the NUFS B nanosuspension produced a higher perilymph drug concentration than Dex-SP 6 h after injection, we injected the two drugs into the middle ear at various concentrations and compared the drug concentration in the perilymph (). When the two groups were compared by Student’s t-test, the NUFS B group showed significantly higher perilymph concentrations at all three dose concentrations. Additionally, to quantify the drug gradients in the perilymph space, sequential sampling of perilymph was taken from 10 mg/mL of NUFS B injected guinea pigs. Six hours after NUFS B injection, serial perilymph sampling was taken from lateral semicircular canal by capillary tube. In each guinea pig, 10 serial capillary tubes were collected (2 μL per each tube). Dexamethasone concentration of capillary tube was analyzed, and the average of three animals is shown in supplement 1. Six hours after NUFS B injection, dexamethasone seems to be well distributed in the perilymph space, even in the apical turn.
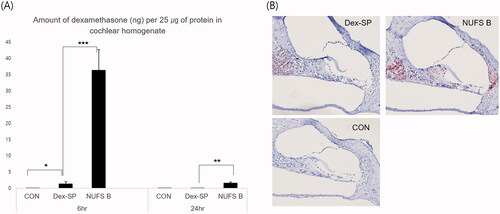
Second, since the amount of drug absorbed into the tissues is important for the effect of the drug, the amount of dexamethasone absorbed into the cochlear tissue was compared between the two groups (). Six hours after drug administration of 10 mg/mL NUFS B nanosuspension or Dex-SP in the middle ear, the amount of dexamethasone per 25 µg of protein in cochlear homogenate was investigated by LC/MS; the result for NUFS B (36.3 ± 17.8 ng), was approximately 26 times the result for Dex-SP (1.4 ± 1.8 ng). Twenty-four hours after drug injection, the levels had decreased to 1.6 ± 1.1 ng in the NUFS B group and 0.0 ± 0.0 ng in the Dex-SP group. On immunochemical staining for dexamethasone in cochleae collected 6 h after drug administration, the NUFS B group was once again found to have a higher uptake of dexamethasone than the Dex-SP group.
Figure 5. Intergroup comparison of the amount of dexamethasone absorbed into the cochlear tissue. (A) For an accurate comparison between animals, the amount of dexamethasone per 25 µg of protein in cochlear homogenate was quantified by LC/MS. Six hours after drug administration, the dexamethasone levels measured 36.3 ± 17.8 ng for NUFS B and 1.4 ± 1.8 ng for Dex-SP; 24 h after drug injection, the measurements were 1.6 ± 1.1 ng in the NUFS B group and 0.0 ± 0.0 ng in the Dex-SP group. (B) According to the immunochemical staining of dexamethasone in the apical turn of cochlea collected 6 h after drug administration, a higher uptake of dexamethasone was found in the NUFS B group than in the Dex-SP group. *p<.05, **p<.01, and ***p<.001.
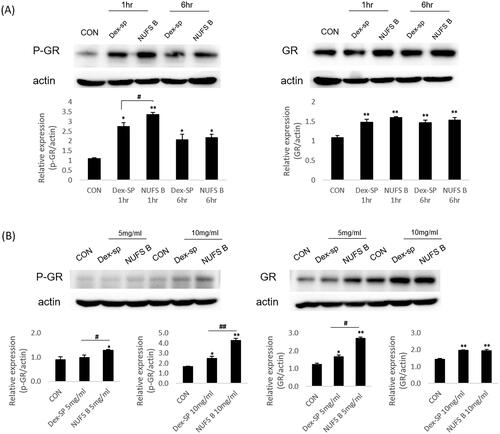
Third, in order to evaluate the efficacy of NUFS B, the degree of P-GR was compared between Dex-SP and NUFS B in in vitro and in vivo experiments (). Western blotting confirmed that the abundance of P-GR was increased in the NUFS B-treated group at both 1 and 6 h after treatment with 50 µg/mL Dex-SP or NUFS B in vitro. In the in vivo experiment, we examined the change in P-GR abundance in the cochlea after 24 h of treatment with NUFS B and Dex-SP at concentrations of 5 mg/mL and 10 mg/mL in the middle ear cavity. The western blotting results also indicated that P-GR abundance was more elevated in the NUFS B group than in Dex-SP group.
Figure 6. Comparison of drug efficacy through phosphorylation of glucocorticoid receptors. In order to predict the comparative effects of NUFS B particles and dexamethasone sodium phosphate (Dex-SP) in clinical use, glucocorticoid receptor phosphorylation was assessed through in vitro and in vivo experiments. (A) In the HEI-OC1 cell line, the phosphorylation of GR increased more in the NUFS B group than in the Dex-SP group at 1 and 6 h after treatment with 50 µg/mL Dex-SP or NUFS B. (B) In the in vivo experiment, the changes in P-GR and total GR levels in cochlear homogenates were investigated after 24 h of treatment with NUFS B and Dex-SP at concentrations of 5 mg/mL and 10 mg/mL in the middle ear cavity of BALB/c mice. P-GR levels increased more in the NUFS B group than in the Dex-SP group.
Therapeutic effects of dexamethasone nanosuspension in a mouse model of ototoxicity
The ABR thresholds of the three groups were measured on day 14, and the Kruskal–Wallis test revealed significant among-group differences at 8 kHz (p=.005) and 16 kHz (p=.028) and in the click test (p=.008). Upon post hoc testing using the Holm–Bonferroni method, the NUFS B group exhibited significantly better hearing than the other two groups at 8 kHz and 16 kHz and in the click test ().
Figure 7. The therapeutic effect of NUFS B in an ototoxic animal model. (A) The auditory brainstem response (ABR) thresholds of the three groups were measured on day 14 after induction of deafness. Only the 10 mg/mL NUFS B group exhibited significantly better hearing than the other two groups at 8 and 16 kHz and the click sound. The statistical results are shown in the text. The data represent the mean ± SD from three independent experiments performed in duplicate. *p<.05, **p<.01, and ***p<.001. (B) We observed the whole organ of Corti using confocal microscopy, focusing on four specific areas of the place-frequency map. The stereocilia of the deaf-sham group and the Dex-SP group were highly damaged at all frequency areas, whereas those of NUFS B group showed less damage than those of the other two groups.
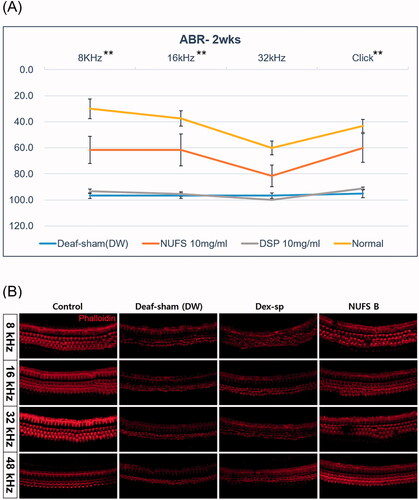
When the whole mount of the organ of Corti was observed, the stereocilia of the deaf-sham group and the Dex-SP group were highly damaged at all frequency areas of 8, 16, 32, and 48 kHz, whereas those of the NUFS B group showed less damage than those of the other two groups ().
In vivo safety evaluation
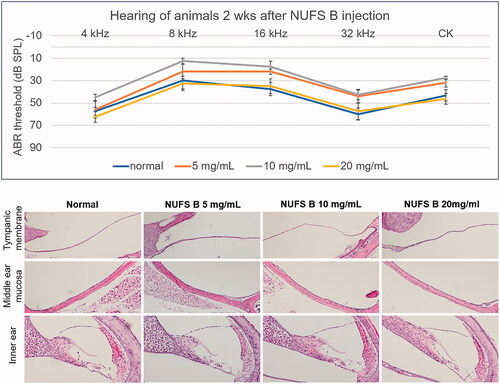
Since Dex-SP is generally injected into the middle ear cavity at 5 mg/mL in clinical practice, the toxicity of NUFS B in the middle and inner ear was evaluated up to a concentration of 20 mg/mL, which is four times the usual Dex-SP concentration. The animals' hearing was evaluated by ABR 2 weeks after injection of the drug into the middle ear, and the NUFS B group showed no hearing loss compared to the normal group up to 20 mg/mL (). Histological evaluation performed after the hearing examination showed no inflammatory reaction in the tympanic membrane or middle ear mucosa in any of the animals treated with NUFS B, and no damage was observed in the inner ear tissue of those animals.
Figure 8. In vivo safety assessment after intratympanic injection of a dexamethasone nanosuspension (NUFS B). NUFS B was injected into the middle ear at concentrations of 5, 10, and 20 mg/mL. Two weeks after drug injection, the animals' hearing was evaluated by measuring the ABR, and histological evaluation was performed after the hearing examination. (A) As indicated by the ABR results, animals treated with NUFS B at up to 20 mg/mL showed no hearing loss compared to the normal group. (B) Histological evaluation showed no inflammatory reaction in the tympanic membrane or the middle ear mucosa in any of the NUFS B animals, and no damage was observed in the inner ear tissue.
Discussion
Dexamethasone nanosuspensions were successfully made by the NUFSTM method. Three kinds of dexamethasone nanoparticles (NUFS A, B, C) were prepared by combining a polymer, a surfactant, and a saccharide. Saccharides contribute to the formation of nanoparticles of API mixtures in the milling process. PVP and poloxamer were used as the basic composition, and the effects of lactose and dextrose were compared by NUFS A and NUFS B (). In the case of dexamethasone, the effect of dextrose appears to be greater than that of lactose as the nanoparticle size of NUFS B maintains a smaller size over time compared to NUFS A (). In addition, dextrose appears to be more favorable for particle stability in suspension than lactose. The polymer ratio and the effect of adding PEG-40 stearate were compared by investigating NUFS B and NUFS C (). The addition of a polymer and surfactant does not appear to have much effect on particle size (NUFS B, C) compared to the addition of lactose (NUFS A). The particles of NUFS B and NUFS C do not markedly change size until 8 h after dispersion of nanoparticles in deionized water; however, NUFS A, which has a high solubilizing agent ratio, increases the particle size after dispersion. After 8 h of dispersion, the particle size of NUFS A increased by more than 100 nm (). Therefore, increasing the polymer and surfactant concentrations seems to have a negative effect on the stability of the nanosuspension.
In all three of the prepared nanosuspensions, the drugs appeared to remain in suspension for more than eight hours. Since an aqueous solution of the drug, as opposed to a gel, escapes from the middle ear cavity to the nasopharynx through the eustachian tube in a short time (Plontke et al., Citation2008; Park et al., Citation2014), our dexamethasone nanosuspensions seem to maintain a suspension state for a sufficient time relative to the time the drug stays in the middle ear cavity.
For the in vitro and in vivo safety evaluations, dexamethasone nanosuspension exhibited safety up to 100 µg/mL (in vitro) and 20 mg/mL (in vivo). In the evaluation of hearing, the hearing of the NUFS 10 mg/mL group seems somewhat better at 16 kHz than that of the normal animals, but this is thought to be due to the small number of animals, and it can be seen that there is no toxicity at all ().
By measuring the drug concentrations in the perilymph, we found that dexamethasone nanosuspensions produced a higher dexamethasone concentration in the perilymph than Dex-SP at six hours after drug injection. Interestingly, Dex-SP produced a high drug concentration in the perilymph at one hour after drug injection, after which the concentration drops rapidly with time, whereas nanosuspensions produced a steady drug concentration for up to 6 h. This pattern also appeared in the results of the micronized dexamethasone powder solution. In view of these results, it is thought that hydrophobic drugs achieve a more effective sustained release than hydrophilic drugs when injected into the middle ear cavity, but additional studies are needed on the mechanism of this phenomenon.
To our knowledge, the therapeutic concentration of dexamethasone in the inner ear has not yet been studied. In the study of Machuca et al. (Citation2006), 10 µM (3.92 μg/mL) dexamethasone resulted in complete protection against the cytotoxic effect of TNF-α in MCF-7 cells. Another study by Urayama et al. (Citation1998) reported that 1 μM (0.392 μg/mL) dexamethasone had a strong protective effect against monochloramine-induced injury of IEC-18 cells. However, the protective dose of dexamethasone (0.39 or 3.92 μg/mL) cannot be maintained continuously in a real perilymph environment because the perilymph constantly circulates with the CSF, and the drug is rapidly washed out of the perilymph. Therefore, within the safe concentration range, maintaining the concentration of dexamethasone above 0.392 µg/mL or 3.92 µg/mL in the perilymph for a long time can be expected to produce a better therapeutic effect. In , even when Dex-SP was injected into the middle ear at a high concentration of up to 10 mg/mL, the concentration of dexamethasone in the perilymph at six hours (0.320 µg/mL) did not reach the therapeutic concentration (0.392 µg/mL). On the other hand, dexamethasone nanosuspension showed a perilymph concentration exceeding 0.392 µg/mL even after injection of 5 mg/mL at six hours (0.453 µg/mL).
The quantity of drug that has been absorbed into the inner ear tissues is important in inner ear drug delivery studies. Dexamethasone binds to intracellular GR to form GR complexes, which are involved in gene transactivation and transrepression in the nucleus, mediating the effect of the drug (Hardy et al., Citation2020). Therefore, dexamethasone must be absorbed into the cochlear tissues to be effective. In addition, since the perilymph is continuous with the cerebrospinal fluid, the drug concentration in the perilymph may differ from the concentration in the tissue (Salt et al., Citation2012).
Unfortunately, however, there are few methods to accurately measure dexamethasone concentrations in cochlear tissue. In this study, the concentration of dexamethasone in cochlear homogenate was evaluated by LC/MS in the manner described by Liebau et al. (Citation2019). For an accurate comparison between animals, the amount of dexamethasone was quantified based on the amount of protein in cochlear homogenate, and surprisingly, at 6 h after drug injection, NUFS B produced a tissue drug concentration approximately 26 times the concentration achieved by Dex-SP. In addition, dexamethasone was detected in tissues until 24 h after NUFS B administration, and the concentration 24 h after NUFS B was similar to the concentration 6 h after Dex-SP.
As dexamethasone nanoparticles are hydrophobic, nanosuspensions of these crystals could be more advantageous than Dex-SP for cell membrane permeation (Al-Awqati, Citation1999). For example, in the western blots from the in vitro testing, NUFS B achieved higher P-GR expression than the same amount of Dex-SP. These results suggest that even if the same amount of drug entered the perilymph, a dexamethasone nanosuspension would have better drug efficacy than Dex-SP because the former would achieve better cell membrane permeation.
In the western blots from the in vivo testing, NUFS B once again produced higher P-GR levels than the same amount of Dex-SP. This advantage is thought to be due to the superior absorption of NUFS B in the tissue and its increased drug efficacy compared to Dex-SP. Furthermore, in the ototoxic animal model, NUFS B showed significantly better hearing protection than Dex-SP against ototoxic drugs. Considering the safety results of the dexamethasone nanosuspension and its much better absorption into the cochlear tissue than Dex-SP, this treatment may have the potential to replace Dex-SP as an intratympanic injection for acute hearing loss.
Conclusions
Nanosuspension of dexamethasone was able to deliver dexamethasone to the cochlea very safely and efficiently and showed potential as a formula for intratympanic injection. In addition, it can be applied in studies on the delivery of various hydrophobic antioxidants to treat acute hearing loss.
Disclosure statement
Zion Kang, Joo Won Park, and Kab Sig Kim work for Bio-Synectics, Inc. The dexamethasone nanosuspension used in this experiment was manufactured by Bio-Synectics, Inc., using their patented method.
Data availability statement
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
Additional information
Funding
References
- Al-Awqati Q. (1999). One hundred years of membrane permeability: does Overton still rule? Nat Cell Biol 1:E201–2.
- Bondi ML, Botto C, Amore E, et al. (2015). Lipid nanocarriers containing sorafenib inhibit colonies formation in human hepatocarcinoma cells. Int J Pharm 493:75–85.
- Dizaj SM, Vazifehasl Z, Salatin S, et al. (2015). Nanosizing of drugs: effect on dissolution rate. Res Pharm Sci 10:95–108.
- Gupta H, Aqil M, Khar RK, et al. (2010). Sparfloxacin-loaded PLGA nanoparticles for sustained ocular drug delivery. Nanomedicine 6:324–33.
- Hardy RS, Raza K, Cooper MS. (2020). Therapeutic glucocorticoids: mechanisms of actions in rheumatic diseases. Nat Rev Rheumatol 16:133–44.
- Jacob S, Nair AB, Shah J. (2020). Emerging role of nanosuspensions in drug delivery systems. Biomater Res 24:3.
- Jung SY, Yoo J, Yang KJ, et al. (2021). Intratympanic administration of alpha-lipoic acid-loaded Pluronic F-127 nanoparticles ameliorates acute hearing loss. Nanomedicine 32:102329.
- Kalinec GM, Webster P, Lim DJ, Kalinec F. (2003). A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol Neurotol 8:177–89.
- King EB, Salt AN, Eastwood HT, O’Leary SJ. (2011). Direct entry of gadolinium into the vestibule following intratympanic applications in Guinea pigs and the influence of cochlear implantation. J Assoc Res Otolaryngol 12:741–51.
- Lemke A, Kiderlen AF, Petri B, Kayser O. (2010). Delivery of amphotericin B nanosuspensions to the brain and determination of activity against Balamuthia mandrillaris amebas. Nanomedicine 6:597–603.
- Liebau A, Schilp S, Mugridge K, et al. (2019). Long-term in vivo release profile of dexamethasone-loaded silicone rods implanted into the cochlea of Guinea pigs. Front Neurol 10:1377.
- Machuca C, Mendoza-Milla C, Córdova E, et al. (2006). Dexamethasone protection from TNF-alpha-induced cell death in MCF-7 cells requires NF-kappaB and is independent from AKT. BMC Cell Biol 7:9.
- Park SH, Park C, Seo JY, et al. (2014). How long should patients remain in the supine treatment position after intratympanic dexamethasone injection? Laryngoscope 124:2807–10.
- Park SY, Kang Z, Thapa P, et al. (2019). Development of sorafenib loaded nanoparticles to improve oral bioavailability using a quality by design approach. Int J Pharm 566:229–38.
- Piao H, Kamiya N, Hirata A, et al. (2008). A novel solid-in-oil nanosuspension for transdermal delivery of diclofenac sodium. Pharm Res 25:896–901.
- Plontke SK, Mikulec AA, Salt AN. (2008). Rapid clearance of methylprednisolone after intratympanic application in humans. Comment on: Bird PA, Begg EJ, Zhang M, et al. Intratympanic versus intravenous delivery of methylprednisolone to cochlear perilymph. Otol Neurotol 2007;28:1124–30. Otol Neurotol 29:732–3.
- Rossi I, Sonvico F, McConville JT, et al. (2018). Nebulized coenzyme Q10 nanosuspensions: a versatile approach for pulmonary antioxidant therapy. Eur J Pharm Sci 113:159–70.
- Rousset F, Kokje VBC, Coelho MDC, et al. (2019). Poly-lactic acid-based biopolymer formulations are safe for sustained intratympanic dexamethasone delivery. Otol Neurotol 40:e739–e46.
- Salt AN, Hartsock JJ, Gill RM, et al. (2012). Perilymph pharmacokinetics of markers and dexamethasone applied and sampled at the lateral semi-circular canal. J Assoc Res Otolaryngol 13:771–83.
- Salt AN, Hartsock JJ, Piu F, Hou J. (2018). Dexamethasone and dexamethasone phosphate entry into perilymph compared for middle ear applications in Guinea pigs. Audiol Neurootol 23:245–57.
- Stachler RJ, Chandrasekhar SS, Archer SM, et al. (2012). Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg 146:S1–S35.
- Sun C, Wang X, Chen D, et al. (2016). Dexamethasone loaded nanoparticles exert protective effects against cisplatin-induced hearing loss by systemic administration. Neurosci Lett 619:142–8.
- Sun C, Wang X, Zheng Z, et al. (2015). A single dose of dexamethasone encapsulated in polyethylene glycol-coated polylactic acid nanoparticles attenuates cisplatin-induced hearing loss following round window membrane administration. Int J Nanomedicine 10:3567–79.
- Urayama S, Musch MW, Retsky J, et al. (1998). Dexamethasone protection of rat intestinal epithelial cells against oxidant injury is mediated by induction of heat shock protein 72. J Clin Invest 102:1860–5.
- Wang L, Du J, Zhou Y, Wang Y. (2017). Safety of nanosuspensions in drug delivery. Nanomedicine 13:455–69.
- Wang X, Chen Y, Tao Y, et al. (2018). A666-conjugated nanoparticles target prestin of outer hair cells preventing cisplatin-induced hearing loss. Int J Nanomedicine 13:7517–31.
- Wang X, Dellamary L, Fernandez R, et al. (2011). Principles of inner ear sustained release following intratympanic administration. Laryngoscope 121:385–91.
- Wang Y, Zhang D, Liu Z, et al. (2010). In vitro and in vivo evaluation of silybin nanosuspensions for oral and intravenous delivery. Nanotechnology 21:155104.
- Wang Y, Zheng Y, Zhang L, et al. (2013). Stability of nanosuspensions in drug delivery. J Control Release 172:1126–41.
- Yang F, Kamiya N, Goto M. (2012). Transdermal delivery of the anti-rheumatic agent methotrexate using a solid-in-oil nanocarrier. Eur J Pharm Biopharm 82:158–63.
- Yang K-J, Son J, Jung SY, et al. (2018). Optimized phospholipid-based nanoparticles for inner ear drug delivery and therapy. Biomaterials 171:133–43.
- Yoon JY, Yang K-J, Park S-N, et al. (2016). The effect of dexamethasone/cell-penetrating peptide nanoparticles on gene delivery for inner ear therapy. Int J Nanomedicine 11:6123–34.
- Zhang L, Xu Y, Cao W, et al. (2018). Understanding the translocation mechanism of PLGA nanoparticles across round window membrane into the inner ear: a guideline for inner ear drug delivery based on nanomedicine. Int J Nanomedicine 13:479–92.
