ABSTRACT
Introduction
Propositional language and underlying executive functions can be impaired post-stroke and affect communication and quality of life. Current stroke screening tools are largely tailored to patients with aphasia, being either non-verbal or focussed on core language skills such as naming and repetition. The Brief Executive Language Screening Test (BELS) is a newly developed cognitive screening tool that assesses memory, oral apraxia, core language, as well as propositional language and associated executive functions that can be impacted and overlooked in stroke patients without aphasia. This study examines BELS sensitivity and specificity, and performance in acute to early sub-acute stroke relative to controls.
Method
Cross-sectional BELS data from 88 acute left and right hemisphere stroke patients (within 7 weeks of stroke) and 116 age-matched healthy controls were compared using independent samples t-tests. ROC Curve Analysis was performed to determine a cutoff score for the BELS.
Results
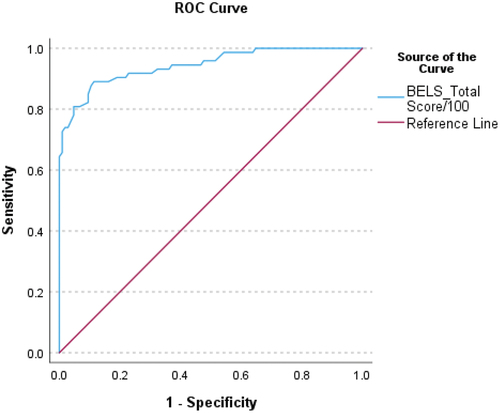
Left and right stroke patients were reduced on all propositional language subtests, and executive function subtests of inhibition, strategy, and selection. Differences were also observed for Oral Apraxia, Naming, and Memory. By contrast, Word Comprehension and Repetition, and Sentence Completion Initiation (after corrections applied) did not differ between groups. A total BELS score of 79.25/100 was highly sensitive (.89) and specific (.89) when classifying stroke patients and healthy controls.
Conclusion
The BELS is brief, sensitive, suitable for bedside administration, and can aid in detection and rehabilitation of subtle executive language impairments. This in turn will help improve relationships and quality of life post-stroke.
Post-stroke cognitive impairments occur in up to 70% of patients and frequently persist long-term (> 1 year), which contributes to disability, social isolation, and reduced quality of lifeCitation1–4 Citation5–7 Executive functioning and language are cognitive domains commonly affected in acute stroke patients that also predict long-term functional, neuropsychological, and emotional outcomesCitation8–11,Citation5,Citation12,Citation13 Current stroke screening tools are largely tailored to patients with aphasia, being either non-verbal or focussed on core language skills [e.g. Cognitive Assessment Scale for Stroke Patients; CASP,Citation14 Cognitive Linguistic Quick Test; CLQT,Citation15 Oxford Cognitive Screen; OCS,Citation16 Oxford Cognitive Screen Plus; OCS-Plus,Citation17 Quick Aphasia Battery;Citation18,Citation19 Western Aphasia Battery – Revised.Citation20 Further, other cognitive screening tools used in the early stages of stroke only capture severe global impairment [e.g. Montreal Cognitive Assessment; MoCA,Citation21 Mini-Mental State Examination; MMSE,Citation22–24 or minimally assess executive functions, which are known to be impacted in early stroke (e.g. CASP, CLQT, OCS, OCS-Plus, MoCA, MMSE). Consequently, subtle communication impairments (e.g. initiation impairment resulting in diminished connected speech) in stroke patients without clear aphasia go undetected, and therefore do not receive appropriate intervention and rehabilitation, which impacts relationships, daily living, and quality of life.Citation9 This study investigates the sensitivity and specificity of the Brief Executive Language Screening Test [BELS;Citation13]; a recently developed cognitive screening tool that assesses memory, oral apraxia, executive functions, and both core language (nominal) skills and spontaneous (propositional) language.
Impairment to executive functions (e.g. initiation, inhibition, planning, problem solving) occur in ~40% of acute stroke patients, and impact successful daily living and mental healthCitation25,Citation8,Citation26 Additionally, it is the strongest predictor of functional and cognitive outcomes one-year post-stroke.Citation5,Citation10,Citation11 In addition, stroke patients often demonstrate impaired propositional language; namely, voluntary, spontaneous, and connected speech that is novel to a context, and crucial for communicating ideas, thoughts, instructions, and feelings.Citation9,Citation13,Citation27–30 Impairment presents as diminished quantity and quality of connected speech,Citation31 and consequently individuals can experience less meaningful communication and strained relationships with others.Citation9
Propositional language impairments highlight the interface of language and executive functioning, which occurs at an early or higher level of language generation.Citation13,Citation31–35 Executive functions are integral to the pre-verbal conceptualization stage of language, and include planning, idea generation, selection (i.e. choosing from competing ideas, inhibiting irrelevant ideas), sequencing ideas, initiation, task-setting and monitoring.Citation31–38 These executive functions have been associated with both left frontal [e.g. initiation, selection; Citation37,Citation39–43 and right frontal [e.g. inhibition;Citation44,Citation45 regions. Importantly, patients with the acquired language disorder of dynamic aphasia demonstrate that disruption to conceptualization processes and severely reduced propositional language can occur despite intact core or nominal language [i.e. naming, repetition, comprehension.Citation46–48,Citation37,Citation43,Citation49 Non-aphasic stroke patients have also shown propositional language impairment, highlighting these deficits are subtle and can be easily missed without sufficient assessment; contributing to a lack of or insufficient intervention and rehabilitation.Citation9
Assessment of propositional language and executive functions in the acute stage of stroke is critical for early detection and rehabilitation. Brief cognitive screens are more practical than large neuropsychology batteries for acute patients who tire quickly; however, tests such as the MMSE and MoCACitation21,Citation22 lack sensitivity and specificity in this population, and underestimate cognitive impairment due to absence or limited measurement of complex, multifaceted domains like language and executive functioning, known to be affected in stroke.Citation23–25,Citation50,Citation51,Citation16,Citation52–56 Recently there has been a shift toward stroke-specific cognitive screening tools (e.g. OCS, OCS-Plus, CASP, QAB, WAB-R), which are tailored to stroke patients with aphasia.Citation14,Citation16–20 Although extremely valuable, these screens either largely remove spoken language, or tap core language only (e.g. naming), meaning higher level language impairments are not captured. Importantly, current screening tools (both aphasia screens and cognitive screens) only very minimally assess the executive function domain, that underpin the pre-verbal message-generation stage of propositional language (e.g. OCS – Trails; MoCA – brief Trails and Verbal Fluency; CASP – Motor Go No-Go; QAB – connected speech topic; CLQT – Verbal Fluency and Design Generation; WAB-R – connected speech topic). Thus, a cognitive screen that more completely captures executive functions and propositional language deficits in non-aphasic stroke patients (in addition to articulation and core language skills) is necessary to improve rehabilitation services, quality of life and long-term stroke outcomes.
Brief executive language screening test (BELS)
The BELSCitation13 is a valid, recently developed brief cognitive test (~15-20 minutes) that can be administered at bedside. It has an Oral Apraxia subtest to screen for articulation and motor speech difficulties, as well as a Nominal Language subsection (Object and Action Naming, Word Comprehension, and Word and Sentence Repetition). A novel feature of the BELS is the Propositional Language and Executive Function subsection, which includes two Spontaneous Speech Scene Descriptions, Phonemic and Semantic Verbal Fluency (with two “Goal” conditions), Sentence Completion (Initiation and Inhibition), and a Motor Go No-Go subtest. This section taps executive functions (initiation, selection, inhibition, and strategy use) known to impact connected speech.Citation13
Aims
This study aimed to determine stroke patients’ performance on the BELS relative to healthy age- and gender-matched controls, as well as sensitivity and specificity of a total BELS score. We also aimed to explore a BELS cutoff score for left and right hemisphere patients (LHS and RHS, respectively). Our first hypothesis was that healthy controls would perform significantly better than stroke patients on the BELS. Secondly, we hypothesized a Receiver Operating Characteristic (ROC) curve analysis would provide a cutoff score on the BELS that was highly sensitive (high true positive rate) and specific (low false positive rate) when classifying stroke and healthy cases. We expected cutoff scores to be similar for LHS and RHS, due to both left and right frontal patients demonstrating impairment to executive processesCitation44 Burgess & Shallice, 1996.Citation37,Citation41,Citation43,Citation45
Method
Data used in this cross-sectional study was obtained via convenience sampling by supervised clinical neuropsychology registrars and doctoral candidates in hospital stroke wards (Princess Alexandra Hospital, Royal Brisbane and Women’s Hospital, Surgical Treatment and Rehabilitation Service) as part of a longitudinal project between 2016 and 2023. This study was approved by the Metro South and Metro North Queensland Health Human Research Ethics Committees (approval number HREC/16/QPAH/793). All participants provided informed written consent. Data and BELS study materials are available on the Open Science Framework (https://osf.io/473g6/). This study conforms to STARD Guidelines.
Participants
Stroke patients
Inclusion criteria were first-time stroke confirmed by brain imaging, over 18 years old and fluent English-speakers. Exclusion criteria were Transient Ischemic Attack, or diagnosis of another neurological disorder. Patients and controls who did not complete the BELS were excluded from analyses. One-hundred-and-nine patients were recruited, with a final sample of 88 stroke patients tested on average 17.39 days (ranging from 2–49 days) post-stroke; acute to early subacute stageCitation57, 43% female; handedness: 90% right, 1% forced right, 9% left; MAGE = 62.88, SDAGE = 14.10; MEDUCATION = 12.03, SDEDUCATION = 2.71]. There were 80 ischemic stroke patients, and eight hemorrhagic patients, 29 left hemisphere patients (LHS), 57 right hemisphere patients (RHS), and two bilateral patients.
Controls
Healthy controls were recruited through the University of Queensland (UQ) networks and included if they spoke fluent English and were 18 years or older. Exclusion criteria was any neurological disorder or performing in the impaired range (<5th percentile) on standard neuropsychology tests. The final control group consisted of 116 healthy adults (47% female; handedness: 92% right, 1% right/ambidextrous, 7% left; MAGE = 63.15, SDAGE = 13.52; MEDUCATION = 15.12, SDEDUCATION = 3.56).
Materials
Due to the verbal nature of the BELS, stroke patients were screened for aphasia via independent neuropsychological tests. These included the National Adult Reading Test to assess reading [NART – 2nd Edition,Citation58 normative data for cutoffs fromCitation59; the Boston Naming Test to assess naming [BNT – Short Form,Citation60 normative data for cutoffs from,Citation61,andCitation62 and the Hayling Sentence Completion Test (Section A) to assess comprehension [HSCT.Citation63 Visual perception was also assessed via the Visual Object and Space Perception Battery Incomplete Letters subtestCitation64 Patients were classified as aphasic if they performed below the 5th percentile on either NART, BNT, or HSCT A.
BELS
The BELS has four subsections.
Oromotor functioning
Oromotor functioning is assessed via the Oral Apraxia subtest, which involves execution of five facial/mouth movements (e.g. whistling).
Nominal language
Ten items (e.g. tiara) are used for Object Naming, Word Repetition and Word Comprehension. Action Naming includes five illustrated actions obtained from Druks and Masterson’sCitation65 Object and Action Naming Battery (e.g. “shooting”), which are subsequently transformed to past tense (e.g. “shot”). There are five Sentence Repetition items.
Propositional language and executive functions
Spontaneous speech is assessed via a complex scene (Australian Beach Scene), with participants instructed to describe the scene for one minute. The Cookie Theft SceneCitation66 is then presented as a “goal” condition, with participants instructed to speak continuously for one minute about the scene.Citation13,Citation67 Word Fluency tasks involve naming as many words as possible in one minute under two conditionsCitation68: phonemic (i.e. words beginning with S and B) and semantic (i.e. animals and fruits/vegetables). “B” and “Fruit/Vegetables” are “goal” conditions, in which participants have a goal of speaking 20% more words than “S” and “Animals,” respectively, which increases language generation.Citation67 Motor Go-No-Go is based on,Citation69 tapping task and requires participants to copy finger tapping sequences performed by the assessor (e.g. assessor taps once, participant taps once), and then reverse tapping rules (e.g. assessor taps once, participant taps twice). Sentence Completion (SC) was developed based on the Hayling Sentence Completion Test,Citation63 and requires participants to complete ten sentences read aloud by the assessor by producing one word, under two conditions: Initiation (the sentence must be meaningful, e.g. “The lecture should last about one … ” hour) and Inhibition (the sentence must be non-meaningful, e.g. “The lecture should last about one … ” plant). Low Constraint Initiation items increase selection demands due to multiple competing responses becoming available, compared to High Constraint items which have a dominant response.Citation13,Citation37,Citation43
Memory
Incidental Verbal Memory involves participants recalling the ten items from Object Naming, Word Repetition and Word Comprehension subtests.
BELS scoring
A total BELS score out of 100 can be calculated, as well as a total score for each subsection. See Supplementary Materials for BELS score sheets, instructions, and scoring manual (including how to calculate a total score).
Statistical analyses
Using IBM SPSS Statistics 27,Citation70 an alpha level of.05 was set for all analyses unless otherwise stated for Bonferroni corrections for multiple comparisons. Differences between stroke and control groups were investigated via independent samples t-tests (and Mann-Whitney U tests for non-parametric data). ROC curve analysis was performed to determine sensitivity and specificity of a total BELS score. To ensure aphasic patients were not driving significant results, inferential statistics and ROC analyses were conducted with and without aphasic patients. ROC curve analyses were also conducted with RHS and LHS separated to determine most appropriate cutoffs.
Results
Descriptive statistics for baseline neuropsychology and BELS subtests are presented in . Patients and controls were matched in age (U = 4927, p =.672). In total, 15 stroke patients (17%; n = 7 LHS, 7 RHS, and 1 bilateral patient) showed signs of aphasia on independent reading (n = 3; 1 LHS, 1 RHS, 1 Bilateral), naming (n = 11; 6 LHS, 5 RHS), and comprehension (n = 2; 1 LHS, 1 RHS) measures. One patient was impaired on both reading and comprehension.
Table 1. Descriptive Statistics for BELS Subtests: Controls and Stroke (LHS & RHS).
The percentage of patients impaired (i.e. performance <5th percentile) on BELS subsections (and proportion of LHS and RHS impaired) were similar when aphasic patients were included and excluded (see ). The largest change was a 5% decrease in impaired patients for Nominal Language, Propositional Language and Executive Functions, and Memory subsections. LHS and RHS were relatively equally impaired (except for Oral Apraxia and Memory, where more RHS were impaired).
Table 2. Percentage of patients (and Proportion of LHS: RHS) Impaired on BELS Subsections.
Inferential statistics comparing patients and controls were conducted with and without aphasic patients (see ). Stroke and control groups did not significantly differ on Word Comprehension, Word Repetition, or SC Initiation number correct (after correction for multiple comparisons; see ). On all other BELS subtests, controls performed significantly better than stroke patients. When aphasic patients were removed from comparisons, the only changes to results were Sentence Repetition and Motor Go No-GO, which became non-significant after correcting for multiple comparisons (U = 3818.50, p =.007; and U = 3732.50, p =.012, respectively). Additionally, to ensure BELS Oromotor Function and Nominal Language impairments were not driving significantly poorer total BELS scores, a second t-test was conducted with controls (N = 105) and patients who were not impaired on these subsections (N = 49). Stroke patients’ total BELS scores remained significantly lower than controls, t(56.89) = 9.87, p <.001, d = 2.20 (equal variances not assumed). The percentage of patients impaired on overall BELS scores also remained high, at 73%.
Table 3. Inferential statistics for BELS subtests: Comparison of controls and stroke groups.
Six ROC curve analyses were conducted (see ). With all stroke and control participants included, AUC was.94, p <.001, 95%CI[.91.98] (see ). A cutoff score of 79.25/100 on the BELS had.89 sensitivity and.89 specificity. The likelihood ratio indicated a score below 79.25 was 8.1 times more likely to be that of a stroke patient than a control. With aphasic patients removed, specificity did not change, and sensitivity reduced slightly (by.02). Sensitivity and specificity were higher for RHS without aphasia (.90 and.89) compared to LHS without aphasia (.83 and.81).
Table 4. ROC curve statistics and cutoffs for BELS total score.
Discussion
Early intervention and rehabilitation of language post-stroke is reliant on administration of sensitive cognitive assessment tools during the early stages of stroke. Screening tools used in stroke are typically either language screens designed to detect aphasia, or non-verbal cognitive screens designed for patients with aphasia (which is undoubtably crucial). However, patients without aphasia may still have subtle propositional language impairments that go undetected on language screens, and current widely used cognitive screens only minimally assess the executive functions that underpin the pre-verbal message-generation of connected speech. Following stroke, propositional language deficits and executive dysfunction impacts interpersonal relationships and quality of life.Citation8,Citation9,Citation26 The BELS is a recently developed, valid screening tool which – in addition to articulation and memory – measures two cognitive domains (language and executive functions) that predict long-term neuropsychological, functional, and emotional outcomes post-stroke.Citation5,Citation8,Citation10–13 We aimed to investigate how acute to early sub-acute stroke patients performed on BELS subtests relative to controls, and to determine sensitivity and specificity of a total BELS cutoff score.
Patients (whole group) and controls did not differ on Word Comprehension or Word Repetition subtests, or on total correct SC Initiation items (after Bonferroni corrections); however, patients performed significantly worse on all other BELS subtests. Impairment on individual subtests ranged from 1% to 52%. Despite largely intact articulation and core nominal language skills, most patients (73%) were impaired on the Propositional Language and Executive Function subscale. With aphasic patients removed from analyses, results remained largely unchanged – the exceptions being Sentence Repetition and Motor Go No-Go becoming non-significantly different between groups (after Bonferroni corrections). Percentages of patients impaired on BELS subsections (and proportions of LHS and RHS groups impaired) remained similar. This aligns with prior evidence that stroke patients without aphasia can experience language impairment at the executive level [i.e. initiation, selection, inhibition, strategy.Citation9,Citation37,Citation43 For the SC Initiation subtest, stroke patients demonstrated greater difficulty than controls with selection of multiple competing responses (SC Initiation Low Constraint), compared to selection of a single dominant response (SC Initiation High Constraint). However, this became non-significant after correcting for multiple comparisons. Notably, controls were significantly faster than patients to initiate responses on both high and low constraint items, highlighting difficulties with both selection, and speed at which speech is initiated in acute to early sub-acute stroke. Deficits to idea selection have also been linked to reduced spontaneous speech in dynamic aphasia patients, meaning assessment of these executive processes are necessary to aid in detection and rehabilitation of connected speech.Citation13,Citation37,Citation43,Citation46,Citation71 Deficits to selection and initiation of speech can be subtle, and although they may be detected in more extensive testing by speech and language therapists, there are currently no cognitive screening tools (to our knowledge) that capture these processes in an acute stroke setting. Importantly, these deficits can arise in the absence of aphasia which means these patients are not referred to speech and language therapists. For instance, one study found that only 10% of acute stroke patients with cognitive communication disorder were referred for community-based rehabilitation when discharged home, compared to 53% of acute stroke patients with aphasia.Citation72
The BELS was highly sensitive (.89) and specific (.89) when classifying stroke patients and healthy controls, particularly compared to the MoCA which is widely used [sensitivity.81, specificity.70.Citation73 When excluding patients with aphasia, specificity remained the same, and sensitivity remained high at.87. Sensitivity and specificity were higher for RHS (without aphasia) and controls (.90 and.89, respectively), compared to LHS (without aphasia) and controls (.83 and.81, respectively), which aligns with research indicating right hemisphere lesions are more susceptible to executive impairments.Citation44,Citation45,Citation74–78 However, we acknowledge the smaller sample of LHS compared to RHS patients, and that with more patients, sensitivity and specificity may increase. For clear first-time lateralized stroke patients, we recommend the LHS and RHS without aphasia cutoffs, and the whole-group cutoff for bilateral or 2nd time stroke patients, or patients with preexisting neurological conditions (e.g. dementia).
The BELS can identify articulation and nominal language impairments, in addition to subtle impairments to executive processes underlying more complex language, making it suitable for a wide range of stroke patients (i.e. non-aphasic patientsCitation13). This is crucial due to subtle language impairments going undetected on other cognitive and non-verbal stroke screens. The BELS therefore compliments tools like the OCS,Citation16 OCS-Plus,Citation17 QABCitation18,Citation19 and CASP,Citation14 by providing a more complete understanding of patients’ propositional language and executive functioning. Consequently, speech and language therapists can intervene early in the rehabilitation process to ensure best possible outcomes and recovery.
Social communication is crucial for interpersonal relationships and the ability to perform social or occupational roles.Citation72,Citation79–81 Reduction or impairment of communication post-stroke can impact reintegration into these roles, leading to social isolation and associated negative impacts on general health and wellbeing.Citation79,Citation82–87 While the impact of post-stroke aphasia is well-established, the impact of subtle, executive-level communication deficits (e.g. Cognitive Communication Disorder) on wellbeing and quality of life are less-researched, potentially due to a paucity of tools that screen or assess more complex language production in an acute stroke setting.Citation72,Citation88,Citation89
The BELS is a valid and valuable bedside screening tool that is sensitive to language and executive impairments experienced by stroke patients in the acute to early sub-acute phase, even in the absence of significant aphasia. A limitation to the current study was that if any BELS subtests were not completed, a total score could not be calculated. This meant 15 stroke patients and 11 controls were excluded from the ROC curve analysis. However, total scores for each BELS subsection can still be calculated, and each subsection (except Memory) is standalone, meaning only the section of interest may be administered. It would therefore be beneficial for future studies to determine a cutoff for each BELS subsection. Due to non-parametric data, we were unable to use ANCOVAs to control for Articulation and Nominal Language scores when examining Propositional Language and Executive Functions, and total BELS scores. However, when patients impaired on Articulation and Nominal Language were excluded, the percentage of patients impaired on Propositional Language and Executive Functions and total BELS scores remained high (~75%), and group comparisons remained significant. Similar results were found when patients indicating aphasia on independent measures were removed. Finally, LHS patients may be underrepresented in our sample, likely because they would be expected to have more core language deficits (e.g. non-fluent aphasia) that impact skills required for consent processes (e.g. comprehension).
Future directions include investigation into whether the BELS (particularly the Propositional Language and Executive Function subsection) is sensitive to left versus right hemisphere stroke impairments, and whether BELS scores can predict long-term neuropsychological and functional outcomes. This will better equip rehabilitation teams to identify language and executive impairments, tailor appropriate interventions, and predict and track improvements post-stroke.Citation90
Disclosure of Interest
The authors report there are no competing interests to declare.
Supplementary Materials_BELS Stimuli_Scoring_Manual.docx
Download MS Word (2.2 MB)Acknowledgments
We wish to thank all participants for their time, especially stroke patients. We also thank Amelia Ceslis, Amelia Hobson, and Katie Veretennikoff for assisting with controls and data collation. This study was supported by the Brazil Family Program for Neurology and an Australian National Health and Medical Research Council Boosting Dementia Research Leadership Fellowship (APP1135769) awarded to GAR.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10749357.2024.2356412
Additional information
Funding
References
- Barker-Collo S, Feigin V. The impact of neuropsychological deficits on functional stroke outcomes. Neuropsychological Review. 2006;16(2):53–64. doi:10.1007/s11065-006-9007-5.
- Hackett ML, Anderson CS. Health outcomes 1 year after subarachnoid hemorrhage: an international population-based study. Neurology. 2000;55(5):658–662. doi:10.1212/WNL.55.5.658.
- Hommel M, Miguel ST, Naegele B, Gonnet N, Jaillard A. Cognitive determinants of social functioning after a first ever mild to moderate stroke at vocational age. J Neurol Neurosurg Psychiatry Res. 2009;80(8):876–880. doi:10.1136/jnnp.2008.169672.
- Hochstenbach JB, Anderson PG, van Limbeek J, Mulder TT. Is there a relation between neuropsychologic variables and quality of life after stroke?. Arch Phys Med Rehabil. 2001;82(10):1360–1366.
- Nys GMS, van Zandvoort MJE, de Kort PLM, Jansen BP, De Haan EH, Kappelle L. Cognitive disorders in acute stroke: prevalence and clinical determinants. Cerebrovascular Dis. 2007;23(5–6):408–416. doi:10.1159/000101464.
- Wagle J, Farner L, Flekkøy K, et al. Early post-stroke cognition in stroke rehabilitation patients predicts functional outcome at 13 months. Dement Geriatr Cogn Disord. 2011;31(5):379–387. doi:10.1159/000328970.
- Warlow CP. Epidemiology of stroke. Lancet. 1998;352:S1–S4. doi:10.1016/S0140-6736(98)90086-1.
- Chahal N, Barker-Collo S, Feigin V. Cognitive and functional outcomes of 5- year subarachnoid haemorrhage survivors: comparison to matched healthy controls. Neuroepidemiology. 2011;37(1):31–38. doi:10.1159/000328647.
- Law B, Young B, Pinsker D, Robinson A. Propositional speech in unselected stroke: the effect of genre and external support. Neuropsychological Rehabilitation: An International Journal. 2015;25(3):374–401. doi:10.1080/09602011.2014.937443.
- Lesniak M, Bak T, Czepiel W, Seniow J, Czlonkowska A. Frequency and prognostic value of cognitive disorders in stroke patients. Dement Geriatr Cogn Disord. 2008;26(4):356–363. doi:10.1159/000162262.
- Nys GMS, van Zandvoort MJE, de Kort PLM, et al. The prognostic value of domain-specific cognitive abilities in acute first-ever stroke. Neurology. 2005a;64(5):821–827. doi:10.1212/01.WNL.0000152984.28420.5A.
- Robertson IH, Ridgeway V, Greenfield E, Parr A. Motor recovery after stroke depends on intact sustained attention: a 2-year follow-up study. Neuropsychology. 1997;11(2):290–295. doi:10.1037/0894-4105.11.2.290.
- Robinson G, Shi L, Nott Z, Ceslis A. A brief executive language screen for frontal aphasia. Brain Sci. 2021a;11(3):1–15. doi:10.3390/brainsci11030353.
- Benaim C, Wauquiez G, Pérennou D, et al. Cognitive assessment scale for stroke patients (CASP): a multicentric validation study. Ann Phys Rehabil Med. 2022;65(3):101594.doi: 10.1016/j.rehab.2021.101594.
- Helm-Estabrooks N. Cognitive Linguistic Quick Test: CLQT. San Antonio, TX: Psychological Corporation; 2001.
- Demeyere N, Riddoch MJ, Slavkova ED, Bickerton WL, Humphreys GW. The Oxford cognitive screen (OCS): validation of a stroke-specific short cognitive screening tool. Psychol Assess. 2015;27(3):883. doi:10.1037/pas0000082.
- Demeyere N, Haupt M, Webb SS, et al. Introducing the tablet-based Oxford cognitive screen-plus (OCS-Plus) as an assessment tool for subtle cognitive impairments. Sci Rep. 2021;11(1):8000. doi:10.1038/s41598-021-87287-8.
- Wilson SM, Eriksson DK, Schneck SM, Lucanie JM. Correction: a quick aphasia battery for efficient, reliable, and multidimensional assessment of language function. PloS One. 2018;13(6):e0199469–e0199469. doi:10.1371/journal.pone.0199469.
- Wilson SM, Entrup JL, Schneck SM, et al. Recovery from aphasia in the first year after stroke. Brain. 2023;146(3):1021–1039. doi:10.1093/brain/awac129.
- Kertesz A. Western aphasia battery–revised. 2007.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x.
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi:10.1016/0022-3956(75)90026-6.
- Chan E, Khan S, Oliver R, Gill SK, Werring DJ, Cipolotti L. Underestimation of cognitive impairments by the Montreal Cognitive Assessment (MoCA) in an acute stroke unit population. J Neurol Sci. 2014;343(1–2):176–179. doi:10.1016/j.jns.2014.05.005.
- Chan E, Altendorff S, Healy C, Werring DJ, Cipolotti L. The test accuracy of the Montreal Cognitive Assessment (MoCA) by stroke lateralisation. J Neurol Sci. 2017;373:100–104. doi:10.1016/j.jns.2016.12.028.
- Blackburn DJ, Bafadhel L, Randall M, Harkness KA. Cognitive screening in the acute stroke setting. Age Ageing. 2013;42(1):113–116.doi:10.1093/ageing/afs116.
- Pohjasvaara T, Leskelä M, Vataja R, et al. Post‐stroke depression, executive dysfunction and functional outcome. Eur J Neurol. 2002;9(3):269–275. doi:10.1046/j.1468-1331.2002.00396.x.
- Barker MS, Nelson NL, Robinson G. Idea formulation for spoken language: the interface of cognition and language. J Int Neuropsychol Soc. 2020;26(2):226–240. doi:10.1017/S1355617719001097.
- Levelt WJM. Speaking from Intention to Articulation. Cambridge, MA: MIT Press; 1989. doi:10.7551/mitpress/6393.001.0001.
- Levelt WJM. Producing spoken language: a blueprint of the speaker. In: Brown CM, Hargoort P, eds. The Neurocognition of Language. Oxford, UK: Oxford University Press; 1999: 83–122. doi:10.1093/acprof:oso/9780198507932.003.0004.
- Sherratt S. Multi-level discourse analysis: a feasible approach. Aphasiology. 2007;21(3– 4):375–393. doi:10.1080/02687030600911435.
- Barker MS, Young B, Robinson GA. Cohesive and coherent connected speech deficits in mild stroke. Brain Lang. 2017;168:23–36. doi:10.1016/j.bandl.2017.01.004.
- Alexander MP. Impairments of procedures for implementing complex language are due to disruption of frontal attention processes. J Int Neuropsychol Soc. 2006;12(2):236–247. doi:10.1017/S1355617706060309.
- Barker MS, Ceslis A, Robinson GA. Idea selection for propositional language production. Aging, Neuropsychology, and Cognition. 2022;29(2):260–283. doi:10.1080/13825585.2020.1862044.
- Robinson G, Shallice T, Cipolotti L. Dynamic aphasia in progressive supranuclear palsy: a deficit in generating a fluent sequence of novel thought. Neuropsychologia. 2006;44(8):1344–1360. doi:10.1016/j.neuropsychologia.2006.01.002.
- Robinson GA, Spooner D, Harrison WJ. Frontal dynamic aphasia in progressive supranuclear palsy: distinguishing between generation and fluent sequencing of novel thoughts. Neuropsychologia. 2015;77:62–75. doi:10.1016/j.neuropsychologia.2015.08.001.
- Barker MS, Robinson GA. Selection for sentence generation in the context of severe anomia: a case series of left temporal patients. J Clin Exp Neuropsychol. 2019;41(4):353–363. doi:10.1080/13803395.2018.1562050.
- Robinson G, Blair J, Cipolotti L. Dynamic aphasia: an inability to select between competing verbal responses. Brain. 1998;121(1):77–89. doi:10.1093/brain/121.1.7.
- Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philosophical Transactions of the Royal Society of London. Series B. Biological Sciences. 2007;362(1481):901–915. doi:10.1098/rstb.2007.2096.
- Burgess PW, Shallice T Response suppression, initiation and strategy use following frontal lobe lesions. Neuropsychologia. 1996;34(4):263–272.
- Madden DL, Sale MV, Robinson GA. Age-related differences in idea generation and selection for propositional language. Aging, Neuropsychology, and Cognition. 2019;26(4):486–506. doi:10.1080/13825585.2018.1476668.
- Robinson GA. Primary progressive dynamic aphasia and Parkinsonism: generation, selection and sequencing deficits. Neuropsychologia. 2013;51(13):2534–2547. doi:10.1016/j.neuropsychologia.2013.09.038.
- Robinson G, Shallice T, Bozzali M, Cipolotti L. Conceptual proposition selection and the LIFG: neuropsychological evidence from a focal frontal group. Neuropsychologia. 2010;48(6):1652–1663. doi:10.1016/j.neuropsychologia.2010.02.010.
- Robinson G, Shallice T, Cipolotti L. A failure of high level verbal response selection in progressive dynamic aphasia. Cogn Neuropsychol. 2005;22(2005):661–694. doi:10.1080/02643290442000239.
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8(4):170–177. doi:10.1016/j.tics.2004.02.010.
- Robinson GA, Cipolotti L, Walker DG, Biggs V, Bozzali M, Shallice T. Verbal suppression and strategy use: a role for the right lateral prefrontal cortex? Brain. 2015b;138(4):1084–1096. doi:10.1093/brain/awv003.
- Crescentini C, Lunardelli A, Mussoni A, Zadini A, Shallice T. A left basal ganglia case of dynamic aphasia or impairment of extra-language cognitive processes? Neurocase. 2008;14(2):184–203. doi:10.1080/13554790802108380.
- Gold M, Nadeau SE, Jacobs DH, Adair JC, Rothi LJG, Heilman KM. Adynamic aphasia: a transcortical motor aphasia with defective semantic strategy formation. Brain Lang. 1997;57(3):374–393. doi:10.1006/brln.1997.1750.
- Luria AR. The Working Brain. London, UK: Penguin; 1973.
- Warren JD, Warren JE, Fox NC, Warrington EK. Nothing to say, something to sing: primary progressive dynamic aphasia. Neurocase. 2003;9(2):140–155.doi: 10.1076/neur.9.2.140.15068.
- Blake ML, Duffy JR, Myers PS, Tompkins CA. Prevalence and patterns of right hemisphere cognitive/communicative deficits: retrospective data from an inpatient rehabilitation unit. Aphasiology. 2002;16(4–6):537–547. doi:10.1080/02687030244000194.
- Chiti G, Pantoni L. Use of montreal cognitive assessment in patients with stroke. Stroke. 2014;45(10):3135–3140.
- Dong Y, Sharma VK, Chan BP-L, et al. The Montreal Cognitive Assessment (MoCA) is superior to the Mini-Mental State Examination (MMSE) for the detection of vascular cognitive impairment after acute stroke. J Neurol Sci. 2010;299(1):15–18. doi:10.1016/j.jns.2010.08.051.
- Mancuso M, Demeyere N, Abbruzzese L, et al. Using the Oxford cognitive screen to detect cognitive impairment in stroke patients: a comparison with the mini-mental state examination. Front Neurol. 2018;9:101. doi:10.3389/fneur.2018.00101.
- Nys GMS, van Zandvoort MJE, de Kort PLM, Jansen BPW, Kappelle LJ, de Haan EHF. Restrictions of the mini-mental state examination in acute stroke. Arch Clin Neuropsychol. 2005b;20(5):623–629. doi:10.1016/j.acn.2005.04.001.
- Salvadori E, Pasi M, Poggesi A, Chiti G, Inzitari D, Pantoni L. Predictive value of MoCA in the acute phase of stroke on the diagnosis of mid-term cognitive impairment. J Neurol. 2013;260(9):2220–2227. doi:10.1007/s00415-013-6962-7.
- Shen Y-J, Wang W-A, Huang F-D, et al. The use of MMSE and MoCA in patients with acute ischemic stroke in clinical. International Journal of Neuroscience. 2016;126(5):442–447. doi:10.3109/00207454.2015.1031749.
- Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the Stroke Recovery and Rehabilitation Roundtable taskforce. Int J Stroke. 2017;12(5):444–450. doi:10.1177/1747493017711816.
- Nelson HE, Willison J. National Adult Reading Test (NART (Pp. 1–26). Windsor: Nfer- Nelson; 1991.
- Bright P, Hale E, Gooch VJ, Myhill T, Van der linde I. The national adult reading test: restandardisation against the Wechsler adult intelligence scale-fourth edition. Neuropsychol Rehabil. 2018;28(6):1019–1027. doi:10.1080/09602011.2016.1231121.
- Mack WJ, Freed DM, Williams BW, Henderson VW. Boston Naming Test: shortened versions for use in Alzheimer’s disease. Journal of Gerontology (Kirkwood). 1992;47(3):154–158. doi:10.1093/geronj/47.3.P154.
- Abeare K, Cutler L, An KY, Razvi P, Holcomb M, Erdodi LA. BNT-15: revised performance validity cutoffs and proposed clinical classification ranges. Cognitive and Behavioral Neurology. 2022;35(3):155–168. doi:10.1097/WNN.0000000000000304.
- Fastenau PS, Denburg NL, Mauer BA. Parallel short forms for the Boston Naming Test: psychometric properties and norms for older adults. J Clin Exp Neuropsychol. 1998;20(6):828–834. doi:10.1076/jcen.20.6.828.1105.
- Burgess P, Shallice T. The Hayling and Brixton Tests: Test Manual. London, UK: Thames Valley Test Company; 1997.
- Warrington EK, James M. The Visual Object and Space Perception Battery. Bury St Edmunds, England: Thames Valley Test Company; 1991.
- Druks J, Masterson J. Object & Action Naming Battery. Hove, U.K: Psychology Press; 2000.
- Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination Booklet. Lea & Febiger; 1983.
- Robinson GA, Campbell L, Ceslis A. A goal intervention improves language fluency: evidence from Parkinson’s disease and healthy aging. Medicines. 2021b;8(3):15. doi:10.3390/medicines8030015.
- Strauss E, Sherman EM, Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. American Chemical Society. 2006.
- Luria AR. Higher Cortical Functions in Man. New York: Basic Books; 1966.
- IBM Corp. Released. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp; 2020.
- Costello A, Warrington EK. Dynamic aphasia: the selective impairment of verbal planning. Cortex. 1989;25(1):103–114. doi:10.1016/S0010-9452(89)80010-3.
- Heweston R, Cornwell P, Shum D. Cognitive-communication disorder following right hemisphere stroke: exploring rehabilitation access and outcomes. Top Stroke Rehabil. 2017;24(5):330–336. doi:10.1080/10749357.2017.1289622.
- Potocnik J, Ovcar Stante K, Rakusa M. The validity of the Montreal cognitive assessment (MoCA) for the screening of vascular cognitive impairment after ischemic stroke. Acta Neurol Belg. 2020;120(3):681–685. doi:10.1007/s13760-020-01330-5.
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 2014;18(4):177–185. doi:10.1016/j.tics.2013.12.003.
- Kawashima R, Satoh K, Itoh H, et al. Functional anatomy of GO/NO-GO discrimination and response selection — a PET study in man. Brain Res. 1996;728(1):79–89. doi:10.1016/0006-8993(96)00389-7.
- Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y. No-go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. Eur J Neurosci. 1998;10(3):1209–1213. doi:10.1046/j.1460-9568.1998.00167.x.
- Volle E, De Lacy Costello A, Coates LM, et al. Dissociation between verbal response initiation and suppression after prefrontal lesions. Cereb Cortex. 2012;22(10):2428–2440. doi:10.1093/cercor/bhr322.
- Wilson BA, Evans JJ, Emslie H, Alderman N, Burgess P. The development of an ecologically valid test for assessing patients with a dysexecutive syndrome. Neuropsychol Rehabil. 1998;8(3):213–228. doi:10.1080/713755570.
- Abusamra V, Côté H, Joanette Y, Ferreres A. Communication impairments in patients with right hemisphere damage. Life Span and Disability. 2009;12:67–82.
- Brady MC, Clark AM, Dickson S, Paton G, Barbour RS. The impact of stroke- related dysarthria on social participation and implications for rehabilitation. Disabil Rehabil. 2011;33(3):178–186. doi:10.3109/09638288.2010.517897.
- Cruice M, Worrall L, Hickson L. Quantifying aphasic people’s social lives in the context of non-aphasic peers. Aphasiology. 2006;20(12):1210–1225. doi:10.1080/02687030600790136.
- Christiansen J, Qualter P, Friis K, et al. Associations of loneliness and social isolation with physical and mental health among adolescents and young adults. Perspect Public Health. 2021;141(4):226–236. doi:10.1177/17579139211016077.
- Evans M, Fisher EB. Social isolation and mental health: the role of nondirective and directive social support. Community Ment Health J. 2022;58(1):20–40. doi:10.1007/s10597-021-00787-9.
- Holt-Lunstad J, Steptoe A. Social isolation: an underappreciated determinant of physical health. Curr Opin Psychol. 2022;43:232–237. doi:10.1016/j.copsyc.2021.07.012.
- Mukherjee D, Levin RL, Heller W. The cognitive, emotional, and social sequelae of stroke: psychological and ethical concerns in post-stroke adaptation. Top Stroke Rehabil. 2006;13(4):26–35. doi:10.1310/tsr1304-26.
- Rohde D, Gaynor E, Large M, et al. The impact of Cognitive Impairment On Poststroke Outcomes: a 5-year follow-up. J Geriatr Psychiatry Neurol. 2019;32(5):275–281. doi:10.1177/0891988719853044.
- Smith KJ, Victor C. Typologies of loneliness, living alone and social isolation, and their associations with physical and mental health. Ageing and Society. 2019;39(8):1709–1730. doi:10.1017/S0144686X18000132.
- Blake ML. Right Hemisphere Strokes. Perspectives of the ASHA Special Interest. Groups. 2016;1(2):63–65. doi:10.1044/persp1.SIG2.63.
- Le K, Mozeiko J, Coelho C. Discourse analyses: characterizing cognitive- communication disorders following TBI. The ASHA Leader. 2011;16(2):18–21. doi:10.1044/leader.FTR4.16022011.18.
- Robinson GA, Butterworth B, Cipolotti L. “my mind is doing it all”: no “brake” to stop speech generation in Jargon Aphasia. Cognitive and Behavioral Neurology. 2015c;28(4):229–241. doi:10.1097/WNN.0000000000000080.