ABSTRACT
Lipophilic persistent organic pollutants (POP) are stored in adipose tissue. Following rapid weight loss such as when induced by bariatric surgery, an increased release of potential harmful lipophilic compounds into the blood circulation may occur. Weight reduction is recommended for overweight and obese individuals in order to decrease risk of weight-related health problems. However, in cases of significant weight reduction POP become mobilized chemicals and consequently may adversely affect health, including endocrine disruption. The objective of the present investigation was to estimate quantitatively the level of mobilization of POP following weight loss over time. According to literature search criteria, 17 studies were identified with 2061 participants. Data from 5 of the studies with 270 participants were used to assess the change in blood levels of POP in percent per kilogram weight loss. Weight loss in the included studies varied from 4.4 to 64.8 kg. In all studies, the majority of POP concentrations in blood were found to rise following weight reduction. Blood concentrations following weight reduction were elevated by 2–4% per kilogram weight loss for most POP examined. The increased POP levels were still elevated 12 mo after intervention. Most research in this field, including animal studies, is carried out on a single compound or group of selected compounds, not taking the “cocktail effect” into consideration. This does not reflect the true range of POP to which humans are actually exposed. Few chronic investigations have been published and, in particular, few studies were available that compared the increase in POP concentrations with clinical consequences as individuals lost weight. These limitations call for caution in interpreting results. The benefits of losing weight still far outweigh the potential adverse health risks. However, further studies are recommended to determine the clinical significance of increased blood levels of POPs following rapid and excessive weight loss, particularly for women attending weight reduction treatment before pregnancy.
Global age-standardized obesity prevalence increased from 6.4 to 12% between 1980 and 2008 (Stevens et al. Citation2012). Further, the prevalence of obesity is still rising globally. By the end of 2020, the incidence of being overweight and obese in the United States and England is estimated to be 74 and 69%, respectively (Wang et al. Citation2011). The most common explanation for this rise is high energy intake combined with lack of physical exercise. Environmental factors, diet quality, fetal environment, stress, and exposure to pharmaceuticals or other chemicals may also be contributing factors (Decherf and Demeneix Citation2011; Grun and Blumberg Citation2009). High obesity rates are linked to the prevalence of cardiovascular disease, metabolic syndrome, type 2 diabetes, and nonalcoholic fatty liver disease (NAFLD) (Cooke et al. Citation2016). Thus, weight reduction is recommended for overweight and obese individuals in order to lower the risk of weight-related adverse health problems.
Persistent organic pollutants (POP) are organic chemical substances and environmental pollutants that accumulate in adipose tissue, liver, brain, and pancreatic tissue and biomagnify in food chains, as well as being resistant to natural degradation processes (Byrne et al. Citation2015; Elabbas et al. Citation2014; Pestana et al. Citation2014; Porta Citation2006; Schafer and Kegley Citation2002). Various POP with documented endocrine-disrupting properties have, in the last 10 years, been suggested as potential contributors to the growing obesity pandemic (Decherf and Demeneix Citation2011; Dirinck et al. Citation2011; Grun and Blumberg Citation2009; Hatch et al. Citation2010; McAllister et al. Citation2009; Newbold et al. Citation2008). POP may bind to different nuclear receptors, displacing endogenous ligands and thereby interacting with signaling pathways and hormonal systems such as the central hypothalamic–pituitary–adrenal axis, the hypothalamic–pituitary–gonadal axis, and the endocrine pancreas (Asp et al. Citation2010; Decherf and Demeneix Citation2011; De Tata Citation2014; Faerch et al. Citation2012; Rylander et al. Citation2006).
Exposure to POP has been associated with diabetes, metabolic syndrome, alterations of thyroid functions, a number of types of cancers, quality of life, infertility, and other neurological, hormonal, and immunological disorders (Casals-Casas and Desvergne Citation2011; Esser et al. Citation2015; Ferrante et al. Citation2014; Grun and Blumberg Citation2009; Hoyer et al. Citation2000; La Merrill et al. Citation2013; Mouritsen et al. Citation2010; Pelletier, Imbeault, and Tremblay Citation2003; Porta Citation2006; Turyk et al. Citation2009). However, many questions on the potential adverse health effects of exposure to environmental pollutants remain unanswered (Henkler and Luch Citation2011; Lee, Jacobs, and Porta Citation2009; Porta et al. Citation2012).
Because of the lipophilic properties of POP, accumulation appears to occur in lipid droplets in adipose tissue and in lipids in biological membranes (Bourez et al. Citation2012). Weight loss increases the concentrations of circulating POP levels since these compounds are released during lipid mobilization, whereas weight gain tends to dilute circulating levels (Vizcaino et al. Citation2014b). The combination of low-calorie diets with enhanced physical activity is the mainstay in obesity treatment (Livingston and Zylke Citation2012). An elevated number of morbidly obese individuals are treated with bariatric surgery. In 2013, in total 468,609 such operations were performed worldwide (Angrisani et al. Citation2015). Bariatric/metabolic surgery includes different surgical procedures involving or combining restrictive and malabsorptive mechanisms. This leads to weight loss (Aasheim et al. Citation2009) and hormonal changes with improvement in metabolic comorbidities (Catoi et al. Citation2015; Poirier et al. Citation2011; Rubino Citation2013).
The aim of this review was to (1) examine the changes in POP concentrations in blood of individuals who had lost weight either by dieting or surgery or for other reasons, and (2) quantify these alterations over time.
Approach
The Medline, Embase, PubMed, and Web of Science databases were searched for scientific publications that reported blood concentrations of POP before and after weight loss. Searches were carried out independently, and included studies published up to April 7, 2016. The Medical Subject Heading (MeSH) terms “weight loss” and “weight reduction” were combined using the Boolean operator “OR,” and this string was combined with the entered MeSH terms “persistent organic pollutants,” “endocrine disrupting chemicals,” “polychlorinated biphenyl,” “polybrominated diphenylether,” “perfluorooctanesulfonic acid,” ” perfluorooctanoic acid,” “PFAS” (perfluoroalkyl sulfonate), and “brominated flame retardant,” using the Boolean operator “AND.” All studies that describe blood concentrations before and after weight loss were included, irrespective of cause and magnitude of the weight loss. The search included investigations of humans, of both genders, that were published in English. The reference lists from all included investigations were also examined to identify articles not found by the database search. The searches were assessed and investigations included in the review were independently and carefully read to uncover any potential biases.
Literature Selection Strategy
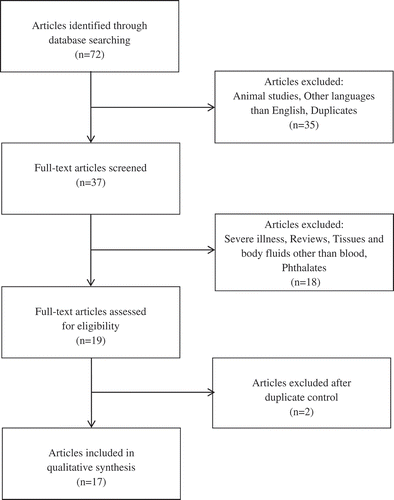
The literature selection strategy is illustrated in (Booth Citation2006; Moher et al. Citation2010). Animal studies, articles in languages other than English, and duplicates were excluded in the first selection. In the second selection, studies of patients with severe illnesses, review articles, and studies that examined tissues and body fluids other than blood were excluded. Investigations reporting on phthalates and of metabolites of phthalates measured in urine were also excluded since their toxicokinetics differ from the kinetics of POP (Lyche et al. Citation2009). In the third selection two studies were identified reporting on the same data (Pelletier et al. Citation2002; Tremblay et al. Citation2004), with only the earlier of the two being included in our review. Another study group also reported on the same data but with different time aspects and number of participants (Dirinck et al. Citation2015, Citation2016). The study with the longest time aspect and the highest number of participants was included (Dirinck et al. Citation2016). Of the remaining 17 studies, 12 were not used for quantitative analysis. These investigations had either less than 16 participants or lacked sufficient data for comparison. However, these were included in the results and discussion. In 5 of the 17 studies, it was possible to use the published data to quantify the increase in blood concentration of POP following weight reduction. These five selected investigations were comparable in terms of number of participants, duration, and methodological quality. Three of five studies reported on lipid-corrected concentrations and two of five on wet weight concentrations. This difference was not expected to significantly influence the presentation of calculated results. The results are calculated as a percent change of POP concentration per kilogram weight loss in each individual study before comparing the averages of all included studies.
Identified Studies
In total, 17 studies were identified according to the literature selection strategy (), encompassing a total of 2061 participants living in France, Belgium, the Czech Republic, Sweden, Finland, Canada, and the United States. summarizes these investigations. Study designs varied in terms of use of control groups, placebos, lipid-corrected/uncorrected concentrations, and division into groups based on criteria. Weight losses were specified in kilograms, pounds, body mass index (BMI) units, or percent weight loss. The highest weight losses over time were achieved by surgery. Males lost on average more weight than females. Two of the studies (73 participants) did not specify gender (Kim et al. Citation2011b; Walford et al. Citation1999). The gender ratio for the remaining 1988 participants was 40% men and 60% women. Weight loss in all studies varied from 4.4 to 64.8 kg. Seven of the 17 studies assessed weight loss over a relative short period of time (3–6 mo) (Arguin et al. 2010; Charlier, Desaive, and Plomteux Citation2002; Chevrier et al. Citation2000; De Roos et al. Citation2012; Imbeault et al. Citation2001; Mullerova et al. Citation2008; Pelletier et al. Citation2002). Ten of the 17 studies assessed weight loss over a period of 1 yr or longer (Backman and Kolmodin-Hedman Citation1978; Dirinck et al. Citation2016; Dirtu et al. Citation2013; Hue et al. Citation2006; Kim et al. Citation2011b; Lim et al. 2011; Mullerova et al. Citation2015; Rantakokko et al. Citation2015; Schildkraut et al. Citation1999; Walford et al. Citation1999); two of these were retrospective studies with self-reported weight changes (Lim et al. 2011; Schildkraut et al. Citation1999).
Table 1. Reported data from the 17 identified studies.
General Observations in the 17 Studies
Blood concentrations of most POP, including organochlorine pesticides (OCP), polychlorinated biphenyls (PCB), and polybrominated diphenyl ethers (PBDE), increased after weight loss. However, no marked changes in the concentration of perfluoroalkyl sulfonates (PFAS) were observed in the single study that analyzed this group of chemicals before and after weight loss. Two studies (Dirtu et al. Citation2013; Rantakokko et al. Citation2015) noted brominated diphenyl ether (BDE) concentrations after weight loss. The results reported by Rantakokko et al. (Citation2015) represented a BDE 47 rise of 0.9%, a BDE 153 increase of 2.6%, a BDE 209 elevation of 1%, and ΣPBDE increase of 1.1% per kilogram weight loss after 12 mo. The study by Dirtu et al. (Citation2013) showed a BDE 47 elevation of 1.9%, a BDE 153 rise of 4.4%, and ΣPBDE increase of 2.3% per kilogram weight loss after 12 mo. This study also detected changes of hydroxylated metabolites of PCB (OH-CB). The metabolites with the highest percent increase per lost kilogram in this study were 3OH-CB118 (13.6%), 4OH-CB107 (6.6%), 3OH-CB138 (6.4%), and 4OH-CB146 (4.9%). Total metabolites rose from 70 to 117 pg/ml during a 12-mo period with a mean weight loss of 22 kg (Supplemental Material, ). Rantakokko et al. (Citation2015) also determined PFAS levels after weight loss. This study demonstrated minor changes in PFAS levels after 1 yr of weight loss: ΣPFCA decreased by 0.02% per kilogram weight loss, while ΣPFSA increased by 0.02% per kilogram weight loss (not shown).
Quantitative Synthesis
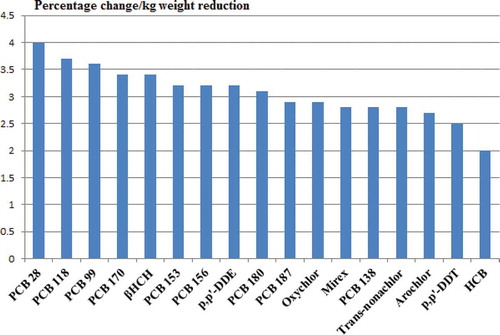
Five studies were selected for quantitative synthesis, including a total of 270 participants (Arguin et al. 2010; Chevrier et al. Citation2000; Dirtu et al. Citation2013; Imbeault et al. Citation2001; Pelletier et al. Citation2002) The percent change in POP concentrations in blood per kilogram body weight loss after 3–4 mo was calculated based on these publications (). Three of the studies reported lipid-corrected concentrations (µg/kg lipid) (Dirtu et al. Citation2013; Imbeault et al. Citation2001; Pelletier et al. Citation2002) and two reported wet weight concentrations (µg/L) (Arguin et al. 2010; Chevrier et al. Citation2000). The weight loss in these five studies was achieved by dietary caloric restriction (Arguin et al. 2010; Imbeault et al. Citation2001), dietary caloric restriction and drug intervention (Chevrier et al. Citation2000; Pelletier et al. Citation2002), and bariatric surgery (n = 66) and dietary caloric restriction (n = 85) (Dirtu et al. Citation2013) (Supplemental Material, ). The POP with the highest increase are shown in . PCB 28, PCB 118, and PCB 99 were the compounds displaying the highest percent rise per kilogram weight reduction, with 4, 3.7, and 3.6%, respectively.
Table 2. Percentage change of POP concentrations per kilogram weight reduction with average change, calculated from the individual five selected studies (average change in declining order).
Weight-Loss-Induced Changes in Blood Levels of POP
Evidence indicates that blood concentrations of most POP increase when humans lose weight. In general, compound levels rise by 2–4% per kilogram weight loss. Our quantitative analysis is essentially in agreement with Chevrier et al. (Citation2000) and Imbeault et al. (Citation2001), who suggested that a moderate weight loss, as obtained by nonsurgical measures, of approximately 10% body weight loss might lead to an elevation of total concentration of POP up to 20% over a period of 4–6 mo (Chevrier et al. Citation2000; Imbeault et al. Citation2001). However, one study that analyzed POP before and after a 10% (moderate) weight loss showed a total plasma concentration elevation of 68.3% (Charlier, Desaive, and Plomteux Citation2002). This increase in circulating lipophilic POP may be attributed to the decrease in fat mass upon weight reduction.
Weight loss amount achieved by surgery normally lies between 30 and 40%, depending upon the methods used (Aasheim et al. Citation2009). Gjevestad et al. (Citation2015) demonstrated that a weight loss of 43 kg (achieved by surgery) also resulted in a skeletal muscle mass loss of 4 kg. On the other hand, diet combined with exercise prevents the loss of muscle mass during weight loss (Gjevestad et al. Citation2015; Nordstrand et al. Citation2013). Rantakokko et al. (Citation2015) found that a weight loss of approximately 30% during 12 mo resulted in a rise of 150–330% for the various POP levels. Hue et al. (Citation2006) noted that a weight loss of 46% resulted in 388% elevation in total POP concentrations in plasma of surgically treated individuals over a period of 1 yr (Hue et al. Citation2006; Rantakokko et al. Citation2015). This constitutes a marked rise, and data indicated that a decrease of body mass index (BMI) >14 kg/m2 induces a faster increase rate than a moderately reduced BMI, suggesting that the risk of adverse health effects are elevated in individuals undergoing excessive weight loss (Hue et al. Citation2006).
In general, organochlorine compound levels increase upon weight reduction, irrespective of initial BMI (Wolff et al. Citation2005). In addition to diet and bariatric surgery, other situations are also associated with substantial weight changes over a relatively short period of time, with pregnancy being an example. Little is known regarding the influence of gestational weight gain on fetal POP concentrations (Vizcaino et al. Citation2014b).
Lipid Correction of Blood Concentrations
The main purpose of lipid correction is to lower intra- and interindividual variations. Phillips et al. (Citation1989) showed that when samples of PCB, hexachlorobenzene (HCB), and p,p'-dichlorodiphenyldichloroethylene (DDE) were lipid corrected, there were no longer significant differences between nonfasting and fasting samples. Most of the studies included in this review specified their results with lipid-corrected concentrations. Three of the investigations reported on a wet weight basis (Arguin et al. 2010; Mullerova et al. Citation2008; Citation2015). According to Brown and Lawton (Citation1984), lipid correction is the preferred measure of PCB congeners because lipid-corrected concentration is equal to the concentration in adipose tissue and mirrors the body burden (Brown and Lawton Citation1984). Arguin et al. (2010) used uncorrected values because the blood samples were not drawn in a weight-stable period and therefore were assumed not to be in a state of equilibrium. Displacements of equilibria may occur both in healthy individuals and in subjects with severe illness, great weight loss, or obstructive jaundice (Porta et al. Citation2009). Physiological changes during a pregnancy, such as blood concentrations of lipids, may influence the concentrations of POP in maternal blood and may account for other toxicokinetic changes attributed to these chemicals (Adetona et al. Citation2013; Jarrell et al. Citation2005). Lipid-adjusted concentrations (Adetona et al. Citation2013) and time-specified sampling of blood during pregnancy may be of importance in order to minimize these differences and more comprehensively interpret the actual exposure of the fetus to POP (Jarrell et al. Citation2005; Wang et al. Citation2009).
Possible Health Effects
Organochlorines (OC)
The circulating concentrations of most organochlorines (OC) increased following weight loss where levels of PCB rose from 1.2% (Chevrier et al. Citation2000) to 7.5% (Dirtu et al. Citation2013) and mean DDE levels were elevated by 3.2% per kilogram weight lost. PCBs 118, 138 and ΣDDT isomers was suspected of increasing the breast cancer risk (Hoyer et al. Citation2000). However, epidemiological data stating a clear cause–effect relationship between exposure to most OC pesticides and breast cancer are still lacking (Salehi et al. Citation2008). In a recent publication, 40 studies since 1993 were reviewed without finding a clear association between breast cancer and DDT or DDE in samples taken from adults. The same publication reported that early-life exposure remains unexplored (Loomis et al. Citation2015).
An association between increased DDE and PCB 153 concentration levels and an enhanced prevalence of type 2 diabetes was reported in 2007; however, only 15 women in this study were diagnosed with type 2 diabetes (Rignell-Hydbom, Rylander, and Hagmar Citation2007). Rylander et al. (Citation2015) found that concentrations of PCB and OC pesticides were associated with type 2 diabetes; however, predicted area under curve (AUC) of PCB 153 (reflecting early life exposure and total life-time exposure using a mechanistic exposure model) did not support an association with type 2 diabetes (Rylander et al. Citation2015). Many studies indicated that there was a link between type 2 diabetes and POP, but because humans are exposed to mixtures of POP that may involve a complex dose-response relationship to type 2 diabetes, critical methodological issues arise when assessing human findings (Lee et al. Citation2014).
POP are transferred from mother to child prenatally via the placenta (Bergonzi et al. Citation2009; Cooke Citation2014; Ferguson, O’Neill, and Meeker Citation2013; Needham et al. Citation2011; Park et al. Citation2008; Rogan et al. Citation1986; Vizcaino et al. Citation2014a; Citation2014b and via breast milk after birth (Elabbas et al. Citation2014; Laug, Kunze, and Prickett Citation1951; Polder et al. Citation2008; Skaare and Polder Citation1990; Skaare, Tuveng, and Sande Citation1988). Mendez et al. (Citation2011) found a positive association between maternal serum DDE concentrations and rapid weight gain in infants after birth, in terms of BMI assessed at 14 mo of age. Similarly, an association between prenatal exposure to DDE and elevated BMI in adult women was also noted (Karmaus et al. Citation2009), suggesting that early life exposure to DDE may affect weight regulation during childhood as well as in adult life.
Various reproductive effects have also been associated with PCB. Mocarelli et al. (Citation2011) demonstrated that children exposed to relative low PCB/dioxin doses prenatally and during breastfeeding periods displayed reduced sperm quality in adulthood (Mocarelli et al. Citation2011). Exposure to PCB during adult life indicates some association to sperm motility (Vested et al. Citation2014). In the 1980s, children born of mothers who consumed PCB-contaminated fish from Lake Michigan demonstrated lower birth weights and smaller head circumferences, even when corrected for 37 confounding variables (Fein et al. Citation1984). A meta-analysis of 7990 mother–child pairs in 12 European birth cohorts found an inverse correlation between low-level PCB 153 exposure and birth weight, suggesting that low-level exposure may exert physiological consequences (Govarts et al. Citation2012). The median cord serum concentration of PCB 153 (reflecting fetal exposure at time of delivery) in this latter study was 140 ng/L (0.14 µg/L). This is lower than the levels observed in the two studies included in the present review with respectively 0.18 and 0.31 µg/L, concentrations measured at baseline (Arguin et al. 2010; Chevrier et al. Citation2000).
Brominated Flame Retardants
Some BDE congeners have a relatively short half-life. Generally, there is a pattern of decreasing half-lives of PBDE congeners with increasing number of bromine substituents. The half-life of BDE 209 in human serum is approximately 15 d, which indicates that humans need to be continuously exposed to sustain levels detected in serum (Thuresson et al. Citation2006). BDE 209 is debrominated into nona- and octabrominated congeners through sunlight exposure (Stapleton and Dodder Citation2008; Wei et al. Citation2013). The 3 nona-BDE and 4 octa-BDE congeners examined by Thuresson et al. (Citation2006) were found to display half-lives of 18–39 and 37–91 d, respectively. When pregnant Sprague-Dawley rats were administered BDE 209 orally, debrominated congeners were detected in blood and placenta of the mother. Even in the presence of lower levels of congeners in pups, both studies still found that a transfer of these chemicals was occurring to both fetuses and suckling infants (Cai et al. Citation2011; Zhang et al. Citation2011). The debromination of BDE 209 with the possible outcome of increased concentration of more toxic and mobile lower brominated congeners constitutes a great concern (Law et al. Citation2014).
Chronic toxicity studies (103 wk) in rats and mice showed that deca-BDE possess the potential to induce pancreatic adenomas and hepatocellular adenomas and carcinomas in mammals, as well as thyroid gland follicular-cell adenomas and carcinomas (Darnerud Citation2003). BDE 153 was found to display an inverted U-shaped association with metabolic syndrome and may be involved in the pathogenesis of diabetes, noted in a cross-sectional study (Lim, Lee, and Jacobs Citation2008). Studies of small cohorts indicated that BDE 153 may reduce sperm concentration and testicular size (Akutsu et al. Citation2008), that congeners BDE 47, BDE 100, and ΣPBDE indicated adverse effect on sperm mobility, and further that congeners BDE 47, BDE 99 and ΣPBDE were negatively associated with thyroxin levels in men (Abdelouahab, Ainmelk, and Takser Citation2011). A study of 62 American men suggested associations between house-dust levels of BDE and hormone levels (thyroid hormones, thyroid-stimulating hormone [TSH], estradiol, free and total testosterone, follicle-stimulating hormone [FSH], luteinizing hormone, and sex-hormone-binding globulin [SHBG]) concluded that these findings were consistent with previous observations and that exposure to contaminants in indoor dust may lead to endocrine disruption in men (Johnson et al. Citation2013). Taken together, data suggest that BDE may exert endocrine-disrupting effects. The widespread use of certain brominated flame retardants and its environmental persistency underline the need for more studies on their potential health implications, particularly at low-exposure doses.
Perfluoroalkyl Substances (PFAS)
Perfluoroalkyl substances (PFAS), such as perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS), are classified as POP. PFAS are not stored in adipose tissues, but bind to proteins in the liver and serum (Casals-Casas and Desvergne Citation2011). In the present review, only one study reported PFAS concentration change during a weight loss period of 1 yr. No significant changes were found in PFAS concentration in blood following an average weight loss of 31.7 kg. However, there was a negative association observed between serum concentrations of several PFAS and lobular inflammation in the liver at baseline (Rantakokko et al. Citation2015). A recent publication by Berg et al. (Citation2015) demonstrated that pregnant women within the highest PFOS quartile had a 24% higher concentration of TSH than women in the first quartile. Associations between perfluorodecanoate (PFDA) and low T3 concentrations and perfluoroundecanoate (PFUnDA) and low FT3 concentrations were also detected. Although the concentrations of thyroid hormones were within normal reference ranges, even subtle changes in maternal thyroid hormone levels may have consequences for fetal health (Berg et al. Citation2015).
Obesity Management and Increased POP Concentrations
This review illustrates that weight loss might lead to increased blood concentrations of environmental pollutants and that the greater the weight loss, the higher are the resulting POP concentrations in blood. Seven of the included studies demonstrated an elevation in blood levels in most POP levels 12 mo after intervention (Backman and Kolmodin-Hedman Citation1978; Dirinck et al. Citation2016; Dirtu et al. Citation2013; Hue et al. Citation2006; Kim et al. Citation2011b; Mullerova et al. Citation2015; Rantakokko et al. Citation2015). The possibility of increased POP levels producing adverse health effects led Imbeault et al. (Citation2001) to suggest moderate rather than great weight loss (Imbeault et al. Citation2001). Other investigators indicated that weight loss might potentially produce undesirable effects in addition to known beneficial health effects and that these should be taken into consideration (Chevrier et al. Citation2000; Lim et al. 2011; Tremblay and Chaput Citation2012). Kim et al. (Citation2011b) reported that POP may be involved in alterations of lipid and energy homeostasis and in dysfunction of the liver, and that even though weight reduction may be beneficial, the benefits appear to be slowed down or decreased by high POP levels. Pelletier et al. (Citation2002) concluded that weight loss is a beneficial treatment for obesity, but high levels of POP might complicate this treatment. Recently, Dirinck et al. (Citation2016) noted that despite elevated levels of PCB, the apparent benefits of losing weight far outweigh the possible health risks, but long-term follow-up studies are needed to confirm whether the increases make individuals more prone to regain weight.
Data collected between 2003 and 2005 showed that 49% of all patients undergoing bariatric surgery were women 18–45 years of age (Maggard et al. Citation2008). Norwegian recommendations specify that women should wait 1 yr after gastric bypass surgery before becoming pregnant, due to the risk of nutritional deficiency and potential malnutrition (Skogoy, Laurini, and Aasheim Citation2009). A retrospective study that included 104 pregnant women who conceived during and 385 who conceived after the first postoperative year demonstrated comparable short-term outcome (Sheiner et al. Citation2011).
Children of overweight and obese pregnant women are exposed to potential health risks, such as miscarriage, gestational diabetes, intrauterine fetal death, macrosomia, and birth complications (Kaplan-Sturk et al. Citation2013; Triunfo and Lanzone Citation2014). A study comparing women with pregnancies before and after bariatric surgery showed a decrease in maternal complications such as diabetes mellitus and hypertensive disorders, and a reduction in frequency of fetal macrosomia (Weintraub et al. Citation2008). Karmon and Sheiner (Citation2008) reported a possible observed rise in cesarean deliveries after bariatric surgery, but were unable to conclude because of confounders (Karmon and Sheiner Citation2008). A retrospective cohort study that compared 221,580 births of obese women and 9587 births in women with bariatric surgery found that in general, women who underwent bariatric surgery had better pregnancy outcome compared with morbidly obese women. However, women who became pregnant after surgery in this study were more likely to (1) display venous thromboembolisms, (2) undergo labor induction, or (3) require a blood transfusion and to experience fetal growth restriction (Abenhaim et al. Citation2016). Pregnancies after bariatric surgery seem to be safe if the women are getting proper postoperative follow-up (Karmon and Sheiner Citation2008; Uzoma and Keriakos Citation2013).
Increased concentrations of POP in blood after a great weight loss directly before a pregnancy may also potentially harm the health of the child. The prenatal exposure period is considered to be one of the most vulnerable periods in human life (Cooke Citation2014; Ferguson, O’Neill, and Meeker Citation2013; Lyche et al. Citation2009, Vizcaino et al. Citation2014a). More knowledge regarding POP toxicokinetics and toxicodynamics, especially during pregnancy, is therefore of great importance.
Limitations
The main limitation of this review is the low number of participants from only two continents, and therefore a limited geographical area. It is a challenge to compare data from different studies using different methodology and designs. A limiting factor as to methodology and interpretation is that not all studies provided lipid-corrected concentrations. In addition, a limit in interpretation of available data is that only 8 of 17 studies reported the use of fasting samples. Further, lack of individual data in available reports precludes assessments of variations in and may also influence the mean concentrations provided.
This review also elucidated that newer investigations can contradict older ones. Global restrictions and bans for use and production of certain POPs (Stockholm Convention 2015, available at http://chm.pops.int) indicate that the concentrations of POP are declining in most parts of the world. This complicates the comparison between older and newer studies. Most research in this field, including animal studies, is carried out on a single compound or group of selected compounds, not taking the “cocktail effect” into consideration. This does not reflect the true range of POPs humans are actually exposed to (Lyche et al. Citation2009; Meeker, Sathyanarayana, and Swan Citation2009). Compounds can interact additively, antagonistically, or multiplicatively (Meeker, Sathyanarayana, and Swan Citation2009). Few long-term studies have been published and in particular few investigations have been published that compare the increase in POP concentrations with clinical consequences as individuals lose weight (De Roos et al. Citation2012). There is a need for longitudinal and well-designed studies based upon a larger number of participants monitoring exposure levels and establishing links between low doses, mixtures of doses, vulnerable time frames, and identification of metabolic active doses. These limitations call for caution in interpreting results.
Conclusions
POP are released from adipose tissue during weight loss, enabling blood levels to rise, for many POP by 2–4% per kilogram weight loss. This may lead to a total POP rise of 150–400% following a 40% body weight loss. Most blood levels are reported to be continuously elevated after a period of 1 yr or more, which may complicate weight loss treatment. POP may potentially alter hormonal, immunological, reproductive, and metabolic systems of mammals. In spite of this, weight reduction is still recommended for overweight and obese subjects, in order to reduce risk of weight-related health problems. The benefits of losing weight still far outweigh the possible adverse health risks. POP are transferred through placenta and breastmilk, and the prenatal period is considered to be one of the most vulnerable time periods in human life. Therefore, the clinical significance of elevated POP concentrations in blood induced by rapid weight loss needs to be explored, particularly in younger women losing weight before pregnancy.
Table 2: Reported data and detailed calculations from the 5 selected studies
Download MS Word (31.8 KB)Table 1: Reported data and detailed calculations from the two studies reporting on BDE and OH-CBs
Download MS Word (17.6 KB)Funding
The financial support of Innlandet Hospital Trust is acknowledged. The funding project number is 150260.
Supplementary Data
Supplemental data for this article can be accessed at publisher’s website
Additional information
Funding
References
- Aasheim, E. T., S. Bjorkman, T. T. Sovik, M. Engstrom, S. E. Hanvold, T. Mala, T. Olbers, and T. Bohmer. 2009. Vitamin status after bariatric surgery: A randomized study of gastric bypass and duodenal switch. [Erratum appears in American Journal of Clinical Nutrition (2010) 91 (1):239–40.] American Journal of Clinical Nutrition 90:15–22. doi:10.3945/ajcn.2009.27583.
- Abdelouahab, N., Y. Ainmelk, and L. Takser. 2011. Polybrominated diphenyl ethers and sperm quality. Reproductive Toxicology 31:546–50. doi:10.1016/j.reprotox.2011.02.005.
- Abenhaim, H. A., N. Alrowaily, N. Czuzoj-Shulman, A. R. Spence, and S. L. Klam. 2016. Pregnancy outcomes in women with bariatric surgery as compared with morbidly obese women. Journal of Maternal-Fetal & Neonatal Medicine 29:3596–601.
- Adetona, O., K. Horton, A. Sjodin, R. Jones, D. B. Hall, M. Aguillar-Villalobos, B. E. Cassidy, J. E. Vena, L. L. Needham, and L. P. Naeher. 2013. Concentrations of select persistent organic pollutants across pregnancy trimesters in maternal and in cord serum in Trujillo, Peru. Chemosphere 91:1426–33. doi:10.1016/j.chemosphere.2013.01.043.
- Akutsu, K., S. Takatori, S. Nozawa, M. Yoshiike, H. Nakazawa, K. Hayakawa, T. Makino, and T. Iwamoto. 2008. Polybrominated diphenyl ethers in human serum and sperm quality. Bulletin of Environmental Contamination and Toxicology 80:345–50. doi:10.1007/s00128-008-9370-4.
- Angrisani, L., A. Santonicola, P. Iovino, G. Formisano, H. Buchwald, and N. Scopinaro. 2015. Bariatric surgery worldwide 2013. Obesity Surgery 25:1822–32. doi:10.1007/s11695-015-1657-z.
- Arguin, H., M. Sanchez, G. A. Bray, J. C. Lovejoy, J. C. Peters, R. J. Jandacek, J. P. Chaput, and A. Tremblay. 2009. Impact of adopting a vegan diet or an olestra supplementation on plasma organochlorine concentrations: Results from two pilot studies. British Journal of Nutrition 103:1433–41. doi:10.1017/S000711450999331X.
- Asp, V., E. Ulleras, V. Lindstrom, U. Bergstrom, A. Oskarsson, and I. Brandt. 2010. Biphasic hormonal responses to the adrenocorticolytic DDT metabolite 3-methylsulfonyl-DDE in human cells. Toxicology and Applied Pharmacology 242:281–89. doi:10.1016/j.taap.2009.10.018.
- Backman, L., and B. Kolmodin-Hedman. 1978. Concentration of DDT and DDE in plasma and subcutaneous adipose tissue before and after intestinal bypass operation for treatment of obesity. Toxicology and Applied Pharmacology 46:663–69. doi:10.1016/0041-008X(78)90311-3.
- Berg, V., T. H. Nøst, S. Hansen, A. Elverland, A.-S. Veyhe, R. Jorde, J. Ø. Odland, and T. M. Sandanger. 2015. Assessing the relationship between perfluoroalkyl substances, thyroid hormones and binding proteins in pregnant women; a longitudinal mixed effects approach. Environment International 77:63–69. doi:10.1016/j.envint.2015.01.007.
- Bergonzi, R., C. Specchia, M. Dinolfo, C. Tomasi, G. De Palma, T. Frusca, and P. Apostoli. 2009. Distribution of persistent organochlorine pollutants in maternal and foetal tissues: Data from an Italian polluted urban area. Chemosphere 76:747–54. doi:10.1016/j.chemosphere.2009.05.026.
- Booth, A. 2006. “Brimful of STARLITE”: Toward standards for reporting literature searches. Journal of the Medical Library Association : JMLA 94:421–429, e205.
- Bourez, S., S. Le Lay, C. Van den Daelen, C. Louis, Y. Larondelle, J. P. Thome, Y. J. Schneider, I. Dugail, and C. Debier. 2012. Accumulation of polychlorinated biphenyls in adipocytes: Selective targeting to lipid droplets and role of caveolin-1. PLoS ONE 7:e31834. doi:10.1371/journal.pone.0031834.
- Brown, J. F.,Jr., and R. W. Lawton. 1984. Polychlorinated biphenyl (PCB) partitioning between adipose tissue and serum. Bulletin of Environmental Contamination and Toxicology 33:277–80. doi:10.1007/BF01625543.
- Byrne, S., P. Miller, V. Waghiyi, C. L. Buck, F. A. Von Hippel, and D. O. Carpenter. 2015. Persistent organochlorine pesticide exposure related to a formerly used defense site on St. Lawrence Island, Alaska: Data from sentinel fish and human sera. Journal of Toxicology and Environmental Health, Part A 78:976–92. doi:10.1080/15287394.2015.1037412.
- Cai, Y., W. Zhang, J. Hu, G. Sheng, D. Chen, and J. Fu. 2011. Characterization of maternal transfer of decabromodiphenyl ether (BDE-209) administered to pregnant Sprague-Dawley rats. Reproductive Toxicology 31:106–10. doi:10.1016/j.reprotox.2010.08.005.
- Casals-Casas, C., and B. Desvergne. 2011. Endocrine disruptors: From endocrine to metabolic disruption. Annual Review of Physiology 73:135–62. doi:10.1146/annurev-physiol-012110-142200.
- Catoi, A. F., A. Parvu, A. Muresan, and L. Busetto. 2015. Metabolic mechanisms in obesity and type 2 diabetes: Insights from bariatric/metabolic surgery. Obesity Facts 8:350–63. doi:10.1159/000441259.
- Charlier, C., C. Desaive, and G. Plomteux. 2002. Human exposure to endocrine disrupters: Consequences of gastroplasty on plasma concentration of toxic pollutants. International Journal of Obesity 26:1465–68. doi:10.1038/sj.ijo.0802144.
- Chevrier, J., E. Dewailly, P. Ayotte, P. Mauriege, J. P. Despres, and A. Tremblay. 2000. Body weight loss increases plasma and adipose tissue concentrations of potentially toxic pollutants in obese individuals. International Journal of Obesity 24:1272–78. doi:10.1038/sj.ijo.0801380.
- Cooke, A. A., R. M. Connaughton, C. L. Lyons, A. M. McMorrow, and H. M. Roche. 2016. Fatty acids and chronic low grade inflammation associated with obesity and the metabolic syndrome. European Journal of Pharmacology 785:207–14. doi:10.1016/j.ejphar.2016.04.021.
- Cooke, G. M. 2014. Biomonitoring of human fetal exposure to environmental chemicals in early pregnancy. Journal of Toxicology and Environmental Health, Part B 17:205–24. doi:10.1080/10937404.2014.898167.
- Darnerud, P. O. 2003. Toxic effects of brominated flame retardants in man and in wildlife. Environment International 29:841–53. doi:10.1016/S0160-4120(03)00107-7.
- De Roos, A. J., C. M. Ulrich, A. Sjodin, and A. McTiernan. 2012. Adiposity, body composition, and weight change in relation to organochlorine pollutant plasma concentrations. Journal of Exposure Science and Environmental Epidemiology 22:617–24. doi:10.1038/jes.2012.43.
- De Tata, V. 2014. Association of dioxin and other persistent organic pollutants (POPs) with diabetes: Epidemiological evidence and new mechanisms of beta cell dysfunction. International Journal of Molecular Sciences 15:7787–811. doi:10.3390/ijms15057787.
- Decherf, S., and B. A. Demeneix. 2011. The obesogen hypothesis: A shift of focus from the periphery to the hypothalamus. Journal of Toxicology and Environmental Health, Part B 14:423–48. doi:10.1080/10937404.2011.578561.
- Dirinck, E., A. C. Dirtu, P. G. Jorens, G. Malarvannan, A. Covaci, and L. F. Van Gaal. 2015. Pivotal role for the visceral fat compartment in the release of persistent organic pollutants during weight loss. Journal of Clinical Endocrinology & Metabolism 100:4463–71. doi:10.1210/jc.2015-2571.
- Dirinck, E., P. G. Jorens, A. Covaci, T. Geens, L. Roosens, H. Neels, I. Mertens, and L. Van Gaal. 2011. Obesity and persistent organic pollutants: Possible obesogenic effect of organochlorine pesticides and polychlorinated biphenyls. Obesity 19:709–14. doi:10.1038/oby.2010.133.
- Dirinck, E. L., A. C. Dirtu, M. Govindan, A. Covaci, P. G. Jorens, and L. F. Van Gaal. 2016. Endocrine-disrupting polychlorinated biphenyls in metabolically healthy and unhealthy obese subjects before and after weight loss: Difference at the start but not at the finish. American Journal of Clinical Nutrition 103:989–98. doi:10.3945/ajcn.115.119081.
- Dirtu, A. C., E. Dirinck, G. Malarvannan, H. Neels, L. Van Gaal, P. G. Jorens, and A. Covaci. 2013. Dynamics of organohalogenated contaminants in human serum from obese individuals during one year of weight loss treatment. Environmental Science & Technology 47:12441–49. doi:10.1021/es400657t.
- Elabbas, L. E., J. Esteban, X. Barber, G. Hamscher, H. Nau, W. J. Bowers, J. S. Nakai, M. Herlin, A. Akesson, M. Viluksela, D. Borg, and H. Hakansson. 2014. In utero and lactational exposure to a mixture of environmental contaminants detected in Canadian Arctic human populations alters retinoid levels in rat offspring with low margins of exposure. Journal of Toxicology and Environmental Health, Part A 77:223–45. doi:10.1080/15287394.2013.861776.
- Esser, A., P. M. Gaum, T. Schettgen, T. Kraus, M. Gube, and J. Lang. 2015. Effect of occupational polychlorinated biphenyls exposure on quality-adjusted life years over time at the HELPcB surveillance program. Journal of Toxicology and Environmental Health, Part A 78:132–50. doi:10.1080/15287394.2014.946165.
- Faerch, K., K. Hojlund, B. F. Vind, A. Vaag, C. Dalgard, F. Nielsen, and P. Grandjean. 2012. Increased serum concentrations of persistent organic pollutants among prediabetic individuals: Potential role of altered substrate oxidation patterns. Journal of Clinical Endocrinology & Metabolism 97:E1705–E1713. doi:10.1210/jc.2012-1342.
- Fein, G. G., J. L. Jacobson, S. W. Jacobson, P. M. Schwartz, and J. K. Dowler. 1984. Prenatal exposure to polychlorinated biphenyls: Effects on birth size and gestational age. Journal of Pediatrics 105:315–20. doi:10.1016/S0022-3476(84)80139-0.
- Ferguson, K. K., M. S. O’Neill, and J. D. Meeker. 2013. Environmental contaminant exposures and preterm birth: A comprehensive review. Journal of Toxicology and Environmental Health, Part B 16:69–113. doi:10.1080/10937404.2013.775048.
- Ferrante, M. C., P. Amero, A. Santoro, A. Monnolo, R. Simeoli, F. Di Guida, G. Mattace Raso, and R. Meli. 2014. Polychlorinated biphenyls (PCB 101, PCB 153 and PCB 180) alter leptin signaling and lipid metabolism in differentiated 3t3-l1 adipocytes. Toxicology and Applied Pharmacology 279:401–08. doi:10.1016/j.taap.2014.06.016.
- Gjevestad, E., J. Hjelmesaeth, R. Sandbu, and N. Nordstrand. 2015. Effects of intensive lifestyle intervention and gastric bypass on aortic stiffness: A 1-year nonrandomized clinical study. Obesity (Silver Spring) 23:37–45. doi:10.1002/oby.20880.
- Govarts, E., M. Nieuwenhuijsen, G. Schoeters, F. Ballester, K. Bloemen, M. de Boer, C. Chevrier, O. M. Eggesb, M. Guxens, U. Kramer, J. Legler, D. Martinez, L. Palkovicova, E. Patelarou, U. Ranft, A. Rautio, M. S. Petersen, R. Slama, H. Stigum, G. Toft, T. Trnovec, S. Vandentorren, P. Weihe, N. W. Kuperus, M. Wilhelm, J. Wittsiepe, and J. P. Bonde. 2012. Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): A meta-analysis within 12 European birth cohorts. Environmental Health Perspectives 120:162–70. doi:10.1289/ehp.1103767.
- Grun, F., and B. Blumberg. 2009. Endocrine disrupters as obesogens. Molecular and Cellular Endocrinology 304:19–29. doi:10.1016/j.mce.2009.02.018.
- Hatch, E. E., J. W. Nelson, R. W. Stahlhut, and T. F. Webster. 2010. Association of endocrine disruptors and obesity: Perspectives from epidemiological studies. International Journal of Andrology 33:324–32. doi:10.1111/(ISSN)1365-2605.
- Henkler, F., and A. Luch. 2011. Adverse health effects of environmental chemical agents through non-genotoxic mechanisms. Journal of Epidemiology & Community Health 65:1–3. doi:10.1136/jech.2008.083881.
- Hoyer, A. P., T. Jorgensen, P. Grandjean, and H. B. Hartvig. 2000. Repeated measurements of organochlorine exposure and breast cancer risk (Denmark). Cancer Causes Control 11:177–84. doi:10.1023/A:1008926219539.
- Hue, O., J. Marcotte, F. Berrigan, M. Simoneau, J. Dore, P. Marceau, S. Marceau, A. Tremblay, and N. Teasdale. 2006. Increased plasma levels of toxic pollutants accompanying weight loss induced by hypocaloric diet or by bariatric surgery. Obesity Surgery 16:1145–54. doi:10.1381/096089206778392356.
- Imbeault, P., J. Chevrier, E. Dewailly, P. Ayotte, J. P. Despres, A. Tremblay, and P. Mauriege. 2001. Increase in plasma pollutant levels in response to weight loss in humans is related to in vitro subcutaneous adipocyte basal lipolysis. International Journal of Obesity and Related Metabolic Disorders 25:1585–91. doi:10.1038/sj.ijo.0801817.
- Jarrell, J., S. Chan, R. Hauser, and H. Hu. 2005. Longitudinal assessment of PCBs and chlorinated pesticides in pregnant women from Western Canada. Environ Health 4:10. doi:10.1186/1476-069X-4-10.
- Johnson, P. I., H. M. Stapleton, B. Mukherjee, R. Hauser, and J. D. Meeker. 2013. Associations between brominated flame retardants in house dust and hormone levels in men. Science of the Total Environment 445–446:177–84. doi:10.1016/j.scitotenv.2012.12.017.
- Kaplan-Sturk, R., H. Akerud, H. Volgsten, L. Hellstrom-Westas, and E. Wiberg-Itzel. 2013. Outcome of deliveries in healthy but obese women: Obesity and delivery outcome. BMC Research Notes 6:50. doi:10.1186/1756-0500-6-50.
- Karmaus, W., J. R. Osuch, I. Eneli, L. M. Mudd, J. Zhang, D. Mikucki, P. Haan, and S. Davis. 2009. Maternal levels of dichlorodiphenyl-dichloroethylene (DDE) may increase weight and body mass index in adult female offspring. Occupational and Environmental Medicine 66:143–49. doi:10.1136/oem.2008.041921.
- Karmon, A., and E. Sheiner. 2008. Pregnancy after bariatric surgery: A comprehensive review. Archives of Gynecology and Obstetrics 277:381–88. doi:10.1007/s00404-008-0608-5.
- Kim, H. S., S. J. Kwack, E. S. Han, T. S. Kang, S. H. Kim, and S. Y. Han. 2011a. Induction of apoptosis and CYP4A1 expression in Sprague-Dawley rats exposed to low doses of perfluorooctane sulfonate. Journal of Toxicological Sciences 36:201–10. doi:10.2131/jts.36.201.
- Kim, M. J., P. Marchand, C. Henegar, J. P. Antignac, R. Alili, C. Poitou, J. L. Bouillot, A. Basdevant, B. Le Bizec, R. Barouki, and K. Clement. 2011b. Fate and complex pathogenic effects of dioxins and polychlorinated biphenyls in obese subjects before and after drastic weight loss. Environmental Health Perspectives 119:377–83. doi:10.1289/ehp.1002848.
- La Merrill, M., C. Emond, M. J. Kim, J. P. Antignac, B. Le Bizec, K. Clement, L. S. Birnbaum, and R. Barouki. 2013. Toxicological function of adipose tissue: Focus on persistent organic pollutants. Environment Health Perspectives 121:162–69.
- Laug, E. P., F. M. Kunze, and C. S. Prickett. 1951. Occurrence of DDT in human fat and milk. Archives of Industrial Hygiene and Occupational Medicine 3:245–46.
- Law, R. J., A. Covaci, S. Harrad, D. Herzke, M. A. E. Abdallah, K. Fernie, L.-M. L. Toms, and H. Takigami. 2014. Levels and trends of PBDEs and HBCDs in the global environment: Status at the end of 2012. Environment International 65:147–58. doi:10.1016/j.envint.2014.01.006.
- Lee, D. H., D. R. Jacobs,Jr., and M. Porta. 2009. Hypothesis: A unifying mechanism for nutrition and chemicals as lifelong modulators of DNA hypomethylation. Environmental Health Perspectives 117:1799–802. doi:10.1289/ehp.0900741.
- Lee, D. H., M. Porta, D. R. Jacobs,Jr., and L. N. Vandenberg. 2014. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocrine Reviews 35:557–601. doi:10.1210/er.2013-1084.
- Lim, J. S., D. H. Lee, and D. R. Jacobs, Jr. 2008. Association of brominated flame retardants with diabetes and metabolic syndrome in the U.S. population, 2003–2004. Diabetes Care 31:1802–07. doi:10.2337/dc08-0850.
- Lim, J. S., H. K. Son, S. K. Park, D. R. Jacobs, Jr., and D. H. Lee. 2010. Inverse associations between long-term weight change and serum concentrations of persistent organic pollutants. International Journal of Obesity 35:744–47. doi:10.1038/ijo.2010.188.
- Livingston, E. H., and J. W. Zylke. 2012. Progress in obesity research: Reasons for optimism. Journal of the American Medical Association 308:1162–64. doi:10.1001/2012.jama.12203.
- Loomis, D., K. Guyton, Y. Grosse, F. El Ghissasi, V. Bouvard, L. Benbrahim-Tallaa, N. Guha, H. Mattock, and K. Straif. 2015. Carcinogenicity of lindane, DDT, and 2,4-dichlorophenoxyacetic acid. Lancet Oncology 16:891–92. doi:10.1016/S1470-2045(15)00081-9.
- Lyche, J. L., A. C. Gutleb, A. Bergman, G. S. Eriksen, A. J. Murk, E. Ropstad, M. Saunders, and J. U. Skaare. 2009. Reproductive and developmental toxicity of phthalates. Journal of Toxicology and Environmental Health, Part B 12:225–49. doi:10.1080/10937400903094091.
- Maggard, M. A., I. Yermilov, Z. Li, M. Maglione, S. Newberry, M. Suttorp, L. Hilton, H. P. Santry, J. M. Morton, E. H. Livingston, and P. G. Shekelle. 2008. Pregnancy and fertility following bariatric surgery: A systematic review. Journal of the American Medical Association 300:2286–96. doi:10.1001/jama.2008.641..
- McAllister, E. J., N. V. Dhurandhar, S. W. Keith, L. J. Aronne, J. Barger, M. Baskin, R. M. Benca, J. Biggio, M. M. Boggiano, J. C. Eisenmann, M. Elobeid, K. R. Fontaine, P. Gluckman, E. C. Hanlon, P. Katzmarzyk, A. Pietrobelli, D. T. Redden, D. M. Ruden, C. Wang, R. A. Waterland, S. M. Wright, and D. B. Allison. 2009. Ten putative contributors to the obesity epidemic. Critical Reviews in Food Science and Nutrition 49:868–913. doi:10.1080/10408390903372599.
- Meeker, J. D., S. Sathyanarayana, and S. H. Swan. 2009. Phthalates and other additives in plastics: Human exposure and associated health outcomes. Philosophical Transactions of the Royal Society B: Biological Sciences 364:2097–113. doi:10.1098/rstb.2008.0268.
- Mendez, M. A., R. Garcia-Esteban, M. Guxens, M. Vrijheid, M. Kogevinas, F. Goni, S. Fochs, and J. Sunyer. 2011. Prenatal organochlorine compound exposure, rapid weight gain, and overweight in infancy. Environmental Health Perspectives 119:272–78. doi:10.1289/ehp.1002169.
- Mocarelli, P., P. M. Gerthoux, L. L. Needham, D. G. Patterson Jr., G. Limonta, R. Falbo, S. Signorini, M. Bertona, C. Crespi, C. Sarto, P. K. Scott, W. E. Turner, and P. Brambilla. 2011. Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environmental Health Perspectives 119:713–18. doi:10.1289/ehp.1002134.
- Moher, D., A. Liberati, J. Tetzlaff, and D. G. Altman. 2010. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. International Journal of Surgery 8:336–41. doi:10.1016/j.ijsu.2010.02.007.
- Mouritsen, A., L. Aksglaede, K. Sorensen, S. S. Mogensen, H. Leffers, K. M. Main, H. Frederiksen, A. M. Andersson, N. E. Skakkebaek, and A. Juul. 2010. Hypothesis: Exposure to endocrine-disrupting chemicals may interfere with timing of puberty. International Journal of Andrology 33:346–59. doi:10.1111/(ISSN)1365-2605.
- Mullerova, D., J. Kopecky, D. Matejkova, L. Muller, J. Rosmus, J. Racek, F. Sefrna, S. Opatrna, O. Kuda, and M. Matejovic. 2008. Negative association between plasma levels of adiponectin and polychlorinated biphenyl 153 in obese women under non-energy-restrictive regime. International Journal of Obesity 32:1875–78. doi:10.1038/ijo.2008.169.
- Mullerova, D., D. Matejkova, J. Dvorakova, L. Muller, J. Rosmus, and K. Kovarova. 2015. Persistent organochlorine pollutants in obese women after diet induced weight loss: Five years follow up study. Central European Journal of Public Health 23:214–17. doi:10.21101/cejph.a4100.
- Needham, L. L., P. Grandjean, B. Heinzow, P. J. Jorgensen, F. Nielsen, D. G. Patterson Jr., A. Sjodin, W. E. Turner, and P. Weihe. 2011. Partition of environmental chemicals between maternal and fetal blood and tissues. Environmental Science & Technology 45:1121–26. doi:10.1021/es1019614.
- Newbold, R. R., E. Padilla-Banks, W. N. Jefferson, and J. J. Heindel. 2008. Effects of endocrine disruptors on obesity. International Journal of Andrology 31:201–08. doi:10.1111/ija.2008.31.issue-2.
- Nordstrand, N., E. Gjevestad, J. K. Hertel, L. K. Johnson, E. Saltvedt, J. Roislien, and J. Hjelmesaeth. 2013. Arterial stiffness, lifestyle intervention and a low-calorie diet in morbidly obese patients-a nonrandomized clinical trial. Obesity 21:690–97. doi:10.1002/oby.20099.
- Park, J. S., A. Bergman, L. Linderholm, M. Athanasiadou, A. Kocan, J. Petrik, B. Drobna, T. Trnovec, M. J. Charles, and I. Hertz-Picciotto. 2008. Placental transfer of polychlorinated biphenyls, their hydroxylated metabolites and pentachlorophenol in pregnant women from Eastern Slovakia. Chemosphere 70:1676–84. doi:10.1016/j.chemosphere.2007.07.049.
- Pelletier, C., E. Doucet, P. Imbeault, and A. Tremblay. 2002. Associations between weight loss-induced changes in plasma organochlorine concentrations, serum T(3) concentration, and resting metabolic rate. Toxicological Sciences 67:46–51. doi:10.1093/toxsci/67.1.46.
- Pelletier, C., P. Imbeault, and A. Tremblay. 2003. Energy balance and pollution by organochlorines and polychlorinated biphenyls. Obesity Reviews 4:17–24. doi:10.1046/j.1467-789X.2003.00085.x.
- Pestana, D., G. Faria, C. Sa, V. C. Fernandes, D. Teixeira, S. Norberto, A. Faria, M. Meireles, C. Marques, L. Correia-Sa, A. Cunha, J. T. Guimaraes, A. Taveira-Gomes, A. C. Santos, V. F. Domingues, C. Delerue-Matos, R. Monteiro, and C. Calhau. 2014. Persistent organic pollutant levels in human visceral and subcutaneous adipose tissue in obese individuals-depot differences and dysmetabolism implications. Environmental Research 133:170–77. doi:10.1016/j.envres.2014.05.026.
- Phillips, D. L., J. L. Pirkle, V. W. Burse, J. T. Bernert, Jr., L. O. Henderson, and L. L. Needham. 1989. Chlorinated hydrocarbon levels in human serum: Effects of fasting and feeding. Archives of Environmental Contamination and Toxicology 18:495–500. doi:10.1007/BF01055015.
- Poirier, P., M. A. Cornier, T. Mazzone, S. Stiles, S. Cummings, S. Klein, P. A. McCullough, C. Ren Fielding, and B. A. Franklin. 2011. Bariatric surgery and cardiovascular risk factors: A scientific statement from the American Heart Association. Circulation 123:1683–701. doi:10.1161/CIR.0b013e3182149099.
- Polder, A., C. Thomsen, G. Lindstrom, K. B. Loken, and J. U. Skaare. 2008. Levels and temporal trends of chlorinated pesticides, polychlorinated biphenyls and brominated flame retardants in individual human breast milk samples from northern and southern Norway. Chemosphere 73:14–23. doi:10.1016/j.chemosphere.2008.06.002.
- Porta, M. 2006. Persistent organic pollutants and the burden of diabetes. Lancet 368:558–59. doi:10.1016/S0140-6736(06)69174-5.
- Porta, M., M. Jariod, T. Lopez, J. Pumarega, E. Puigdomenech, E. Marco, N. Malats, J. O. Grimalt, and F. X. Real. 2009. Correcting serum concentrations of organochlorine compounds by lipids: Alternatives to the organochlorine/total lipids ratio. Environment International 35:1080–85. doi:10.1016/j.envint.2009.06.004.
- Porta, M., T. Lopez, M. Gasull, M. Rodriguez-Sanz, M. Gari, J. Pumarega, C. Borrell, and J. O. Grimalt. 2012. Distribution of blood concentrations of persistent organic pollutants in a representative sample of the population of Barcelona in 2006, and comparison with levels in 2002. Science of the Total Environment 423:151–61. doi:10.1016/j.scitotenv.2012.02.001.
- Rantakokko, P., V. Mannisto, R. Airaksinen, J. Koponen, M. Viluksela, H. Kiviranta, and J. Pihlajamaki. 2015. Persistent organic pollutants and non-alcoholic fatty liver disease in morbidly obese patients: A cohort study. Environ Health 14:79. doi:10.1186/s12940-015-0066-z.
- Rignell-Hydbom, A., L. Rylander, and L. Hagmar. 2007. Exposure to persistent organochlorine pollutants and type 2 diabetes mellitus. Human & Experimental Toxicology 26:447–52. doi:10.1177/0960327107076886.
- Rogan, W. J., B. C. Gladen, J. D. McKinney, N. Carreras, P. Hardy, J. Thullen, J. Tinglestad, and M. Tully. 1986. Neonatal effects of transplacental exposure to PCBs and DDE. The Journal of Pediatrics 109:335–41. doi:10.1016/S0022-3476(86)80397-3.
- Rubino, F. 2013. From bariatric to metabolic surgery: Definition of a new discipline and implications for clinical practice. Current Atherosclerosis Reports 15:369. doi:10.1007/s11883-013-0369-x.
- Rylander, C., T. M. Sandanger, T. H. Nøst, K. Breivik, and E. Lund. 2015. Combining plasma measurements and mechanistic modeling to explore the effect of POPs on type 2 diabetes mellitus in Norwegian women. Environmental Research 142:365–73. doi:10.1016/j.envres.2015.07.002.
- Rylander, L., E. Wallin, B. A. Jonssson, M. Stridsberg, E. M. Erfurth, and L. Hagmar. 2006. Associations between CB-153 and p,p’-DDE and hormone levels in serum in middle-aged and elderly men. Chemosphere 65:375–81. doi:10.1016/j.chemosphere.2006.02.012.
- Salehi, F., M. C. Turner, K. P. Phillips, D. T. Wigle, D. Krewski, and K. J. Aronson. 2008. Review of the etiology of breast cancer with special attention to organochlorines as potential endocrine disruptors. Journal of Toxicology and Environmental Health, Part B 11:276–300. doi:10.1080/10937400701875923.
- Schafer, K. S., and S. E. Kegley. 2002. Persistent toxic chemicals in the US food supply..” Journal of Epidemiology & Community Health 56:813–17. doi:10.1136/jech.56.11.813.
- Schildkraut, J. M., W. Demark-Wahnefried, E. DeVoto, C. Hughes, J. L. Laseter, and B. Newman. 1999. Environmental contaminants and body fat distribution. Cancer Epidemiology, Biomarkers & Prevention 8:179–83.
- Sheiner, E., A. Edri, E. Balaban, I. Levi, and B. Aricha-Tamir. 2011. Pregnancy outcome of patients who conceive during or after the first year following bariatric surgery. American Journal of Obstetrics and Gynecology 204:50.e1–50.e6. doi:10.1016/j.ajog.2010.08.027.
- Skaare, J. U., and A. Polder. 1990. Polychlorinated biphenyls and organochlorine pesticides in milk of Norwegian women during lactation. Archives of Environmental Contamination and Toxicology 19:640–45. doi:10.1007/BF01183978.
- Skaare, J. U., J. M. Tuveng, and H. A. Sande. 1988. Organochlorine pesticides and polychlorinated biphenyls in maternal adipose tissue, blood, milk, and cord blood from mothers and their infants living in Norway. Archives of Environmental Contamination and Toxicology 17:55–63. doi:10.1007/BF01055154.
- Skogoy, K., R. Laurini, and E. T. Aasheim. 2009. [Pregnancy shortly after bariatric surgery]. Tidsskrift for Den Norske Laegeforening: Tidsskrift for Praktisk Medicin, Ny Raekke 129:534–36.
- Stapleton, H. M., and N. G. Dodder. 2008. Photodegradation of decabromodiphenyl ether in house dust by natural sunlight. Environmental Toxicology and Chemistry / SETAC 27:306–12. doi:10.1897/07-301R.1.
- Stevens, G. A., G. M. Singh, Y. Lu, G. Danaei, J. K. Lin, M. M. Finucane, A. N. Bahalim, R. K. McIntire, H. R. Gutierrez, M. Cowan, C. J. Paciorek, F. Farzadfar, L. Riley, M. Ezzati, & the Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group. 2012. National, regional, and global trends in adult overweight and obesity prevalences. Population Health Metrics 10:22. doi:10.1186/1478-7954-10-22.
- Thuresson, K., P. Hoglund, L. Hagmar, A. Sjodin, A. Bergman, and K. Jakobsson. 2006. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environmental Health Perspectives 114:176–81. doi:10.1289/ehp.8350.
- Tremblay, A., and J. P. Chaput. 2012. Obesity: The allostatic load of weight loss dieting. Physiology & Behavior 106:16–21. doi:10.1016/j.physbeh.2011.05.020.
- Tremblay, A., C. Pelletier, E. Doucet, and P. Imbeault. 2004. Thermogenesis and weight loss in obese individuals: A primary association with organochlorine pollution. International Journal of Obesity 28:936–39. doi:10.1038/sj.ijo.0802527.
- Triunfo, S., and A. Lanzone. 2014. Impact of overweight and obesity on obstetric outcomes. Journal of Endocrinological Investigation 37:323–29. doi:10.1007/s40618-014-0058-9.
- Turyk, M., H. A. Anderson, L. Knobeloch, P. Imm, and V. W. Persky. 2009. Prevalence of diabetes and body burdens of polychlorinated biphenyls, polybrominated diphenyl ethers, and p,p’-diphenyldichloroethene in Great Lakes sport fish consumers. Chemosphere 75:674–79. doi:10.1016/j.chemosphere.2008.12.035.
- Uzoma, A., and R. Keriakos. 2013. Pregnancy management following bariatric surgery. Journal of Obstetrics and Gynaecology 33:109–14. doi:10.3109/01443615.2012.736550.
- Vested, A., A. Giwercman, J. P. Bonde, and G. Toft. 2014. Persistent organic pollutants and male reproductive health. Asian Journal of Andrology 16:71–80. doi:10.4103/1008-682X.122345.
- Vizcaino, E., J. O. Grimalt, A. Fernandez-Somoano, and A. Tardon. 2014a. Transport of persistent organic pollutants across the human placenta. Environment International 65:107–15. doi:10.1016/j.envint.2014.01.004.
- Vizcaino, E., J. O. Grimalt, B. Glomstad, A. Fernandez-Somoano, and A. Tardon. 2014b. Gestational weight gain and exposure of newborns to persistent organic pollutants. Environment Health Perspectives 122:873–79.
- Walford, R. L., D. Mock, T. MacCallum, and J. L. Laseter. 1999. Physiologic changes in humans subjected to severe, selective calorie restriction for two years in biosphere 2: Health, aging, and toxicological perspectives. Toxicological Sciences 52:61–65.
- Wang, R. Y., R. B. Jain, A. F. Wolkin, C. H. Rubin, and L. L. Needham. 2009. Serum concentrations of selected persistent organic pollutants in a sample of pregnant females and changes in their concentrations during gestation. Environmental Health Perspectives 117:1244–49. doi:10.1289/ehp.0800105.
- Wang, Y. C., K. McPherson, T. Marsh, S. L. Gortmaker, and M. Brown. 2011. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378:815–25. doi:10.1016/S0140-6736(11)60814-3.
- Wei, H., Y. Zou, A. Li, E. R. Christensen, and K. J. Rockne. 2013. Photolytic debromination pathway of polybrominated diphenyl ethers in hexane by sunlight. Environmental Pollution 174:194–200. doi:10.1016/j.envpol.2012.11.035.
- Weintraub, A. Y., A. Levy, I. Levi, M. Mazor, A. Wiznitzer, and E. Sheiner. 2008. Effect of bariatric surgery on pregnancy outcome. International Journal of Gynecology & Obstetrics 103:246–51. doi:10.1016/j.ijgo.2008.07.008.
- Wolff, M. S., J. A. Britton, S. L. Teitelbaum, S. Eng, E. Deych, K. Ireland, Z. Liu, A. I. Neugut, R. M. Santella, and M. D. Gammon. 2005. Improving organochlorine biomarker models for cancer research. Cancer Epidemiology Biomarkers & Prevention 14:2224–36. doi:10.1158/1055-9965.EPI-05-0173.
- Zhang, W., Y. Cai, G. Sheng, D. Chen, and J. Fu. 2011. Tissue distribution of decabrominated diphenyl ether (BDE-209) and its metabolites in sucking rat pups after prenatal and/or postnatal exposure. Toxicology 283:49–54. doi:10.1016/j.tox.2011.02.003.