Abstract
In this article, formulation studies for terbinafine hydrochloride nanoemulsions, prepared by high-energy ultrasonication technique, are described. Pseudo-ternary phase diagram was constructed in order to find out the optimal ratios of oil and surfactant/co-solvent mixture for nanoemulsion production. Clove and olive oils were selected as oil phase. Based on the droplet size evaluation, maximum nanoemulsion region were determined for formulation development. Further characterization included polydispersity index (PDI), zeta potential, Fourier transform infrared (FT-IR) spectroscopy, morphology, pH, viscosity, refractive index, ex vivo skin permeation, skin irritation, and histopathological examination. Droplet sizes of optimized formulations were in colloidal range. PDI values below 0.35 indicated considerably homogeneous nanoemulsions. Zeta potential values were from 13.2 to 18.1 mV indicating good stability, which was also confirmed by dispersion stability studies. Ex vivo permeation studies revealed almost total skin permeation of terbinafine hydrochloride from the nanoemulsions (96–98%) in 6 hours whereas commercial product reached only 57% permeation at the same time. Maximum drug amounts were seen in epidermis and dermis layers. Skin irritation and histopathological examination demonstrated dermatologically safe formulations. In conclusion, olive oil and clove oil-based nanoemulsion systems have potential to serve as promising carriers for topical terbinafine hydrochloride delivery.
1. Introduction
Terbinafine hydrochloride (TF-HCl) belongs to allylamines family of antifungal drugs. It causes fungal cell death by hindering the ergosterol synthesis in the fungal cells (Trichophyton mentagrophytes and Trichophyton rubrum). This is due to the inhibition of squalene monooxygenase enzyme activity, which prohibits the growth of fungus (Barot et al., Citation2012).
TF-HCl is used in the treatment of fungal nail infections and ringworm as well as in jock itch and athlete’s foot. Based on previous clinical findings, TF-HCl has a better therapeutic profile (low minimum inhibitory concentration (MIC) for various fungal species, short duration of therapy, and low relapse rate) when compared to other antifungals such as imidazoles, triazoles, polyene antimycotics, and pyrimidine analogs (Hinojosa et al., Citation2007).
Therapeutic effectiveness of topical antifungal treatment depends on the penetration of drug into different skin layers and formation of an effective drug reservoir into these layers (Güngör et al., Citation2013). However, therapeutic efficacy of antifungal drugs, like TF-HCl, has its limitations due to the unfavorable physicochemical properties of drugs and dosage vehicles. Terbinafine hydrochloride is soluble in organic solvents, solubility to ethanol is approximately 30 mg/mL, to dimethyl sulfoxide (DMSO) 12.5 mg/mL, and to dimethylformamide 14 mg/mL, but its aqueous solubility is very low, 0.738 ng/mL (Vejnovic et al., Citation2010; Terbinafine Hydrochloride Product Information, 26.1., Citation2022).
Poor aqueous solubility and slow permeation rate of TF-HCl are the main drawbacks in its use, which lead to need for an extended duration of topical therapy even up to two weeks. In some cases, combination therapy with oral tablets (250–500 mg) is required. TF-HCl is commercially available for oral (tablets) and topical (creams, ointments, gels) administration. Oral administration of TF-HCl may cause adverse effects such as colestatic toxic hepatitis, taste loss, hepatotoxicity resulting in chronic biliary ductopenia with portal fibrosis, cholestatic liver injury, and hepatic malfunctioning (Burstein et al., Citation2004). In the case of topical delivery, poor permeability means prolonged therapy, which increases overall costs and lowers patient compliance (Elmataeeshy et al., Citation2018).
In topical drug delivery, effective diffusion across the stratum corneum requires small particle sizes, and a wide range of colloidal carriers (nanosystems) have been explored as potential drug delivery vehicles. Nanosized systems can enhance the permeation of drug molecules, which otherwise are not able to permeate through stratum corneum by utilizing conventional dermal dosage forms (such as ointments, creams, and gels), and nanosystems help these drugs to reach therapeutic drug levels (Gupta et al., Citation2012). With efficient formulation, topical dosage forms can improve patient compliance and drug bioavailability by avoiding gut and hepatic first-pass metabolism, reducing dose frequency and lowering side effects.
One example of nanosized drug delivery systems is nanoemulsions, which are single phase, optically isotropic, and kinetically stable colloidal dispersion systems. In nanoemulsions, small colloidal droplet sizes with large surface area to volume ratio increase drug solubility (Rai et al., Citation2018), which often leads to improved permeability. Besides, nanoemulsion formulations can protect bioactive compounds against degradation, improve diffusion across skin layers and enhance permeability and bioavailability of drug molecules.
Nanoemulsions have droplet sizes below 500 nm, which enhances drug penetration through stratum corneum lipid bilayer in the skin. This means increased drug bioavailability and reduced dosing frequency (Sosnowska et al., Citation2017). Nanoemulsions may be oil-in-water (O/W) or water-in-oil (W/O) type emulsions, depending on whether the oil is dispersed as droplets in the bulk water, or vice versa. Combined with efficient solubilization capacity, nanoemulsions can encapsulate either hydrophilic or hydrophobic drugs (depending on the emulsion type) in order to improve the drug solubility properties and, hence, to reach higher diffusion/absorption rates (McClements, Citation2012; Salim et al., Citation2016). Small droplet size and low oil/water interfacial tension due to the presence of small amount of surfactant, result lower agglomeration tendency, and, hence, decreased stability related problems such as creaming and sedimentation tendency (Elmataeeshy et al., Citation2018).
The overall aim of this study was to improve TF-HCl permeability by developing topical nanoemulsion formulation. Pseudo-ternary phase diagram was utilized for formulation optimization. For the optimized nanoemulsions, thorough physicochemical analysis as well as in vitro permeation studies using Franz diffusion cells was completed and the optimized nanoemulsion systems were compared to a commercial TF-HCl product. Finally, skin irritation and histopathological examination were done in order to demonstrate the safety of the formulations.
2. Materials and methods
2.1. Materials
TF-HCl was a generous donation from Mass Pharma Pvt. Ltd Lahore (Pakistan). Castor oil, clove oil, olive oil, and coconut oil were purchased from Loba Chemie (India). Ethanol and isopropyl alcohol were from Merck (Germany) and Tween 80 and Tween 20 from BDH (India). All the chemicals used were of analytical grade. The purified water was obtained from RO system Jawa Pharmaceuticals Pvt. Ltd Lahore (Pakistan) and distillation plant (Riphah Institute of Pharmaceutical Sciences, Riphah International University, Lahore, Pakistan).
2.2. Screening studies for nanoemulsion development
For screening studies in nanoemulsion development, the solubility of TF-HCl to different nanoemulsion components was determined: oils including olive oil, clove oil, castor oil, coconut oil; surfactants like Tween 80 and Tween 20; and co-solvents such as ethanol and isopropyl alcohol.
TF-HCl solubility to nanoemulsion components was accomplished with a shake flask method by placing excess amount of TF-HCl together with 2 mL of solvent into a small vial. Vials were closed tightly with rubber stoppers and subjected to continuous stirring by magnetic stirrer at 37 °C for 72 hours. After 72 hours, vials were centrifuged (Centrifuge YJ03-043-15000, China) at 10 000 rpm for 10 min time. Supernatant was separated by using glass pipette and it was diluted with a methanol:phosphate buffer solution (PBS: pH 6.8). Drug concentration was determined using UV spectrophotometer (UV-1800 Shimadzu, Japan) at 220 nm wavelength.
2.3. Construction of pseudo-ternary phase diagram
Pseudo-ternary phase diagram was constructed using Microsoft Excel (version 2013) to find out the best amounts for selected oils and surfactant:co-solvent ratios, Smix. The amounts/ratios, which provided maximum zone of nanoemulsion region, were selected for further formulation development. For this purpose, surfactant (Tween 80) and ethanol (co-solvent) were mixed for 30 min using magnetic stirrer at ratios 1:1, 1:2, and 2:1. Smix ratio 1:1 was further utilized for developing placebo formulations. Pseudo-ternary phase diagram of placebo formulations was constructed with different ratios of oil to Smix (1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, 9:1) with either clove oil or olive oil as an oil phase (). Then these samples were checked for transparency and uniformity after gradual addition of water (Kaur & Ajitha, Citation2019).
Table 1. Compositions of nanoemulsion formulations for formation of pseudo-ternary phase diagrams.
2.4. Preparation of TF-HCl loaded nanoemulsions
TF-HCl loaded nanoemulsions were prepared using ultrasonication technique (Carpenter & Saharan, Citation2017). Accurately weighed amount of TF-HCl (100 mg) was dissolved either into clove oil or olive oil. Batch size was 10 mL of nanoemulsion. Different concentrations of Tween 80 (surfactant) and ethanol (co-solvent) were added in three different ratios (1:1, 1:2, and 2:1) under continuous stirring. Dropwise addition of water was done in each formulation with continuous stirring until a clear, homogeneous oil in water (O/W) emulsion was obtained. After the emulsion formation, emulsion was subjected to ultrasonication for 2 hrs time.
Based on the pseudo-ternary phase diagrams, maximum nanoemulsion region (5:5 and 6:4) was selected for drug-loaded nanoemulsion analysis. All the Smix ratios (1:1, 1:2, and 2:1) were studied in the selected region. The exact compositions of the studied nanoemulsions are presented in .
Table 2. Compositions of TF-HCl-loaded nanoemulsions formed with olive oil (F1–F6) and clove oil (F7–F12).
2.5. Characterization of nanoemulsions
2.5.1. Drug–excipient interaction studies
Interaction studies between the different composites of the formulations were performed using Fourier Transform Infrared spectrophotometer (FT-IR, IR Affinity-1 Shimadzu, Japan). Pure oils (clove oil and olive oil), surfactant, co-solvent, pure TF-HCl, physical mixtures of the nanoemulsion composites, and nanoformulations were subjected to FT-IR measurements. Attenuated total reflectance (ATR) mode of spectrophotometer was operated and analytes were placed directly on diamond crystal. Spectral area analyzed was 400–4000 cm−1.
2.5.2. Droplet size and polydispersity index (PDI) determinations
Droplet sizes and PDI values of all the nanoemulsions were evaluated using dynamic light scattering measurements (Zetasizer ZS90, Nanoseries, Malvern, UK). For the determinations, formulations were diluted 10-fold with double distilled water and put into a quartz cuvette. The analysis was executed at 25 °C with a 90° angle. Fixed refractive index values (1.398 for olive oil and 1.489 for clove oil formulations) were used in all the analysis. Measurements were performed triplicate and average values were calculated.
2.5.3. Zeta potential measurement
Surface charges of the nanoemulsions with olive oil and clove oil were determined using Zetasizer (Zetasizer ZS90, Nano series, Malvern, UK). Measurements were done in triplicate. For the measurements, samples were diluted 10-fold with water, and measurements were performed in disposable cuvettes (DTS 107).
2.5.4. Morphological studies
The morphology of nanoemulsion droplets was studied using a scanning electron microscope (SEM, Quanta 250 FEG FEI, USA). Optimized nanoemulsions (based on droplet size measurements) of olive oil (formulation F1) and clove oil (formulation F7) were mounted on an aluminum stub using silver adhesive tape. Aluminum stubs were stored overnight under vacuum. Stubs were sputter-coated with gold film (0.20 µm thickness). Images were taken at accelerated voltage of 10 kV at diverse amplification.
2.5.5. pH measurement
pH of the optimized nanoemulsions of olive oil (F1) and clove oil (F7) was measured using the digital pH meter (Model CG-820, Germany). Prior to measurements, pH meter was calibrated using buffer solutions of pH values 4.0, 7.0 and 9.0 (Gaur et al., Citation2014).
2.5.6. Viscosity
Viscosity of optimized nanoemulsions F1 and F7 was determined using Brookfield viscometer (Model DV-E, Brookfield Engineering Laboratories, Middleborough, MA, USA). The shear rate was 30 rpm at 25 °C. Measurements were performed in triplicate.
2.5.7. Refractive index
Refractive indexes of optimized nanoemulsions F1 and F7 were measured using a refractrometer (ATAGO, Tokyo, Japan). Calibration of instrument was done with distilled water before the measurements. The measurements were performed at room temperature (Bakshi et al., Citation2018).
2.5.8. Entrapment efficiency studies
An accurately measured amount of optimized nanoemulsions F1 and F7 was diluted in 10 mL volumetric flask with methanol. The mixture was subjected to sonication for 15 minutes at 15 °C. The system was centrifuged at 13,000 rpm for 10 min time and drug amount in the supernatant was analyzed using UV spectrophotometer (UV-1800 Shimadzu, Japan) at 220 nm wavelength with earlier validated method (Jain et al., Citation2011; Baheti et al., Citation2016).
2.5.9. Skin permeation studies
The experimental protocol for skin permeation studies was approved by the Research Ethics Committee (REC) of Riphah Institute of Pharmaceutical Sciences, Riphah International University Lahore, Pakistan. In vitro skin permeation studies were performed with well-established method using mice skin on Franz diffusion cells, and phosphate buffer saline (pH 6.8) including 0.01%(w/v) Tween 80 was used as dissolution medium in the tests (Patel et al., Citation2011; Rajan & Vasudevan, Citation2012; Arzani et al., Citation2015). Mice skin was excised from abdominal region and hairs were shaved using razor to prepare the skin properly. After the preparation, skin samples were stored in freezer at −21 °C until the use (Hussain et al., Citation2017). For the permeation tests, appropriately sized pieces of skin were mounted on Franz diffusion cells with a surface area of 1.77 cm2 and a receiver compartment capacity of 8 mL. Both the optimized nanoemulsions F1 and F7 as well as one commercially available conventional TF-HCl cream formulation (Lamisil) were tested. For the skin permeation tests, phosphate buffer saline (pH 6.8) with 0.1% w/v Tween 80 was filled in to the receptor compartment. Tested formulations were applied on skin in the donor compartment. Temperature at the receiver compartment was maintained at 37 °C and receiver compartment was constantly stirred using magnetic stirrer. Samples were drawn from receiver compartment at different time intervals (0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, and 12 hrs) and withdrawn amount was replaced with fresh phosphate buffer solution. Samples were analyzed at UV visible spectrophotometer at wavelength of 220 nm after filtration with 0.45 µm syringe membrane filter with an earlier validated method (Jain et al., Citation2011; Baheti et al., Citation2016).
2.5.10. Drug retention studies
For drug retention studies, skin samples from permeation study were cleaned using phosphate buffer solution and cut in to pieces having the area of 1 cm2. Using cryotome, skin samples were sectioned separately to stratum corneum, epidermis and dermis layers. Each skin layer was then separately treated with phosphate buffer solution (pH 5.5) at 37 °C for 24 h time in a shaking incubator in order to extract the drug. After centrifugation, drug content was determined using UV spectrophotometer at 220 nm wavelength (Jain et al., Citation2011; Baheti et al., Citation2016).
2.5.11. Skin irritation studies
Skin irritation studies were performed on albino rats, the weight of the rats being 200–250 g. The experimental protocol was approved by the Research Ethics Committee (REC) of Riphah Institute of Pharmaceutical Sciences, Riphah International University, Lahore, Pakistan. Three separate groups, each containing 6 rats were used in the following way: Group 1 animals were untreated without application of any of the formulations, Group 2 was treated with the nanoemulsion F7, and Group 3 animals were treated with commercially available conventional cream (Lamisil). For Groups 2 and 3, 15 mg/kg bodyweight of either nanoemulsion F7 (Group 2) or Lamisil (Group 3) was applied on shaved skin.
After 24 h of application, skin was carefully checked for any signs of edema and erythema. The mean erythemal and edemal scores were recorded based on their degree of severity caused by application of formulations in the following way: no erythema/edema = 0, slight erythema/edema = 1, well-defined erythema/edema = 2, moderate erythema/edema = 3, and scar formation = 4 (Hussain et al., Citation2016).
2.5.12. Histopathological assessment of treated rat skin
In order to check the changes in complete blood count (CBC) after application of conventional cream and TF-HCl loaded nanoemulsion, blood samples were collected from the rats used in the irritation studies. Moreover, animals were sacrificed 24 hours after sample application and skin was excised for histopathological examination. Dorsal portion of rat skin of control group (Group 1), nanoemulsion treated group (Group 2) and conventional cream treated group (Group 3) was preserved for checking topical toxicity. Preserved skin tissues were sectioned using a microtome and stained with acidic dye (eosin) and basic dye (hematoxylin) for visualization under microscope.
2.5.13. Dispersion stability studies
Dispersion stability studies for both the optimized formulations (F1 and F7) were carried out in order to confirm the good stability of the systems. For this purpose, developed formulations were subjected to stressed conditions: heating cooling, centrifugation and freeze-thaw tests (Shafiq et al., Citation2007). In heating cooling stress, six cycles between refrigerator temperature (4 °C) and 45 °C with storage at each temperature at least 48 h were studied. In centrifugation, formulations were centrifuged at 3500 rpm for 30 min. And, in freeze-thaw stressing, three freeze-thaw cycles between −21 °C and +25 °C with storage at each temperature not less than 48 h was done. After the stressing, formulations were checked for creaming, phase separation, and cracking.
2.6. Statistical analysis
The results were illustrated as mean ± SD. All statistical comparisons were performed using Microsoft Excel 2010. The data obtained from repeated measurements were subjected to Student's t-test and a value of p < 0.05 was considered as significant.
3. Results
3.1. Screening of oils, surfactants and co-solvents for nanoemulsion formulation development
Oil phase drug solubilizing capacity is a very important property, as it is related to formulation’s drug loading potential. Accordingly, in order to select the best oils for TF-HCl formulations, solubilizing capacity of four oils (castor oil, clove oil, coconut oil, and olive oil) for TF-HCl was screened. In solubility testing, it was found that TF-HCl had 90.31 mg/mL solubility in clove oil, 82.42 mg/mL solubility in olive oil ( very close to literature value (Baheti et al., Citation2016; Barot et al., Citation2012), 75.15 mg/mL in castor oil and 12.22 mg/mL in coconut oil (). Based on the higher solubility values, clove oil and olive oil were chosen for further investigation for nanoemulsion studies as oil bases.
Table 3. Solubility of TF-HCl to different oils, surfactants, and co-solvents (n = 3).
For development of less toxic nanoemulsions, nonionic surfactants in low concentrations are preferred over ionic surfactants (Abdou et al., Citation2017). In this study, two different nonionic surfactants, Tween 80 and Tween 20, were studied. Solubility of TF-HCl was higher to Tween 80 (50.24 mg/mL) as compared to Tween 20 (45.87 mg/mL), based on which Tween 80 (HLB = 14.5) was selected as a surfactant for further studies (). Isopropyl alcohol and ethanol were tested as possible co-solvents. TF-HCl was more soluble to ethanol (30.28 mg/mL; literature value 30 mg/mL (Vejnovic et al., Citation2010)) as compared to isopropyl alcohol (20.43 mg/mL). Solubility value for ethanol in this study was in very good agreement with the literature value 30 mg/mL (Vejnovic et al., Citation2010). Therefore, ethanol was selected as a co-solvent in this study.
3.2. Pseudo-ternary phase diagram studies for optimizing the nanoemulsion formulations
For development of more stable and effective nanoemulsions, surfactant:co-solvent ratio plays very important role. For this purpose, pseudo-ternary phase diagrams were constructed with both the clove and olive oils to determine the nanoemulsion formation area. Phase diagrams were formed with drug-free nanoemulsions.
Pseudo-ternary phase diagrams were constructed by using Smix (surfactant:co-solvent) ratios of 1:1, 1:2 and 2:1 (). The equal concentration ratio of surfactant and co-solvent (1:1) yielded remarkable region of nanoemulsion formation. Nanoemulsion region was increased with increase in co-solvent concentration (Smix 1:2), while the region was decreased with increase in surfactant concentration (Smix 2:1).
Figure 1. Pseudo-ternary phase diagrams for drug free nanoemulsions with Smix values of (A) 2:1, (B) 1:2 and (C) 1:1. Both the oils showed similar kind of nanoemulsion behavior. The circles presented in the figure are showing successful nanoemulsion formation area.
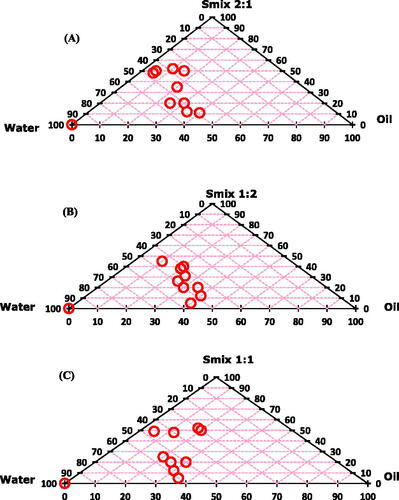
Based on the pseudo-ternary phase diagrams, oil:Smix ratios 5:5 and 6:4 were on the maximum nanoemulsion region. Accordingly, these two ratios with Smix values of 1:1, 1:2 and 2:1 were selected for further studies for preparation of drug-loaded nanoemulsions ().
3.3. Characterization of nanoemulsions
3.3.1. Droplet sizes and polydispersity index (PDI) values
The droplet sizes of all the nanoemulsion batches ranged from 222 nm to 924 nm. Smallest droplets were with olive oil formulation F1 (490 nm) and with clove oil formulation F7 (222 nm).
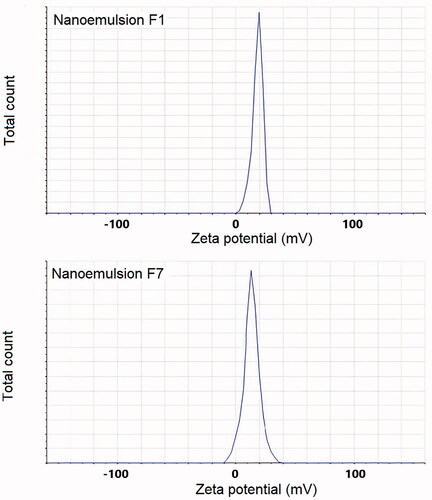
PDI values of all the studied formulations were below 0.691 (). With the smallest droplet size formulations (F1 and F7) also the PDI values were considerably low (0.339 and 0.349, correspondingly), indicating good monodispersity and good stability. This is also seen in the size distribution curves (). Based on particle size and PDI values, these two formulations (F1 and F7) were selected as optimized formulations for further studies.
Figure 2. Size distributions by intensity of nanoemulsion formulations (A) batch F1, and (B) Batch F7.
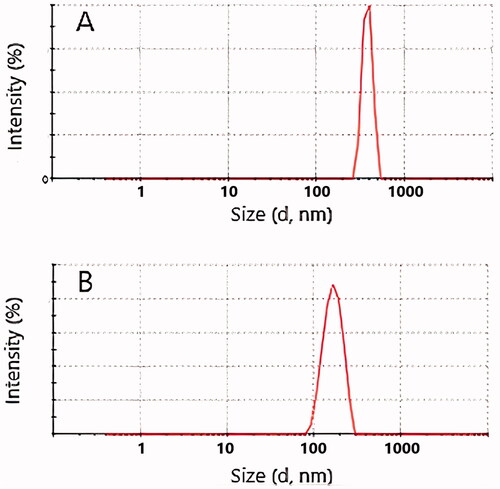
Table 4. Droplet sizes and PDI values of 5:5 oil:SMix nanoemulsions.
3.3.2. Drug–excipient interaction studies
For interaction studies, FT-IR spectrum of pure drug, TF-HCl, was compared with the spectra obtained from drug loaded nanoemulsions ().
Figure 3. FTIR spectra from top to down: TF-HCl, olive oil, clove oil, Tween 80, ethanol, nanoemulsion F1, and nanoemulsion F7. Wavelength areas representing functional groups of TF-HCl are framed in the figure: C = C stretching (green), C–N bending (black), and aromatic alkenyl C = C–H (blue) stretching bands (Anju and Kuriachan, Citation2017). These peaks were found either unchanged or very slightly shifted in nanoemulsion spectra.
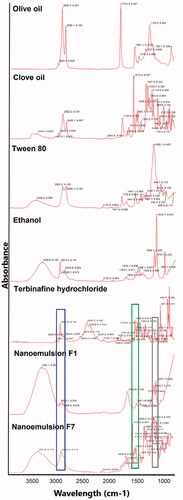
FTIR spectrum of pure TF-HCl showed C=C stretching band at 1513 cm−1, C–N band at 1073 cm−1, and aromatic alkenyl C=C–H stretching band at 2967 cm−1. Peaks in nanoemulsions spectra showed that functional groups of TF-HCl, namely C=C stretching (marked with green frames in ), C–N bending (black frames in ), and aromatic alkenyl C=C–H (blue frames in ) stretching bands (Anju & Kuriachan, Citation2017), were found either unchanged or very slightly shifted in nanoemulsion spectrum (). Nanoemulsion formulation F1 showed C=C stretching band at 1457 cm−1, C–N band at 1084 cm−1, and aromatic alkenyl C=C–H stretching band at 2978 cm−1, while nanoemulsion formulation F7 showed C=C stretching band at 1513 cm−1, C–N band at 1088 cm−1, and aromatic alkenyl C=C–H stretching band at 2922 cm−1. Besides the above-mentioned drug-specific peaks, in nanoemulsion spectrum for formulation F1 (with olive oil), asymmetrical and symmetrical stretching vibration of methylene (-CH2) group at 2922 cm−1 was found, like it was also detected in the original spectrum of olive oil.
3.3.3. Zeta potential measurements
Zeta potential values for all the nanoemulsions ranged from 6.4 to 18.1 mV. The optimized nanoemulsions of olive oil (F1) and clove oil (F7) showed zeta potential values of 18.1 and 13.2 mV, respectively (). These values of zeta potential can be seen to be enough in order to create sufficient stability to nanoemulsion systems.
3.3.4. Morphology
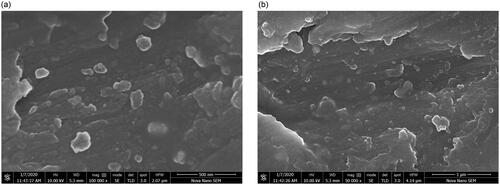
SEM images of nanoemulsions F1 and F7 revealed irregularly shaped droplets with clear outlines and cores (). Slight aggregation was observed. The sizes of the nanodroplets corresponded to the ones measured with DLS.
3.3.5. Determination of pH, viscosity, and refractive index values
pH of TF-HCl loaded optimized nanoemulsion formulations F1 and F7 were found to be 5.8 ± 1.1 and 6.0 ± 2.1, respectively (). Viscosity of optimized nanoemulsions F1 and F7 was low, being in the range of 0.9–1.2 cP. Refractive index of the optimized nanoemulsions F1 and F7 was found to be 1.33 ± 0.08 and 1.33 ± 0.05, respectively (). The refractive index values of nanoemulsions F1 and F7 were comparable to water indicating a clear and transparent nature of oil in water (O/W) type nanoemulsions.
Table 5. pH, viscosity, refractive index, and entrapment efficiency values for the two optimized nanoemulsion formulations F1 and F7 (n = 3).
Table 6. Skin irritation results 24 h after the application.
3.3.6. Entrapment efficiency studies
For the two optimized nanoemulsion formulations, entrapment efficiencies were 97.53% (formulation F1) and 96.83% (formulation F7) as shown in . This demonstrated minimum loss of TF-HCl during the production steps, which is typical for nanoemulsion formulations (Acharya et al., Citation2017).
3.3.7. Skin permeation studies
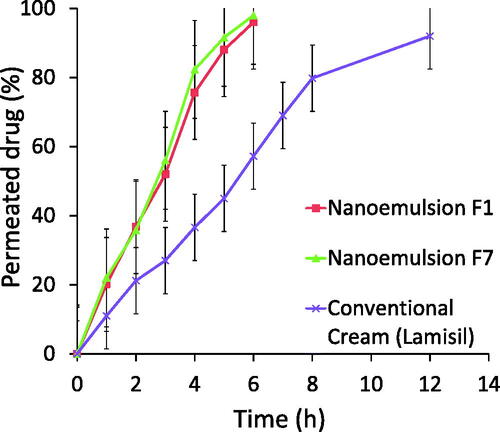
In vitro skin permeation study was performed in order to check the permeation of TF-HCl from two optimized nanoemulsion formulations (F1 and F7) and one commercial reference product. In vitro skin permeation profile for both the optimized nanoemulsions F1 and F7 were very similar, and showed improved permeation of the drug (96% for F1 and 98% for F7, respectively) in six hours compared to commercial product with the same drug load (57% drug penetration in six hours, and 92% during the 12 hours study) (). (Commercial product contained sodium hydroxide, benzyl alcohol, sorbitan monostearate, cetyl palmitate, cetyl alcohol, stearyl alcohol, polysorbate 60, isopropyl myristate and water as excipient, but the exact composition is not known.)
3.3.8. Drug retention studies
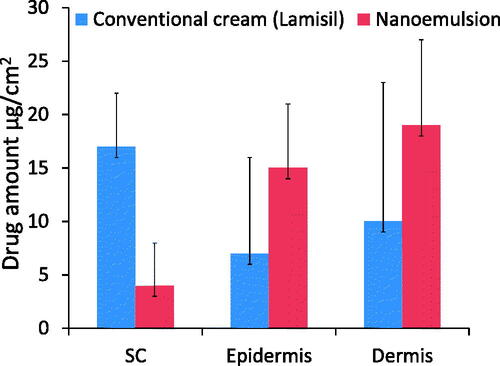
Drug retention studies showed higher quantities of TF-HCl retention in epidermis and dermis layers of skin treated with optimized nanoemulsion (F7) as compared to skin treated with conventional commercial cream ().
3.3.9. Skin irritation studies
Skin irritation studies were performed in order to check any signs of edema or erythema after application on rat skin. Skin irritation was determined visually by inspecting the treated skin area. Any of the images of rat skin from untreated control group (group 1), group treated with nanoemulsion formulation F7 (group 2) and group treated with conventional commercial cream (Lamisil cream) (group 3) showed no signs of edema nor erythema 24 hours after treatment ().
Figure 8. Photographs of skin sections of control group (Group 1), group treated with nanoemulsion F7 (Group 2), and group treated with conventional commercial cream (Lamisil) (Group 3). No signs of edema or erythema were seen (the size of shaved area is approximately 2 × 2 cm2).
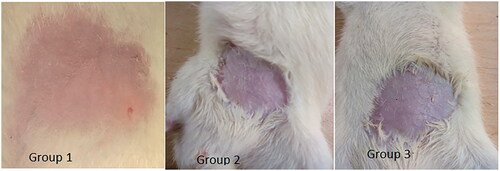
Convenience and acceptability by the patients had been limited when any irritation or erythema is observed after topical drug application (Khurana et al., Citation2013). Hence, any topical drug delivery system should be free of these erythematic reactions. In this study, erythema scale and edema scale showed for nanoemulsion formulation F7 values below one indicating no signs of erythema and edema. This was the case also for the conventional commercial cream (Lamisil) (). Accordingly, nanoemulsion formulation was safe and well tolerated for topical use.
3.3.10. Histopathological assessment of treated rat skin
Histopathological assessment was performed in order to check changes in complete blood count (CBC) after topical penetration of TF-HCl. CBC results of the blood from Group 2 (animals treated with nanoemulsion formulation F7) and Group 3 (animals treated with conventional cream) are presented in .
Table 7. CBC results for control group animals (Group 1), nanoemulsion F7 treated animals (Group 2), and conventional cream (Lamisil) treated animals (Group 3) (n = 3).
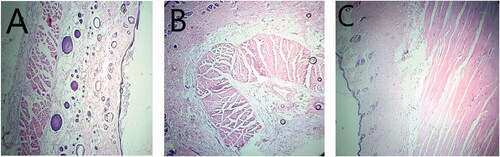
Microscopic examination showed that Group 1 skin (control group animals) had compacted layer of corneocytes with normal epidermis, dermis and sub-cutaneous tissues (El-Naggar et al., Citation2017). Histological examination of skin treated with commercial cream (Group 3) or nanoemulsion formulation (Group 2) showed similarity with skin of the control animals in Group 1. Results showed no alteration in CBC count in nanoemulsion treated animals and the values were similar to normal values (). CBC values of the animals treated with conventional cream (Lamisil) were lower, which is associated with induced side effects (Elsherif et al., Citation2017).
Finally, all the animal groups were subjected to histopathological examination (). Results showed excellent skin tolerance when treated with optimized nanoemulsion formulation F7 for 24 hours.
3.3.11. Dispersion stability studies
Dispersion stability studies of optimized nanoemulsions F1 and F7 were carried out under stressed conditions. In this study, the nanoemulsions showed good stability and no coalescence, phase separation, creaming nor cracking upon the stress tests were seen.
4. Discussion
As explained earlier, selection of the components for the nanoemulsion formulation studies was based on TF-HCl solubility properties. Clove oil and olive oil were selected as oil phase candidates for nanoemulsions due to the high solubility of TF-HCl to these oils. Besides, both the oils have different kind of advantageous medical properties.
Olive oil has good penetration-enhancing properties for topical drug delivery. The higher permeation is based on the fact that it enhances the fluidity of the intercellular lipid barriers in the stratum corneum by creating the separate domains that cause interference with the continuity of the stratum corneum and, thus, induce highly penetrable channels through the stratum corneum (Abdellatif & Abou-Taleb, Citation2015). Olive oil is also enriched with high amounts of polyphenols and thereby it is effective in topical infections. These beneficial effects occur due to the major secondary metabolite of olive oil, oleuropein, a ester of β-glycosylated eleanolic acid and hydroxytyrosol (Smeriglio et al., Citation2019).
Clove oil has in the same way many advantageous medical properties, e.g. it is analgesic, antiseptic, carminative and it behaves as natural stimulant (Shahbazi, Citation2019). Clove oil is safe to use in topical preparations for pain relief and wound healing: it has been found safe by the US food and drug administration (US FDA) with no side effects. Moreover, clove oil has also fungicidal activity (Kheawfu et al., Citation2018).
Nanoemulsion droplet sizes and PDI values were used as the main critical quality attributes (CQAs) for selection of the optimized formulations, and small particle size (below 500 nm) and low PDI value (preferably less than 0.35) was considered best combination. One optimized formulation was selected for both of the oils (clove oil and olive oil), because both of the oils provide a different kind of advantageous properties for topical drug formulations (as explained above). Penetration of the drugs encapsulated in emulsions is enhanced when the droplet size is in nanometric range (less than 500 nm) (Singh et al., Citation2017). Reducing the droplet size below 500 nm produces higher penetration through different skin layers and absorption of drug ingredients with higher particle uptake by enhancing the mechanism of passive transport. Among the olive oil nanoemulsions, F1 was considered as optimized nanoemulsion formulation with droplet size value of 490 nm, while for clove oil nanoemulsion F7 was optimized formulation with droplet size value of 222 nm. Due to the droplet sizes below 500 nm, both these nanoemulsions are expected to face no barrier in passage through biological barriers, as explained above (Alzorqi et al., Citation2016).
Both the optimized formulations had Smix value of 1:1 with an equal concentration of surfactant and co-solvent. Formulations with Smix values 1:2 and 2:1 had relatively high concentrations of surfactant and co-solvent as compared to oil, which entered into the oil phase and increased the droplet size (Khani et al., Citation2016). Statistically difference between droplet sizes in formulations F1 and F7 was significant (p < 0.05) indicating that the type of oil used affected significantly the droplet sizes of the nanoemulsions.
Polydispersity index (PDI) describes the uniformity (in size) of droplets in the formulation. It is a unitless value ranging from 0 to 1 (Khurana et al., Citation2013); the lower the value, the more homogeneous is the sample. In drug delivery applications using lipid-based carriers, PDI value 0.3 indicates homogenous system (Danaei et al., Citation2018). PDI values of the optimized nanoemulsions were close to 0.3 (0.339 and 0.349) indicating relatively homogeneous nature of the nanoemulsions, and meaning more stable nanoemulsions. Besides, small nanodroplet sizes and low PDI values have been shown to enhance drug permeation through epidermis and dermis layers of the skin (Sharma et al., Citation2019).
Based on SEM images, slight aggregation was observed, which is a common feature for emulsion systems in SEM analysis, and it is due to the sample drying process before the analysis (Klang et al., Citation2012). However, droplet sizes in SEM images correlated well with the ones measured by DLS.
FT-IR spectroscopy is a very important analytical tool to determine any possible interactions between drug and excipient used in the formulation (Pathan & Mallikarjuna Setty, Citation2012). In this study, no major interactions between drug and other formulation components used in the nanoemulsions were noticed, and intact TF-HCl peaks of pure drug were detected in FT-IR spectrum of tested nanoemulsions. Accordingly, based on FT-IR analysis, nanoemulsion excipients were compatible with TF-HCl and there were no interactions between the drug and the excipient used in the formulations.
Zeta potential is an important parameter that directly affects the stability of nanoemulsions as well as skin irritation and drug penetration. High values of zeta potential (±30 mV) are associated with more stable nanoemulsion systems as charge creates a high-energy barrier toward agglomeration of dispersed nanoemulsion droplets (Acharya et al., Citation2017). Zeta potential values in the range of −5 to +5 mV indicate fast aggregation of globules, if stabilization is based only on electric stabilization. Higher values of zeta potential help in maintaining the globules in Brownian motion for a longer time due to repulsive forces between similar charges, which separate the particles farther away (Rai et al., Citation2018). Zeta potential values measured for both the nanoemulsion formulation in this study were high enough for stable emulsion formation.
Both the optimized nanoemulsions of olive oil (formulation F1) and clove oil (formulation F7) showed positive zeta potential values. The interaction of nanoemulsions with skin depends upon a number of factors including also the electrical charge of the droplets and the positive zeta potential is beneficial for permeation: in a study by Hoeller et al., positively charged nanoemulsion containing fludrocortisone acetate showed better permeation compared to negatively charged nanoemulsion (Hoeller et al., Citation2009). Accordingly, zeta potential values in this study indicated that both the optimized formulations were stable with less tendency to globular aggregation, as well as the overall positive charge enhanced skin permeation.
pH of the topical formulations is related to the skin irritability and extent of drug delivery (Oliveira et al., Citation2018). pH of the nanoemulsion formulations was found to be in the acceptable range for skin preparations (pH 5–7), which meant that developed nanoemulsion formulations were compatible with skin, causing no irritation due to the pH value, and were safe for topical use.
Viscosity of nanoemulsions is related to the oil concentration (in this study olive oil and clove oil) and surfactant (here Tween 80) level in nanoemulsion formulations. With an increase in concentration levels of oil and surfactant in the formulations, viscosity of nanoemulsions increased. Similarly, viscosity decreased with an increase in co-solvent (ethanol) concentration. Viscosity values of optimized nanoemulsions F1 and F7 were low (0.9–1.2 cP) due to the low amount of surfactant and small oil phase volume (Kanke et al., Citation2019).
Refractive index is the measure of transparency of nanoemulsions. Nanoemulsions were found to have refractive indexes similar to water (1.33), which was due to the high amount of water in the nanoemulsions. Transparency of emulsion system is also macroscopic indicator of its physical stability. Transparent appearance of emulsions has become an important feature in their various uses, such as for cosmetics and pharmaceuticals.
Both the tested oils entrapped efficiently the drug into the nanoemulsion system, and difference between the drug contents of the formulations F1 and F7 was statistically insignificant (p > 0.05). This reflected well the similar solubility values of TF-HCl with both the oils.
Skin permeation studies of optimized nanoemulsions F1 and F7 showed that permeation rate through skin was higher with nanoemulsions as compared to conventional cream formulation. There were no differences in permeation rates between the two studied nanoemulsions. The difference between these two nanoemulsions was the oil component (olive oil vs. clove oil), while the relative amounts of the components were the same. Small droplet size provided increased surface area for permeation. Also, in nanoemulsion formulations, external aqueous phase caused swelling of cells in stratum corneum due to hydration, making the transport channels wider for drug passage and thus enhancing the penetration of a lipophilic drug (Elmataeeshy et al., Citation2018). Enhanced permeation of TF-HCl from nanoemulsion formulations involves different factors, namely, globule size in nano range, as well as favorable formulation components and their concentrations. Selected oils, surfactant and co-solvent in nanoemulsion formulations provided enhanced drug penetration through the skin by disruption effects. Permeation enhancement may lead to improved efficacy (Imam et al., Citation2015, Citation2017; Sita & Vavia, Citation2020).
Drug retention studies showed higher quantities of TF-HCl in epidermis and dermis layers of skin treated with optimized nanoemulsion (F7) as compared to conventional commercial cream. This was due to the easier penetration of nanoemulsion through the skin layers. Higher drug amounts in dermis after the nanoemulsion application on the skin will increase the efficacy by producing prolonged therapeutic effect.
Skin permeation and drug retention studies were based on UV spectrophotometric analysis. Though HPLC would be more recommended technique, UV spectrophotometry is widely utilized in many earlier studies (Patel et al., Citation2011; Rajan & Vasudevan, Citation2012). UV spectrophotometry is fast and it avoids use of organic solvents being more environmental friendly technique. The challenge with UV spectrophotometric analysis is that lipid components released from the skin may give some interference to UV spectrophotometric analysis. In this study, the drug was TF-HCl, which has a very strong absorption maximum at utilized analytical wavelength, and that can be seen to minimize the interfering effect.
Convenience and acceptability by the patients is lowered, if any irritation or erythema is observed after topical drug administration (Khurana et al., Citation2013). Hence, any topical drug delivery system should be free of these erythematic reactions. In this study, erythema scale and edema scale showed for nanoemulsion formulation F7 values below one indicating no signs of erythema and edema, which was the case also for the conventional commercial cream. Accordingly, nanoemulsion formulation was safe and well-tolerated for topical use.
Finally, dispersion stability studies of optimized nanoemulsions F1 and F7 were carried out under stressed conditions. Nanoemulsions are basically thermodynamically stable when formed with specific quantities of oil, water and surfactant. The instability of nanoemulsions are originated from Ostwald ripening and coalescence (Capek, Citation2004). In this study, the nanoemulsions showed good stability and no coalescence, phase separation, creaming nor cracking upon the stress tests were seen.
5. Conclusions
In this study, olive oil and clove oil-based terbinafine hydrochloride nanoemulsions were prepared successfully using high-energy ultrasonication method. Terbinafine hydrochloride and pharmaceutically accepted excipient used in the study were found to be compatible with each other as shown by FT-IR studies. Olive oil and clove oil (oils), Tween 80 (surfactant), and ethanol (co-solvent) were screened out as best formulation components for nanoemulsion formation. Further, nanoemulsion composition optimization was performed with the aid of pseudo-ternary phase diagrams. Based on droplet size, one olive oil and one clove oil-based formulation were considered as optimized nanoemulsion and they were studied further. In the skin penetration studies, terbinafine hydrochloride-loaded nanoemulsions exhibited rapid penetration of drug through mice skin (up to 98%) compared to commercial product (57% permeation at the same time). Small droplet size and low viscosity values of the nanoemulsions were the main factors for enhanced drug penetration. Skin irritation studies and histopathological examination proofed that nanoformulations were safe and efficacious to use against topical infections. The overall results of the study concluded that olive and clove oil-based nanoemulsion systems are promising carriers for improved topical delivery of terbinafine hydrochloride.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Research ethics committee (REC) of Riphah Institute of Pharmaceutical Sciences, Riphah International University Lahore, Pakistan.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data presented in this study are available on request from the corresponding author (Mohammad Imran Khan).
Additional information
Funding
References
- Abdellatif A, Abou-Taleb HA. (2015). Optimization of nano-emulsion formulations for certain emollient effect. World J Pharm Pharm Sci 4:1314–28.
- Abdou EM, Kandil SM, El Miniawy HM. (2017). Brain targeting efficiency of antimigrain drug loaded mucoadhesive intranasal nanoemulsion. Int J Pharm 529:667–77.
- Acharya U, Rajarajan S, Acharya R, Acharya R, Chaudhary G, Ghimire S. (2017). Formulation, and evaluation of nanoemulsion based system for transdermal delivery of the antipsoriatic drug. WJPPS 6:732–48.
- Alzorqi I, Ketabchi MR, Sudheer S, Manickam S. (2016). Optimization of ultrasound induced emulsification on the formulation of palm-olein based nanoemulsions for the incorporation of antioxidant β-d-glucan polysaccharides. Ultrason Sonochem 31:71–84.
- Anju V, Kuriachan M. (2017). Formulation and evaluation of terbinafine hydrochloride loaded microsponge based gel for topical sustained delivery. Int J Pharm Pharm Res 10:69–93.
- Arzani, G, Haeri, A, Daeihamed, M, Bakhtiari-Kaboutaraki, H, Dadashzadeh, S. (2015). Niosomal carriers enhance oral bioavailability of carvedilol: effects of bile salt-enriched vesicles and carrier surface charge. Int J Nanomed 10:4797–4813.
- Baheti A, Srivastava S, Sahoo D, Lowalekar R, Panda B.P, Padhi B.K, Raghuvanshi R. (2016). Development and pharmacokinetic evaluation of industrially viable self-microemulsifying drug delivery systems (SMEDDS) for terbinafine. CDD 13:65–75.
- Bakshi P, Jiang Y, Nakata T, Akaki J, Matsuoka N, Banga AK. (2018). Formulation development and characterization of nanoemulsion-based formulation for topical delivery of heparinoid. J Pharm Sci 107:2883–90.
- Barot BS, Parejiya PB, Patel HK, Gohel MC, Shelat PK. (2012). Microemulsion-based gel of terbinafine for the treatment of onychomycosis: optimization of formulation using D-optimal design. AAPS PharmSciTech 13:184–92.
- Burstein Z, Vildósola H, Lozano Z, Verona R, Vargas G. (2004). Colestasic toxic hepatitis caused by terbinafine: case report. Revista de gastroenterologia del Peru: organo oficial de la Sociedad de Gastroenterologia del Peru 24:357–62.
- Capek I. (2004). Degradation of kinetically-stable o/w emulsions. Adv Colloid Interface Sci 107:125–55.
- Carpenter J, Saharan VK. (2017). Ultrasonic assisted formation and stability of mustard oil in water nanoemulsion: effect of process parameters and their optimization. Ultrason Sonochem 35:422–30.
- Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, Khorasani S, Mozafari M. (2018). Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10:57.
- Elmataeeshy ME, Sokar MS, Bahey-El-Din M, Shaker DS. (2018). Enhanced transdermal permeability of terbinafine through novel nanoemulgel formulation: development, in vitro and in vivo characterization. Future J Pharm Sci 4:18–28.
- El-Naggar ME, Shaarawy S, Shafie AE, Hebeish A. (2017). Development of antimicrobial medical cotton fabrics using synthesized nanoemulsion of reactive cyclodextrin hosted coconut oil inclusion complex. Fibers Polym 18:1486–95.
- Elsherif NI, Shamma RN, Abdelbary G. (2017). Terbinafine hydrochloride trans-ungual delivery via nanovesicular systems: in vitro characterization and ex vivo evaluation. AAPS PharmSciTech 18:551–62.
- Gaur S, Garg A, Yadav A, Beg M, Gaur K. (2014). Nanoemulsion gel as novel oil based colloidal nanocarrier for topical delivery of bifonazole. Indian Res J Pharm Sci 1:36–54.
- Güngör S, Erdal MS, Aksu B. (2013). New formulation strategies in topical antifungal therapy. JCDSA 03:56–65.
- Gupta M, Agrawal U, Vyas SP. (2012). Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert Opin Drug Deliv 9:783–804.
- Hinojosa JR, Hitchcock K, Rodriguez JE. (2007). Which oral antifungal is best for toenail onychomycosis? J Fam Pract 56:581–82.
- Hoeller S, Sperger A, Valenta C. (2009). Lecithin based nanoemulsions: a comparative study of the influence of non-ionic surfactants and the cationic phytosphingosine on physicochemical behaviour and skin permeation. Int J Pharm 370:181–6.
- Hussain A, Samad A, Singh S, Ahsan M, Haque M, Faruk A, Ahmed F. (2016). Nanoemulsion gel-based topical delivery of an antifungal drug: in vitro activity and in vivo evaluation. Drug Deliv 23:642–47.
- Hussain A, Singh S, Webster TJ, Ahmad FJ. (2017). New perspectives in the topical delivery of optimized amphotericin B loaded nanoemulsions using excipients with innate anti-fungal activities: a mechanistic and histopathological investigation. Nanomedicine 13:1117–26.
- Imam SS, Ahad A, Aqil M, Akhtar M, Sultana Y, Ali A. (2017). Formulation by design based risperidone nano soft lipid vesicle as a new strategy for enhanced transdermal drug delivery: in-vitro characterization, and in-vivo appraisal. Mater Sci Eng C Mater Biol Appl 75:1198–1205.
- Imam SS, Aqil M, Akhtar M, Sultana Y, Ali A. (2015). Formulation by design-based proniosome for accentuated transdermal delivery of risperidone: in vitro characterization and in vivo pharmacokinetic study. Drug Deliv 22:1059–70.
- Jain P, Chaudhari A, Patel S, Patel Z, Patel D. (2011). Development and validation of the UV-spectrophotometric method for determination of terbinafine hydrochloride in bulk and in formulation. Pharm Methods 2:198–202.
- Kanke PK, Pathan IB, Jadhav A, Usman MRM. (2019). Formulation and evaluation of febuxostat nanoemulsion for transdermal drug delivery. J Pharm BioSci 7:1–7.
- Kaur R, Ajitha M. (2019). Formulation of transdermal nanoemulsion gel drug delivery system of lovastatin and its in vivo characterization in glucocorticoid induced osteoporosis rat model. J Drug Deliv Sci Technol 52:968–78.
- Khani S, Keyhanfar F, Amani A. (2016). Design and evaluation of oral nanoemulsion drug delivery system of mebudipine. Drug Deliv 23:2035–43.
- Kheawfu K, Pikulkaew S, Rades T, Müllertz A, Okonogi S. (2018). Development and characterization of clove oil nanoemulsions and self-microemulsifying drug delivery systems. J Drug Deliv Sci Technol 46:330–38.
- Khurana S, Jain N, Bedi P. (2013). Nanoemulsion based gel for transdermal delivery of meloxicam: physico-chemical, mechanistic investigation. Life Sci 92:383–92.
- Klang V, Matsko NB, Valenta C, Hofer F. (2012). Electron microscopy of nanoemulsions: an essential tool for characterisation and stability assessment. Micron 43:85–103.
- McClements DJ. (2012). Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter 8:1719–29.
- Oliveira CA, Gouvêa MM, Antunes GR, de Freitas ZMF, de Carvalho Marques FF, Ricci-Junior E. (2018). Nanoemulsion containing 8-methoxypsoralen for topical treatment of dermatoses: development, characterization and ex vivo permeation in porcine skin. Int J Pharm 547:1–9.
- Patel MR, Patel RB, Parikh JR, Solanki AB, Patel BG. (2011). Investigating effect of microemulsion components: in vitro permeation of ketoconazole. Pharm Dev Technol 16:250–58.
- Pathan IB, Mallikarjuna Setty C. (2012). Nanoemulsion system for transdermal delivery of tamoxifen citrate: design, characterization, effect of penetration enhancers and in vivo studies. Dig J Nanomater Biostructures 7:1373–87.
- Rai VK, Mishra N, Yadav KS, Yadav NP. (2018). Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: formulation development, stability issues, basic considerations and applications. J Control Release 270:203–25.
- Rajan R, Vasudevan DT. (2012). Effect of permeation enhancers on the penetration mechanism of transfersomal gel of ketoconazole. J Adv Pharm Technol Res 3:112–16.
- Salim N, Ahmad N, Musa SH, Hashim R, Tadros TF, Basri M. (2016). Nanoemulsion as a topical delivery system of antipsoriatic drugs. RSC Adv 6:6234–50.
- Shafiq S, Shakeel F, Talegaonkar, S, Ahmad FJ, Khar RK, Ali M. (2007). Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm 66:227–43.
- Shahbazi Y. (2019). Antioxidant, antibacterial, and antifungal properties of nanoemulsion of clove essential oil. Nanomed Res J 4:204–08.
- Sharma B, Iqbal B, Kumar S, Ali J, Baboota S. (2019). Resveratrol-loaded nanoemulsion gel system to ameliorate UV-induced oxidative skin damage: from in vitro to in vivo investigation of antioxidant activity enhancement. Arch Dermatol Res 311:773–93.
- Singh Y, Meher J.G, Raval K, Khan F.A, Chaurasia M, Jain N.K, Chourasia MK. (2017). Nanoemulsion: concepts, development and applications in drug delivery. J Control Release 252:28–49.
- Sita VG, Vavia P. (2020). Bromocriptine nanoemulsion-loaded transdermal gel: optimization using factorial design, in vitro and in vivo evaluation. AAPS PharmSciTech 21:80.
- Smeriglio A, Denaro M, Mastracci L, Grillo F, Cornara L, Shirooie S, Nabavi SM, Trombetta D. (2019). Safety and efficacy of hydroxytyrosol-based formulation on skin inflammation: in vitro evaluation on reconstructed human epidermis model. Daru 27:283–93.
- Sosnowska K, Szymanska E, Winnicka K. (2017). Nanoemulsion with clotrimazole: design and optimalization of mean droplet size using microfluidization technique. Acta Pol Pharm 74:519–26.
- Terbinafine hydrochloride product information, Cayman Chemicals, Ann Arbor, MI, USA.Taken from the websites 26.1. 2022. https://cdn.caymanchem.com/cdn/insert/10011619.pdf
- Vejnovic I, Huonder C, Betz G (2010). Permeation studies of novel terbinafine formulations containing hydrophobins through human nails in vitro. Int J Pharm 397:67–76.