ABSTRACT
Caenorhabditis elegans has emerged as a major model in biomedical and environmental toxicology. Numerous papers on toxicology and pharmacology in C. elegans have been published, and this species has now been adopted by investigators in academic toxicology, pharmacology, and drug discovery labs. C. elegans has also attracted the interest of governmental regulatory agencies charged with evaluating the safety of chemicals. However, a major, fundamental aspect of toxicological science remains underdeveloped in C. elegans: xenobiotic metabolism and transport processes that are critical to understanding toxicokinetics and toxicodynamics, and extrapolation to other species. The aim of this review was to initially briefly describe the history and trajectory of the use of C. elegans in toxicological and pharmacological studies. Subsequently, physical barriers to chemical uptake and the role of the worm microbiome in xenobiotic transformation were described. Then a review of what is and is not known regarding the classic Phase I, Phase II, and Phase III processes was performed. In addition, the following were discussed (1) regulation of xenobiotic metabolism; (2) review of published toxicokinetics for specific chemicals; and (3) genetic diversity of these processes in C. elegans. Finally, worm xenobiotic transport and metabolism was placed in an evolutionary context; key areas for future research highlighted; and implications for extrapolating C. elegans toxicity results to other species discussed.
Introduction
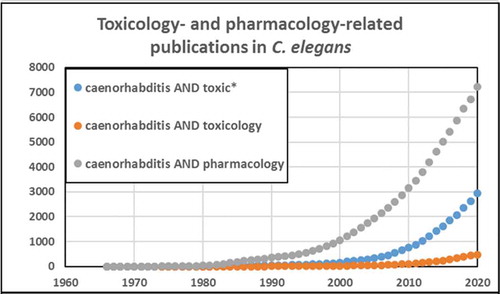
Caenorhabditis elegans has emerged as an important model in biomedical and environmental toxicology. C. elegans was first described over 100 years ago by Maupas (Citation1900) and was intermittently studied thereafter until coming to prominence as an exceptionally powerful model organism for developmental biology, neurobiology, and genetics, as a result of pioneering efforts by Sydney Brenner and colleagues in the 1970s (Nigon and Felix Citation2017). Although there was some early research in toxicologically relevant areas such as DNA damage and repair (Hartman and Herman Citation1982) and antioxidant defenses (Blum and Fridovich Citation1983), the first explicit efforts to develop C. elegans as a model for toxicological research were carried out by Phil Williams, David Dusenberry, and colleagues beginning in the 1980s (Williams and Dusenbery Citation1987, Citation1988, Citation1990a, Citation1990b). In the early 1990s, Jonathan Freedman’s lab worked on heavy metal response (Freedman et al. Citation1993; Slice, Freedman, and Rubin Citation1990), toxicogenomic analysis (Cui et al. Citation2007), and medium-throughput toxicity testing (Boyd, McBride, and Freedman Citation2007), and went on to establish a worm toxicology lab at the United States National Toxicology Program (Behl et al. Citation2015; Boyd et al. Citation2010, Citation2015, Citation2009; Xia et al. Citation2018). Starting in the mid-1990s, Christian Sternberg’s group carried out ecotoxicological studies with aquatic and sediment exposures (Hoss et al. Citation1997, Citation1999; Traunspurger et al. Citation1997), ultimately leading to a number of academic reports (Hagerbaumer et al. Citation2015; Hoss et al. Citation2009). Two standardized toxicology testing protocols have been published (International Standard Organization (ISO) Citation2020, ((ASTM), American Society of Testing and Materials. Citation2001). Richard Nass established the use of C. elegans for chemical-induced neurodegeneration (Nass, Miller, and Blakely Citation2001, Citation2002). Further, C. elegans has been used to study transgenerational and environmental epigenetics (Kelly Citation2014; Weinhouse et al. Citation2018). C. elegans is now a well-established model for human and ecological toxicology employed by many labs (a non-comprehensive sampling identifies approximately two dozen: (Leung et al. Citation2008; Boyd et al. Citation2010; Steinberg, Sturzenbaum, and Menzel Citation2008; Helmcke, Avila, and Aschner Citation2010; Meyer and Williams Citation2014; Tejeda-Benitez and Olivero-Verbel Citation2016; Hunt Citation2017; Choi et al. Citation2014; Honnen Citation2017; Ferreira and Allard Citation2015; Allard et al. Citation2013; Lenz, Pattison, and Ma Citation2017; Nass, Miller, and Blakely Citation2001a; Harrington et al. Citation2010; Cooper and Van Raamsdonk Citation2018; Liao and Yu Citation2005; Fitsanakis, Negga, and Hatfield Citation2014; Menzel et al. Citation2005; Harlow et al. Citation2018; Zhao et al. Citation2013; Brady et al. Citation2019; Anbalagan et al. Citation2013; Clavijo et al. Citation2016; Hoss et al. Citation2009; Horsman and Miller Citation2016; Shomer et al. Citation2019; Zhang et al. Citation2020; Haegerbaeumer et al. Citation2018b; Lee et al. Citation2020; Shen et al. Citation2019; Harlow et al. Citation2016; Dietrich et al. Citation2016; Xiong, Pears, and Woollard Citation2017)). The number of publications on toxicology and related fields in C. elegans has grown rapidly in recent years, with an even more rapid growth in pharmacology-related publications ().
Figure 1. There has been a rapid increase in the use of C. elegans for pharmacology and toxicology research
This toxicological research is complemented by extensive investigations of other stress-response pathways by non-toxicologists, including heat shock unfolded protein, DNA damage, antioxidant defense, osmotic stress, hormesis, insulin-responsive pathways, hypoxia, caloric stress, regulation of apoptosis and necrosis, and autophagy (Baugh Citation2013; Baumeister, Schaffitzel, and Hertweck Citation2006; Blackwell et al. Citation2015; Hengartner and Horvitz Citation1994; Melendez and Levine Citation2009; Murphy Citation2006; Nikoletopoulou and Tavernarakis Citation2014; Prahlad and Morimoto Citation2009; Rieckher et al. Citation2018; Ristow and Schmeisser Citation2011; Rodriguez et al. Citation2013). Importantly, as reviewed by Hunt (Citation2017) of the U.S. Food and Drug Administration (Center for Food Safety and Applied Nutrition), there is a significant correspondence between C. elegans and higher eukaryotes in rank-order acute toxicity of chemicals as noted in multiple studies in several labs.
Explicit discussion of the strengths and limitations of C. elegans as a model for toxicological research may be found in several review papers and books (Hunt Citation2017; Maurer, Luz, and Meyer Citation2018; Queiros et al. Citation2019; Wang Citation2019a, Citation2019b, Citation2020; Weinhouse et al. Citation2018), and thus will not be repeated here. The focus of this review will be on one very important and oft-cited limitation: the absence of detailed understanding of xenobiotic transport and metabolism processes that regulate concentrations of chemicals and metabolites that reach molecular targets. The study of these processes is fundamental to toxicology, pharmacology, and drug discovery. A truism in toxicology is that “the dose makes the poison,” and it is not possible to truly understand the toxicity of a chemical without understanding how much of it (or its metabolites) has reached specific molecular sites of action. Similarly, it is not possible to extrapolate effects observed in one species (e.g., worms) to another (e.g., humans) without being able to compare internal toxicant concentrations. In toxicology, a distinction is made between toxico(pharmaco)kinetics (understanding the absorption, distribution, metabolism, and excretion (ADME) processes that regulate xenobiotic transport and transformation processes) and toxico(pharmaco)dynamics (understanding the interactions of xenobiotics with molecular targets including receptors, DNA, and proteins).
Toxicodynamics (TD) appear to be relatively similar between C. elegans and higher eukaryotes based on conservation of genes that encode molecular targets such as proteins and signaling pathways, as reviewed in some detail previously (Hunt Citation2017; Leung et al. Citation2008; Wang Citation2019a). Further, the great majority of worm toxicology papers to date studied TD, and these investigations generally support similar mechanisms of action for many chemicals in worms compared to higher eukaryotes. However, genetic, biochemical, and other differences that qualitatively alter potential chemical toxicity exist (reviewed in Maurer, Luz, and Meyer Citation2018; Queiros et al. Citation2019; Weinhouse et al. Citation2018) and need to be considered. For example, worms (1) do not synthesize heme or cholesterol; (2) lack an adaptive immune system; and (3) lack homologs of some important receptors such as estrogen receptors. Therefore, chemicals that act directly on proteins in these pathways in higher eukaryotes might exert differing or no effects in worms.
Toxicokinetics (TK), in contrast, the focus of this review, is relatively poorly studied in C. elegans. Further, based upon the large physiological differences between worms and humans TK are expected to be different in C. elegans. For example, the nematode cuticle and eggshell are effective barriers to some chemicals. In addition, ADME processes are likely different in an organism that has a simple gut, but lacks a circulatory system and many discrete organs such as the liver. Significant uncertainty also exists in our understanding of the regulation and catalytic activity of xenobiotic metabolic enzymes and transporters. This review is intended to highlight what is or is not known regarding these processes in C. elegans, with the goal of stimulating research to fill in these knowledge gaps and improve the utility of this powerful model organism for toxicology and pharmacology.
Uptake of chemicals by the nematode occurs predominantly either (1) across the cuticle, which has few openings and thus presents an effective barrier against many chemicals and particles, or (2) via ingestion. Description of these barriers is provided in the next section. Emerging evidence suggests that the worm’s microbiome exhibits the potential to alter chemicals prior to intracellular uptake, and the following section summarizes our nascent understanding of the role of the worm microbiome in xenobiotic and drug metabolism. The state of knowledge of the biochemical toxicological processes classically referred to as “Phase I, II, and III” (Casarett, Doull, and Klaassen Citation2008) are present in worms. These processes, which are critically important for organic xenobiotics, but also in some cases important for metals and metalloids, are conceptualized as:
–Phase I: enzymatically exposing or adding reactive moieties in parent xenobiotic;–Phase II: conjugating the Phase I-modified (or in some cases, parent) xenobiotic to large, water-soluble molecules, which facilitates excretion;–Phase III: transport of these metabolized compounds out of the cell (some transporters may act to import a parent compound, sometimes described as “Phase 0”).
What is known of transcriptional regulation of these processes, including the role of the large number of nuclear hormone receptors present in C. elegans, are described below. An important caveat to keep in mind in the context of our discussion of specific genes is that most of what is presented is for the historically heavily-studied N2 Bristol strain. Subsequently a comprehensive catalog from the literature in which actual chemical measurements have been made in worms that can inform us about chemical uptake, transformation, and excretion is provided. The impacts of genetic diversity present across C. elegans populations (C. elegans is found globally), its use to identify sources of natural variation in toxicological processes, and its role in an evolutionary toxicology context are discussed. Finally, knowledge gaps are summarized and future directions discussed.
Physical barriers and tissue-specific xenobiotic transport and metabolism
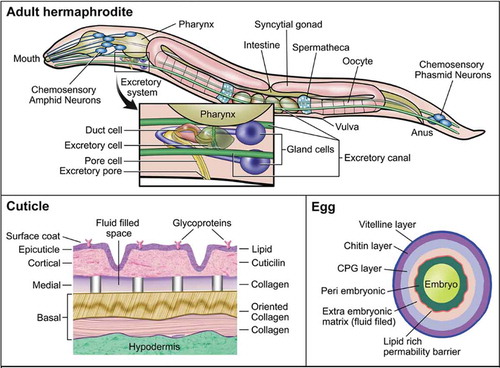
A simple cell membrane consists of a lipid bilayer composed primarily of phospholipids, glycolipids, and cholesterol. The phospholipids arrange in such a way that their hydrophobic tails are pointed inwards and their hydrophilic heads are oriented toward the outer and inner membrane surfaces. The membrane barrier is differentially permeable to various xenobiotics based upon their physicochemical properties (movement by transporters is addressed below). Some xenobiotics might passively diffuse across cell membranes; hydrophilic molecules might enter through aqueous pores in the membrane, and hydrophobic molecules might diffuse directly through the lipid domain of the membrane (Benz, Janko, and Läuger Citation1980). The smaller the molecule, the more rapidly it moves across a membrane, either by aqueous pores or simple diffusion. For large organic molecules, the octanol/water partition coefficient P dictates the rate at which the molecule moves across the membrane, with higher lipophilicity (positive log P) corresponding to higher membrane permeability, except at extreme levels of lipophilicity. In the case of weak organic acids and bases, ionic compounds move slowly and inefficiently through aqueous pores whereas non-ionized forms diffuse across the lipid membrane. Thus, the rate at which non-ionized organic acids and bases permeate the membrane depends on pKa/pKb of the compound and the pH of the surrounding environment (Avdeef Citation2001; Klaassen Citation2019; Manallack et al. Citation2013). However, C. elegans is not just a simple cell model system with a single membrane for protection; it is a complex organism with many physical barriers that prevent the passive transport of molecules into its body. In this section, the physical barriers that may impact uptake and release of xenobiotics by C. elegans are presented ().
The worm’s principle physical barrier is its cuticle (). The cuticle of the nematode provides structure to maintain body shape, along with protection from its environment (Johnstone Citation1994; Page Citation2007; Lints and Hall Citation2009). The cuticle consists of a complex matrix of collagen and serves as a physical barrier against chemicals and pathogens. The cuticle is well known for protecting the worm from uptake of xenobiotics, and is often viewed as a drawback to using C. elegans in high-throughput toxicity assays (Xiong, Pears, and Woollard Citation2017). Mutants with defective cuticle collagen proteins, of which there are more than 150, were employed to increase uptake in pharmacological screens (Burns et al. Citation2010; Johnstone Citation2000; Xiong, Pears, and Woollard Citation2017). In adult C. elegans, the cuticle is roughly 0.5 µm thick and contains 5 distinct zones: surface coat, epicuticle layer, cortical zone, medial zone, and basal zone (Cox, Kusch, and Edgar Citation1981a; Citation1981b). The width of the cuticle increases with the age of the worm as the basal zone expands, and in older worms it often wrinkles, potentially due to weakening of the muscles and hypodermis (Herndon et al. Citation2002). The cuticle might also be damaged from physical interactions with the environment and the process of mating (Woodruff et al. Citation2014). Each cuticle layer has a distinct composition, suggesting a high degree of specialization between layers (Johnstone Citation2000). The structure of the cuticle varies at each larval stage along with the composition of each of the layers (Lints and Hall Citation2009; Page, Citation2007). C. elegans undergoes molting periodically until adulthood. Molting occurs in two stages: lethargus, a period of relative behavioral dormancy and extracellular matrix remodeling in preparation for cuticle shedding, and ecdysis, when the cuticle is undergoing a molt cycle (Lažetić and Fay Citation2017; Singh and Sulston Citation1978). At each larval stage, C. elegans undergoes ecdysis, in which it sheds the current cuticle and forms a new one. The process of molting enables rapid growth and potentially depuration of any xenobiotic compounds on or within the current cuticle. The existence of varying cuticle structures throughout C. elegans development also suggests that critical windows of sensitivity to cuticular xenobiotic uptake exist. Dauer larvae, which are highly resistant to many stressors (Erkut et al. Citation2011; Fielenbach and Antebi Citation2008), possess thickened basal and epicuticle layers that might offer enhanced protection from their environment (Cassada and Russell Citation1975; Wolkow and Hall Citation2011). Early developmental exposures may result in more uptake of xenobiotics because of the difference in protein composition within the cuticle and thinness. Uptake might also be greater in older worms as the cuticle begins to wrinkle and weaken, although little research has been done to address alterations in uptake kinetics throughout the lifespan of C. elegans. It is also important to note that many of these physical processes and barriers have been extensively studied in C. elegans hermaphrodites but are rarely studied in males. Given the differences in size and morphology of male C. elegans, particularly around the tail region, it is possible that the different sexes might have altered uptake kinetics of xenobiotics, but this hypothesis has not apparently been addressed (Emmons Citation2005; Sulston, Albertson, and Thomson Citation1980).
Another important barrier is the eggshell, which in C. elegans begins to form as soon as the oocyte enters the spermatheca () and is fertilized (Stein and Golden Citation2015). The eggshell of C. elegans is the dominant physical barrier when the nematode is in utero and externally (after the egg is laid at approximately 30-cell stage), prior to hatching. The trilaminar outer eggshell is made of an outer vitelline layer, a chitin layer, and chondroitin proteoglycan (CPG) layer (Johnston and Dennis Citation2012; Olson et al. Citation2012). Beneath these layers resides the fluid-filled extra-embryonic matrix (EEM), a lipid-rich layer that acts as a permeability barrier to the developing embryo, and an amorphous space referred to as the peri-embryonic layer (Olson et al. Citation2012). Maternal environment and food supply impact the structure and size of the eggshells produced (Harvey and Orbidans Citation2011). Production of the egg might serve as a way to excrete xenobiotics, although no apparent research has been done to address this. Similarly, transfer of xenobiotics from mother to egg via other maternally-loaded components, such as vitellogenin is likely to occur. Vitellogenin contains both hydrophobic and hydrophilic domains and is an important vector for the transfer of xenobiotics to eggs in other species (Monteverdi and Di Giulio Citation2000). After egg laying occurs, the eggshell is exposed to the environment where it serves to protect the developing worm. Molecules that are able penetrate the various layers of the eggshell may be taken up by the developing worm.
C. elegans might also be exposed to xenobiotic compounds through ingestion. C. elegans feeds on microbes in the lab, either bacterial lawns or suspension in liquid cultures, through pharyngeal pumping. The pharynx is the neuromuscular pump that connects the mouth to the intestine and contracts and relaxes in order to take in bacteria and expel liquids (Avery and You Citation2012). The pharynx is lined with a specialized cuticle that helps to form structural elements of the pharynx such as the flaps, sieve, and the grinder, all of which are necessary for initial processing as well as transport of food to the intestine, and protection against diffusion of compounds in the pharynx (Page Citation2007; Altun and Hall Citation2009a). The pharyngeal cuticle and the pumping mechanism that expels liquids might serve as a protective barrier from xenobiotic uptake. However, xenobiotics that are bound to or taken up by bacterial food source would still be ingested by the worm. For chemicals that make their way into the intestine, uptake through epithelial cells lining the intestine might occur, as well as transport and trafficking to other locations in the organism. The intestine exhibits high expression of P-glycoproteins, involved in trafficking hydrophobic molecules, as well as cytochrome P450s, metallothioneins, and other phase I–III enzymes (McGhee Citation2013). It is important to note the availability and uptake of xenobiotics in the intestine varies by physicochemical properties and the chemical properties of the gut. As previously noted, membrane permeability to these compounds in the gut is determined by chemical structure, pKa, and pH of the environment (Casarett, Doull, and Klaassen Citation2008). Alterations in the C. elegans diet and microbiome also lead to changes in intestine pH or metabolites, which might alter the availability of certain compounds (Höss, Schlottmann, and Traunspurger Citation2011). Other cell types may also play specialized roles; for example, the six coelomocytes are macrophage-like cells that occupy the body cavity, are highly endocytotic, and may, similar to intestinal cells, play a “liver-like” role. Investigators demonstrated their importance in metal detoxification via endocytosis and metal transport and binding proteins (Maurer et al. Citation2016; Schwartz et al. Citation2010; Tang et al. Citation2020).
C. elegans possesses an excretory system comprising four main cell types. The excretory system includes one pore cell, one duct cell, one canal cell (excretory cell), and a fused pair of gland cells (Altun and Hall Citation2009b) (). The excretory system plays a role in osmoregulation and waste elimination, somewhat similar to the renal system in vertebrates (Sundaram and Buechner Citation2016). In the adult worm, the excretory canal cell extends from the head to the tail of the animal with the cell body located in the head region. The excretory canal cell is connected to the excretory gland cells, duct cell, and pore cell. The canal cell collects and guides fluids from within the worm toward the cell body for excretion through the duct and pore cells (Nelson, Albert, and Riddle Citation1983; Nelson and Riddle Citation1984; Stone, Hall, and Sundaram Citation2009). The duct cell and pore cell form a channel that opens to the environment, through which fluid might be excreted. Both the duct and pore cells possess a specialized cuticle that provides structure and protects them from the surrounding environment (Lints and Hall Citation2009; Sundaram and Buechner Citation2016). Nonetheless, the excretory system might also permit entry as well as exit for xenobiotics.
C. elegans also have a sophisticated chemosensory system that mediates detection and avoidance of noxious conditions. Chemosensation is vital to survival of C. elegans, enabling them to find food, mate, avoid harmful environments, and enter or exit the dauer stage (Bargmann Citation2006). Chemosensory neurons are found in the head and tail regions of the worm. There are 16 pairs of bilaterally symmetric neurons (approximately 10% of the total number of neurons, with neurons comprising approximately 1/3 of all somatic cells) that are involved with chemosensation (Bargmann Citation2006; Sengupta Citation2007). These neurons are bipolar, containing a single axon, a single dendrite, and cilia, which are exposed to the surrounding environment through small openings in the cuticle (Sengupta Citation2007; Ward et al. Citation1975; Ware et al. Citation1975). The ability of C. elegans to sense and respond to chemical stimuli enables them to avoid potentially toxic environments in the wild and protect themselves from exposures to xenobiotic compounds via chemotaxis. At the same time, the exposure of cilia to the external environment may also be a route of exposure for xenobiotics to infiltrate the neurons (Perkins et al. Citation1986).
Overall, physical and behavioral barriers play key roles in protecting C. elegans from xenobiotic uptake and accumulation, but knowledge of the manner by which most specific xenobiotics and even classes of xenobiotics cross these barriers and alter behavior is lacking.
C. elegans microbiome
The gut microbiome is a complex mixture of microorganisms (Koontz et al. Citation2019). Changes and alterations to the composition of the gut microbiota are associated with several diseases, including irritable bowel disease, obesity, type 2 diabetes, and neurodegenerative disorders in humans (Scotti et al. Citation2017). Recently, more research attempted to elucidate the complex interactions between host and the associated gut microbiome. This theme has also increased in toxicology research with many review papers emphasizing the importance and significance of understanding the role of the gut microbiome in toxicology (Claus, Guillou, and Ellero-Simatos Citation2016; Koontz et al. Citation2019; Koppel, Maini Rekdal, and Balskus Citation2017; Mesnage et al. Citation2018; National Academies of Sciences, Engineering, and Medicine Citation2018; Pryor et al. Citation2019).
Humans possess several distinct microbiomes, including the skin, respiratory, reproductive system, and gut (National Academies of Sciences, Engineering, and Medicine Citation2018). The gut microbiome is the most abundant (National Academies of Sciences, Engineering, and Medicine Citation2018; Sender, Fuchs, and Milo Citation2016). When chemicals enter the gut, bidirectional interactions occur: the chemicals may be transformed by microbiota present to be more or less toxic or effective, or chemicals might alter the composition of the microbiome (Koontz et al. Citation2019; National Academies of Sciences, Engineering, and Medicine Citation2018). Microbes within the gut primarily use hydrolytic and reductive reactions but are capable of utilizing a wide range of metabolic enzymes including azoreductases, nitroreductases, beta-glucuronidases, sulfatases, and beta-lyases (Koontz et al. Citation2019; Koppel, Maini Rekdal, and Balskus Citation2017) to metabolize or transform environmental pollutants such as some heavy metals, pesticides, polycyclic aromatic hydrocarbons, and polychlorinated biphenyls. Conversely, toxicant exposure might lead to dysbiosis by altering abundance and diversity of the microbiota present (Claus, Guillou, and Ellero-Simatos Citation2016; Tsiaoussis et al. Citation2019).
Investigating the human microbiota may be challenging and complex; model organisms provide the opportunity to simplify these studies. C. elegans is particularly useful for studying the microbiome because of the ease of creating germ-free progeny through bleaching ability to (1) grow and develop on a monoculture of bacteria, (2) grow worms on bacteria-free media, and (3) use mutant strains (Gerbaba, Green-Harrison, and Buret Citation2017; Zhang et al Citation2017). Although, as described above, C. elegans has been increasingly employed to study toxicological impacts of xenobiotics, most of these studies were performed without considering what role their gut microbiome might play (Dirksen et al. Citation2016). Further, most experiments to date were conducted by feeding only with standard strains of bacteria such as the common lab food source, Escherichia coli strain OP50. None of the commonly used lab food strains are found in nature; thus, these food sources presumably generate an artificial microbiome, although as discussed below, there is some limited evidence for non-food source bacteria colonizing the worm intestine in the lab.
A few studies examined gut microbiomes in C. elegans found in the wild. Zhang et al. (Citation2017) presented the first three papers that document non-lab microbiomes in C. elegans. The dominant taxa across varying substrates were Proteobacteria (approximately 80%) while the remaining approximately 20% were Bacteriodetes, Firmicutes, and Actinobacteria. These investigators also showed that the composition of the worm gut microbiome was distinct from and to a large extent independent of their environment (Zhang et al. Citation2017; Berg et al. Citation2016). The degree to which worm microbiomes vary by geography has yet to be determined. Lee et al. (Citation2020) examined the gut microbiome of N2 Bristol worms grown in lab conditions with OP50 and found that despite the lab culture conditions, these worms also contained a complex microbiome that consisted mainly of Proteobacteria, Firmicutes, and Actinobacteria (Stenotrophomonas, Bacillus, Microbacterium). Non-OP50 bacteria might be present because typical worm culture lab techniques are not sterile, such that other bacteria may be found and able to colonize the gut of the worm. However, this study contrasts with other reports that noted that when grown under lab conditions, the N2 strain with OP50 bacteria did not contain microbes other than OP50 within their gut (Dirksen et al. Citation2016). Clarifying this discrepancy, and the degree to which it depends upon lab culture conditions, is important, because certain bacterial strains influence worm development and reproduction (Dirksen et al. Citation2020, Citation2016).
The relationship of C. elegans with standard OP50 changes throughout aging. During development, bacteria consumed are destroyed in the worm’s grinder, leaving them inactivated in their intestine. However, during middle age, a small number of bacteria consumed pass through the grinder and colonize the intestine. As the worm ages, live bacteria found in the intestine begin to proliferate, eventually contributing to their death (Cabreiro and Gems Citation2013). Therefore, the microbiome may exert a greater effect on xenobiotic metabolism in older versus younger worms. Presumably, other bacteria might colonize the worm gut at earlier and later ages.
Tools for studying the worm microbiome
With advancing sequencing technology, analyzing the worm’s gut microbiome has become more rapid and cost-efficient. However, understanding how differences in diversity and abundance directly affect the worm’s health is more challenging. Recently, tools were developed that might help elucidate how microbes impact the health of C. elegans.
Dirksen et al. (Citation2020) created CeMbio, a kit of 12 bacterial strains that colonize the gut of worms in one native European environment. Worms may be grown on individual strains or on a strain community. When worms were grown on different individual strains, growth rates changed. Compared to OP50, nine of the strains initiated worms to develop faster, two strains significantly delayed growth, and one strain resulted in similar growth rates. Quantification of bacteria within the gut of worms might be accomplished by PCR, using strain-specific primers. Thus, this resource facilitates testing how exposures influence single or combined microbial populations, and the manner by which these microbiome changes affect host health.
Another tool, developed by Rutter et al. (Citation2019) permits an in vivo analysis of worm-bacterial-chemical interactions by using a bacterial food source engineered to serve as a sensor This sensor is a strain of E. coli carrying a plasmid encoding constitutively-expressed mCherry, plus GFP expressed upon exposure to isopropyl β-D-1-thiogalactopyranoside (IPTG). This enables simultaneous visualization of bacteria in the intestine via mCherry, and GFP fluorescence to detect response to IPTG. The ratio of GFP to mCherry therefore provides information both on the bacterial population present, and the response of the bacteria to chemical exposure.
The effect of specific bacterial genes on microbiome-chemical interactions might also be tested via the use of libraries of bacterial mutants. For example for E. coli K-12 there are 3985 mutant strains available (Baba et al. Citation2006); for E. coli OP50 (Govindan et al. Citation2015), approximately 2000; for Pseudomonas aeruginosa, approximately 4901 (Jacobs et al. Citation2003); and for Comamonas aquatica DA1877, approximately 5760 (Watson et al. Citation2014).
Finally, several methods have been described to reduce or eliminate the influence of bacteria on chemical toxicity. These include heat-killing bacteria (Powell and Ausubel Citation2008); inactivating bacteria with ultraviolet radiation, sometimes using a DNA-damage-deficient strain of bacteria (Meyer et al. Citation2010b); feeding standardized, lyophilized bacteria lysate (Garcia-Gonzalez et al. Citation2017); and culturing in axenic media, for which explicit toxicological protocols were provided (Nass and Hamza Citation2007; Sprando et al. Citation2009). It is important to note that each of these methods has its own caveats including in many cases reducing caloric intake and/or food quality (axenic media, heat-killed bacteria) or incomplete abrogation of biochemical metabolism (ultraviolet light inactivation).
Gut microbiome and toxicological studies using C. elegans
To date, relatively few studies explored how xenobiotics alter the C. elegans gut microbiome, or if transformation of xenobiotics occurs within the worm gut. (Garcia-Gonzalez et al. Citation2017) investigated how microbiota transformed drugs, and how this affected worm health, in order to better understand drug efficacy variability often seen in clinical studies. Other studies examined the manner in which the worm microbiome was aletered after exposure to environmental pollutants.
Cabreiro et al. (Citation2013) using standard lab strain OP50 found that metformin decreased microbial folate production and altered methionine synthesis, which led to an elevation in worm lifespan. However, if worms were grown on metformin-resistant bacteria, or no bacteria, metformin exerted no significant effect on folate production, and reduced lifespan, indicating toxicity. Moreover, worms grown with metformin and OP50 displayed a significant reduction in S-adenosyl methionine levels, which led to diminished in methionine biosynthesis. Since C. elegans' main source of methionine is the diet, a decrease in microbial folate and methionine biosynthesis led to a dietary deficiency in methionine. Cabreiro et al. (Citation2013) postulated that deficiency in methionine is the underlying mechanism causing metformin-mediated extended lifespan. Scott et al. (Citation2017) found that different strains of bacteria significantly influenced the minimum inhibitory range of 5 different fluoropyrimidines and that live bacteria were necessary to activate fluoropyrimidines. Supplementation with a vitamin B6 precursor, pyridoxal enhanced the efficacy of 5-FU in worms (Scott et al. Citation2017). The addition of pyridoxal to E. coli enhances ribonucleotide metabolism of 5-FU thereby increasing the efficacy to the host. Further, data demonstrated that exposure to metformin altered bacterial one-carbon metabolism which is believed to modulate fluoropyrimidines activation. If worms were given both drugs concurrently, activation of 5-FU was inhibited, resulting in a decreased efficacy. These findings show the importance of understanding how the host microbiome, diet, and drug exposure alter drug efficacy and impact host health.
Lee et al. (Citation2020) examined how environmental chemicals alter gut microbiome. OP50-fed worms were exposed to cadmium (Cd) and marked effects on gut microbial composition were noted. Without Cd exposure, Proteobacteria dominated the gut, followed by Firmicutes and Actinobacteria. After Cd exposure, Firmicutes dominated the microbiota. Firmicutes are postulated to be resistant to Cd, which enables them to proliferate within the gut once the Proteobacteria and Actinobacteria were reduced (Lee et al. Citation2020). However, when fed a more diverse population that was isolated from an organic farm, Cd exposure resulted in non-significant alterations in taxa by increasing the amount of Actinobacteria present. Lee et al. (Citation2020) demonstrated that worms that possessed a more diverse gut microbiome were more resistant to Cd exposure as evidenced by having a longer lifespan, more progeny, and fewer changes to gut microbial communities compared to worms fed OP50.
Arsenic (As) is biotransformed by microorganisms in the environment where reduction and methylation reactions are particularly important in altering transport and toxicity. In order to investigate how the microbiome might alter As speciation (redox status and methylation) and toxicity, Zhou et al. (Citation2020) used three different types of E. coli that either lacked the ability to reduce or methylate arsenic, or might reduce but not methylate As, or might both reduce and methylate As. Zhou et al. (Citation2020) found that bacteria biotransformed As, and that the worm responses (gene expression and reproduction) varied depending upon the strain of bacteria, supporting the importance of the microbiome in mediating As-induced toxicity.
Thus data demonstrated how C. elegans might be used as a powerful tool to elucidate the manner in which microbiota alter chemicals to which worms are exposed, as well as how environmental chemicals impact worm microbiota, potentially impacting worm physiology and stress response. It is noteworthy there are caveats associated with differences in C. elegans biology that need to be taken into account. For example, the above-mentioned lack of an adaptive immune system in C. elegans limits the ability to model mammalian mucosal-microbiota interactions, which when perturbed subsequently might alter xenobiotic metabolism, transport, and gut permeability.
Xenobiotic detoxification in C. elegans – Phase I
Phase I metabolic enzymes perform biotransformation of a parent organic compound to introduce or expose functional groups. This typically increases polarity and thus water solubility and reactivity, ultimately permitting conjugation of the compound (Ioannides Citation2001). Phase I reactions are broadly grouped into three categories: oxidation, reduction, and hydrolysis, with oxidation reactions being the most common and well-studied (Ioannides Citation2001). In C. elegans, all three types of phase I reactions are represented (Lindblom and Dodd Citation2006), and phase I enzymes are broadly expressed in somatic cells with some cell type specificity for particular isoforms likely driven by exogenous roles such as intestinal expression and endogenous roles such as hormone and/or neurotransmitter synthesis in neurons and other tissues.
Oxidative reactions are mainly carried out by the cytochrome P450 superfamily of monooxygenase enzymes, but also by flavin-containing monooxygenases, alcohol and aldehyde dehydrogenases, monoamine oxidase, and by peroxidases that perform co-oxidation (Ioannides Citation2001). These oxidation reactions are presumed to have evolved to increase water solubility and thus reduce toxicity from accumulation of chemicals in hydrophobic biological environments and structures. However, many oxidative reactions of benign hydrophobic substrates paradoxically result in formation of a toxic reactive metabolite (also termed ‘bioactivation’)(Guengerich Citation2006). A well-known example of this phenomenon is the pollutant benzo[a]pyrene (BaP), which is bioactivated by CYP1A/1B enzymes to a reactive epoxide metabolite that ultimately forms carcinogenic DNA adducts (Shiizaki, Kawanishi, and Yagi Citation2017).
Reduction reactions are carried out by cellular reductases and have not been well-characterized for their xenobiotic metabolizing activities, even in mammalian systems. In C. elegans, several genes have been predicted to exhibit reductase activity but have not been closely studied. Finally, hydrolytic metabolism also occurs by enzymes termed hydrolases. These enzymes cleave their substrates using hydrolysis at functional groups, commonly at esters, amides, and epoxides.
Cytochromes P450 in C. elegans
Cytochrome P450 enzymes carry out the majority of oxidative Phase I reactions and, as illustrated by the example of BaP, are responsible for the majority of bioactivation reactions (Guengerich Citation2008; McDonnell and Dang Citation2013). Cytochromes P450 are monooxygenases that consume one molecule of molecular oxygen and one molecule of NAD(P)H to add one oxygen atom to a substrate to release water and NADP+ as byproducts. Cytochromes P450 are localized in the endoplasmic reticulum, where they associate with the co-enzyme cytochrome P450 reductase (emb-8 in C. elegans) to carry out their reactions. In mammals, P450s also localize to the mitochondria where they associate with adrenodoxin/adrenodoxin reductase (Ahn and Yun. Citation2010; McDonnell and Dang Citation2013) (possibly Y73F8A.27 and Y62E10A.6 in C. elegans) although the presence of mitochondrial P450s in C. elegans is relatively understudied.
The C. elegans genome contains genes for at least 86 cytochromes P450 (compared to 60 in humans), and eight of those are predicted to be pseudogenes (Menzel, Bogaert, and Achazi Citation2001). Three genome-wide RNAi screens collectively identified roles for several of these P450 enzymes (). Perhaps the most well-studied P450 is daf-9, an orthologue of human CYP2S1 that is involved in the modification of hormones involved in dauer signaling (Jia, Albert, and Riddle Citation2002).
Table 1. C. elegans P450 genes with reported human orthologues, phenotypes, substrates, inhibitors, and/or expression patterns
Of the 86 cytochrome P450 genes in the C. elegans genome, many are induced upon exposure to xenobiotics (, ‘Inducers’), suggesting that at least some of the P450 isoforms encoded in the genome are xenobiotic metabolizing enzymes. For the vast majority of these enzymes, the evidence for induction was based upon mRNA levels quantified by microarray, RNA-seq, and/or qPCR or GFP signals in a strain expressing GFP under the control of the CYP promoter. Due to the lack of availability of antibodies for most C. elegans proteins, there have not to the authors’ knowledge been any studies where P450 induction was quantified on the protein level. This is potentially problematic, because some P450 isoforms are highly regulated post-transcriptionally and mRNA levels often do not correlate well with protein and activity (Chang et al. Citation2003; Song et al. Citation1986; Sumida et al. Citation1999). Further, Abbass et al (Citation2021) recently provided indirect evidence that C. elegans might metabolize the commonly studied polycyclic aromatic hydrocarbon benzo[a]pyrene to more-reactive forms, despite confirming previous phenotypic and sequence homology-based evidence (Harris et al. Citation2020; Leung et al. Citation2010) that this species lacks the family 1 P450s that canonically produce metabolites that generate bulky DNA adducts. This result highlights the need to study the catalytic activity of C. elegans xenobiotic transformation enzymes.
There are a few examples, however, of more direct measurement of P450 metabolism of xenobiotics in the literature (, ‘Substrates’). In those cases, the expected metabolite from P450 monooxygenase activity was detected and attributed to a particular isoform. In addition to those examples, P450 metabolites of phenacetin (metabolized by human CYP1A2, abbreviated hCYP1A2), diclofenac (hCYP2C9 substrate), amitriptyline (hCYP2C19/2D6 substrate), clomipramine (hCYP2C19 substrate), dextromethorphan (hCYP2D6/3A4 substrate), and nifedipine (hCYP3A4 substrate) were detected in C. elegans but not attributed to a particular isoform (Harlow et al. Citation2018). Unfortunately, none of those studies compared metabolic activity with and without an inducer, such that the inducibility of C. elegans P450 enzyme activity is unclear. In addition, it is interesting that in the case of tolbutamide metabolism, the isoforms identified by RNAi to be metabolizers of tolbutamide were not the closest isoforms identified by sequence homology to the human tolbutamide hydroxylases (CYP2C8/9/19)(Harlow et al. Citation2018). This observation is an illustrative example of how sequence homology is a poor predictor of substrate specificity for P450 enzymes, and is supported by the observations that although several P450 isoforms have sequence homology to CYP2E1 (), one is not able to detect any CYP2E1-like activity for multiple substrates in C. elegans (unpublished observations, JHH and JNM).
Cytochrome P450 enzymes require a heme cofactor (bound to iron) and a coenzyme (cytochrome P450 reductase) to carry out their reactions (McDonnell and Dang Citation2013). Unlike humans, C. elegans cannot synthesize heme, and need to scavenge this component from their diet (bacteria) to incorporate it into newly synthesized cytochrome P450 polypeptides (Sinclair and Hamza Citation2015). C. elegans have a suite of specialized enzymes that deliver environmental heme into the intestine and into other tissues (Chen et al. Citation2012). Heme availability during xenobiotic challenge might limit the xenobiotic response, but this hypothesis has not been explored experimentally. Cytochrome P450 enzymes also require co-expression of cytochrome P450 reductase, also termed P450 oxidoreductase (POR), which is encoded by emb-8 gene in worms. The emb-8 protein is expressed in highest levels in intestine, neurons, and pharynx and is also expressed in coelomocytes, hypodermis, and germline (essentially the same tissues where P450 enzymes are expressed). Loss of emb-8 produces temperature-sensitive embryonic lethality (Miwa et al. Citation1980), likely due to its role in embryonic polarization (Rappleye et al. Citation2003).
Finally, longevity associated with reduced mitochondrial function in C. elegans requires induction of cytochrome P450 enzymes through Kruppel-like factor 1, suggesting that protection from endobiotic and/or xenobiotic insults is part of the longevity mechanism (Herholz et al. Citation2019). This hypothesis fits with data from other species illustrating upregulation of xenobiotic detoxification programs in long-lived mutants. An open question is which endo- and xenobiotic compounds underlie this longevity phenotype.
Flavin Monooxygenases
Similar to humans, C. elegans possess 5 identified flavin monooxgenase (FMO) genes (fmo-1 to fmo-5). FMO enzymes consume oxygen and NADPH to incorporate a single oxygen atom into substrates, usually soft nucleophiles at nitrogen or sulfur atoms. C. elegans FMO enzymes are expressed mainly in two patterns: fmo-1, −2, and −5 in intestinal cells and excretory gland cells, and fmo-3 and −4 in the hypodermis (Petalcorin et al. Citation2005). In addition, expression was reported in duct cells (fmo-4), pore cells (fmo-4), body-wall muscle (fmo-3,), germline (fmo-4), hypodermal cells (fmo-3, −4), pharyngeal muscle (fmo-1), ventral nerve cord (fmo-4, −5) and neurons (fmo-2, −3, −4, −5).
Similar to cytochromes P450, FMOs contain both endogenous and exogenous substrates and exhibit functions. Nematodes lacking fmo-1, −4, and −5 induce neurodevelopmental defects (Gujar, Stricker, and Lundquist Citation2017). Further, animals deficient in fmo-4 are extremely sensitive to hypoosmotic stress (Hirani et al. Citation2016; Petalcorin et al. Citation2005). C. elegans fmo-4 is orthologous to human FMO4, and both contain similar protein structures. However, human FMO4 was not able to rescue hypoosmotic stress sensitivity phenotype in the fmo-4 deletion strain (Hirani et al. Citation2016). C. elegans fmo-2 is upregulated by starvation (Goh et al. Citation2018) and dietary restriction (Leiser et al. Citation2015), is involved in dietary restriction-induced lifespan extension, and might extend lifespan when overexpressed (Leiser et al. Citation2015). In addition, both oxidative stress (Goh et al. Citation2018) and stress from infection with Pseudomonas aeruginosa (PA14)(Dasgupta et al. Citation2020) or Staphylococcus aureus (Wani et al. Citation2020) induce fmo-2, which is required for pathogen resistance (Wani et al. Citation2020). Further, both fmo-2 and fmo-4 are upregulated by hypoxia (Shen et al. Citation2005).
In terms of catalytic activity, C. elegans FMOs (fmo-1 and −4) are functional for S-oxidation of the prototypical FMO substrate methimazole when expressed recombinantly in insect cells. The overall specific activities (per mg microsomal protein) were approximately 10-fold lower than human enzymes expressed in the same background. However, lack of antibodies for C. elegans FMOs precluded estimation of protein expression efficiency in each case (Hirani et al. Citation2016). To date, this study is the only one that has functionally characterized C. elegans FMO activities, and further studies are needed to determine endogenous and xenobiotic substrates of each isoform.
Alcohol and Aldehyde Dehydrogenases
C. elegans possesses two alcohol dehydrogenases, sodh-1 and sodh-2. Both isoforms are widely expressed in the worm including in neurons, muscle, hypodermis, germline, and intestine. In biochemical assays, alcohol dehydrogenase activity appeared as a single band on a polyacrylamide gel. However, the two isoforms are close in sequence and size (349 vs. 351 amino acids for sodh-1 and sodh-2, respectively) and thus may be expected to co-migrate on a gel (Williamson, Long, and Theodoris Citation1991). C. elegans ADH activity is higher for longer-chain alcohols (propanol and butanol) compared to ethanol, and similar to the human enzyme, the worm has a preference for primary over secondary alcohols (Williamson, Long, and Theodoris Citation1991).
C. elegans contains several aldehyde dehydrogenase genes (alh-1 through alh-13), most of which have not been functionally characterized and do not have an observable knockdown phenotype (Alaimo et al. Citation2012). Using gas chromatographic methods to measure internal ethanol accumulation, Alaimo et al. (Citation2012) indicated that alh-6 and alh-13 metabolize the ethanol metabolite acetaldehyde to acetic acid.
Monoamine Oxidases
Monoamine neurotransmitters such as dopamine and serotonin are degraded in humans by monoamine oxidases MAO-A and MAO-B. These enzymes also act on xenobiotics that are structurally similar to their natural substrates. C. elegans has a single ortholog of these genes, amx-2 (Schmid et al. Citation2015), which was demonstrated to metabolize serotonin in the worm (Wang et al. Citation2017) and presumably would have overlapping specificity with MAO-metabolized xenobiotics identified in mammalian systems.
Hydrolases
Hydrolysis reactions use water to break a chemical bond. Enzymes that perform hydrolysis reactions are referred to as hydrolases. Xenobiotic-metabolizing hydrolases include esterases, amidases, and epoxide hydrolases. Of those, only epoxide hydrolases were studied in detail in C. elegans. Although humans express four epoxide hydrolase isoforms including both membrane-bound (microsomal) and soluble (cytosolic) forms, C. elegans possesses only two isoforms, ceeh-1 and ceeh-2 (Harris et al. Citation2008). These are most orthologous with the EH3 and EH4 human isoforms, which are the most recently discovered and least well characterized among human isoforms but are postulated to predominantly metabolize lipids. The C. elegans enzymes were confirmed to exhibit endobiotic and xenobiotic metabolizing activities, with ceeh-1 displaying higher activity toward substrates compared to ceeh-2 (Harris et al. Citation2008). Further studies are needed to establish the substrate specificity of both isoforms, particularly for xenobiotic substrates.
Phase II in C. elegans
Phase II reactions involve conjugation of a substrate with a large, water-soluble group, facilitating excretion. There are 4 major families of phase II enzymes: UDP-glucuronosyltransferases (UGTs), sulfotransferases (SULTs), glutathione S-transferases (GSTs), and N-acetyltransferases (NATs). Except in rare cases, phase II reactions produce safe and easily excreted metabolites, and therefore this phase of metabolism mainly impacts toxicity by facilitating clearance of the compound. Mutations in phase II enzymes in humans result in various phenotypes, including hyperbilirubinemia from mutations in the UGT1 family and elevated risk of cancers from mutations in various UGT and GST isozymes. In C. elegans, Phase II enzymes, similar to phase I, are expressed broadly in somatic cells and enriched in intestine, neurons, and other specialized cells depending upon isoform.
UDP-glucuronosyltransferases (UGTs) are endoplasmic reticulum-localized enzymes that catalyze the conjugation of pollutants, drugs, and endogenous compounds to a sugar group, glucuronic acid. The site of metabolism for UGTs is usually at hydroxyl, carboxyl, or amine functional groups. Individual UGT isoforms are classified into families based upon sequence identity, and substrate specificity for individual UGTs tends to be broad compared to Phase I enzymes (Burchell et al. Citation1998). Mammals express 22 UGTs from 4 families: UGT1, UGT2, UGT3, and UGT8 (Meech et al. Citation2019). In contrast, C. elegans possess 66 UGT genes (named ugt-1 through ugt-66) which have all been classified into families UGT1, UGT2, UGT3, and UGT8 based upon sequence homology, and most of the C. elegans UGT genes are each homologous to multiple human isoforms. Knockouts of individual UGT isoforms did not produce phenotypes other than drug hypersensitivity in some cases (Cui et al. Citation2007; Fontaine and Choe Citation2018).
A few studies reported induction of UGT after chemical exposures. Hasegawa et al. (Citation2010) found that allyl isothiocyanate exposure induces expression of ugt-13 (ortholog of human UGT1A1/2B28/2B7). Di, Zhang, and Lawton (Citation2018) noted that the mycotoxin deoxynivalenol induced ugt-26 (ortholog of human UGT1A4/1A6/2B10) and ugt-28 (ortholog of human UGT1A10/1A6/1A8). The insecticide albendazole also stimulated expression of ugt-16 (ortholog of human UGT3A1/3A2)(Laing et al. Citation2010), ugt-22 (ortholog of human UGT1A5/1A6/2B17) and ugt-63 (ortholog of human UGT1A10/1A5/1A8)(Fontaine and Choe Citation2018). The latter study found that ugt-22 upregulation was downstream of the transcription factor skn-1 and noted that mutation of ugt-22 markedly protected against albendazole-mediated toxicity. The chemical acrylamide was also reported to induce several UGT isoforms in C. elegans (Hasegawa et al. Citation2007). The chemical inducers fluoranthene and β-naphthoflavone also stimulated several UGT isoforms including ugt-13 and ugt-63, as well as other phase I and II xenobiotic metabolizing enzymes (Taubert et al. Citation2008). Notably, induction was only detected at the transcriptional level, likely due in part to lack of validated antibodies against C. elegans UGT proteins. Further, biochemical activities of UGT enzymes have not been directly measured in C. elegans to date.
C. elegans exposed to the insecticide albendazole also produced conjugated albendazole-glucose metabolites, which Laing et al. (Citation2010) suggested may have been formed by UGT enzymes utilizing UDP-glucose as a substrate. The same glucose-conjugated albendazole metabolites were also detected in the helminth H. contortus (Cvilink et al. Citation2008). Further, glycosylated metabolites of the bacterial toxins 1-hydroxyphenazine and indole were also reported in C. elegans (Stupp et al. Citation2013). Taken together, these studies provide support to the postulation that UDP-glucose may be utilized by invertebrate UGT enzymes, although this substrate is uncommon in mammals. A similar concept was previously suggested based upon sequence analysis, that is, some C. elegans isoforms that were identified as UGTs may be more correctly termed glycosyltransferases that catalyze the transfer of galactose, glucose, or glucuronic acid (Kapitonov and Yu Citation1999).
Sulfotransferase enzymes (SULTs) catalyze the conjugation of a sulfonate group from a donor molecule, typically 3-phosphoadenosine-5ʹ-phosphosulfate or PAPS, to a substrate (at a hydroxyl or amino functional group). SULTs might either be membrane-bound in the Golgi apparatus, where they metabolize endogenous molecules, or soluble in the cytosol, where they metabolize xenobiotic and endogenous substrates. The human genome encodes up to 14 SULTs, while the C. elegans genome encodes a single cytosolic sulfotransferase enzyme, ssu-1 (also termed Y113G7A.11 and ceST1)(Hattori et al. Citation2006). There is no apparent knockdown phenotype, although it is a suppressor of unc-1 and unc-24 phenotypes (thus the naming ssu, or suppressor of stomatin mutant uncoordination)(Carroll et al. Citation2006). Its enzymatic properties were investigated biochemically by recombinant protein expression, and the C. elegans SULT enzyme sulfonated prototypical hydroxylated substrates including 4-nitrophenol and 2-naphthol as well as bisphenol A but did not metabolize monoamines or hydroxysteroids. Hattori et al. (Citation2006). created antibodies against the recombinant protein and were able to detect expression in cytosolic fractions of C. elegans lysate and induction by the substrate isophenylpropanol. The mRNA is also highly induced in dauer larvae, indicating a potential role for sulfonation in dauer signaling (Hattori et al. Citation2006).
Glutathione S-transferases (GSTs) conjugate glutathione to a wide variety of endogenous and xenobiotic substrates and are divided into two types: membrane-bound and soluble family members. Membrane-bound GSTs are found in the endoplasmic reticulum and mitochondria and form homo- and hetero-trimers with a single active site, and typically metabolize endogenous leukotrienes and prostaglandins. Human cytosolic GSTs (11 total) are expressed at high levels, are highly polymorphic, and classified into 6 groups: α, µ, ω, π, ς, θ, and ζ. The C. elegans genome contains 56 GST genes with most categorized within the ς family, which are the most abundant and conserved within insects. However, there are also GSTs in the C. elegans genome classified in the α, π, ζ, and θ subfamilies.
Induction of GSTs by xenobiotics was reported. Wu et al. (Citation2015) noted that BaP exposure resulted in induction of SKN-1 as well as gst-24 (homolog of human GST ς enzyme hematopoietic prostaglandin D synthase or HPGDS). The toxin indole up-regulated gst-5, gst-6, and gst-33 (all orthologous to human HPGDS)(Lee et al. Citation2017), and the natural compound ursolic acid upregulated gst-7 (also orthologous to human HPGDS)(Negi et al. Citation2017). Interestingly, exogenous heme induced expression of gst-19, gst-7, and gst-5, and these isoforms were proposed to play an endogenous role in heme trafficking (Perally et al. Citation2008).
Humans have two N-acetyltransferase enzymes that are xenobiotic-metabolizing, NAT1 and NAT2. To date, no N-acetyltransferase genes in C. elegans that are homologous to the human xenobiotic-metabolizing NATs have been identified. In addition to the 4 major families of phase II enzymes described here, it is also possible to conjugate xenobiotics to other cellular substrates including amino acids, but these reactions are less studied and less common.
Phase III in C. elegans
Phase III of xenobiotic metabolism is the export of xenobiotics and metabolites out of the cell, typically via transporters, processes that are especially critical for those xenobiotics whose physicochemical properties make them unable to pass the cell membrane itself (Kell Citation2020a; Xu, Li, and Kong Citation2005). Transporters vary vastly in mechanism, function, substrate, and sequence, and are classified largely along those lines into the families of ATP-binding cassette (ABC) transporters, solute carriers (SLCs), pumps, ion channels, and water channels or aquaporins (Hediger et al. Citation2013). Mutations in each of these transporter families were associated with altered individual sensitivity to xenobiotic or drug exposures and various diseases (DeGorter et al. Citation2012).
ATP-binding cassette (ABC) transporters comprise a broad family of transmembrane proteins defined by their two nucleotide binding domains and two transmembrane domains. Serving as both importers and exporters in prokaryotes and predominantly exporters in eukaryotes, ABC transporters bind and hydrolyze ATP to move substrates across the membrane (Rees, Johnson, and Lewinson Citation2009). ABC transporters have been implicated in drug resistance, including to chemotherapeutic drugs, efflux of many xenobiotics including phase I and especially phase II metabolites, as well as endogenous processes such as lipid and cholesterol transport, pathogen response, and mitochondrial iron homeostasis (DeGorter et al. Citation2012; Glavinas et al. Citation2004; Jin et al. Citation2012; Klaassen Citation2019; Rees, Johnson, and Lewinson Citation2009).
With 60 sequences encoding ABC transporters, compared to only 49 in humans, these are the most prevalent family of transporters in C. elegans representing 0.3% of the N2 reference gene content (Sheps et al. Citation2004). Of the 60 identified genes, 15 encode full P-glycoproteins (PGPs), 8 encode PGP half-molecules, 8 encode other multidrug resistance proteins (MRPs), 7 encode members of the abt subfamily, and 1 encodes a cystic fibrosis transporter homolog. The remaining 21 were classified according to HUGO nomenclature into groups D-H but lack a corresponding subfamily name in C. elegans and are not discussed in depth. Eight are apparently not expressed, and one may be a pseudogene (Sheps et al. Citation2004). Though 16 ABC transporter encoding genes are found in tandem duplications, promoter linked fluorescent protein detection revealed differential expression patterns for 15 of the 16 duplicates, indicating they have different roles (Zhao et al. Citation2004). Notably, these patterns can overlap. Although PGPs are expressed in various cell types, many are expressed more or solely in intestinal cells, highlighting the intestine as a critical detoxification organ (Lincke et al. Citation1993).
In examining orthology to human ABC transporters, only eight were orthologous (Sheps et al. Citation2004). With such low conservation, it seems logical that those transporters that are conserved play critical roles in biological functions. Thus, unsurprisingly, two are mitochondrial half transporters that support iron homeostasis, and subfamilies E and F, with the highest conservation between humans, Saccharomyces cerevisiae, and Drosophila melanogaster, play roles in formation of ribosome associated proteins (subfamily F) and RNase L inhibition (subfamily E) (Sheps et al. Citation2004).
Knockouts of several of these ABC transporters were examined, and led to increased sensitivity to heavy metals, xenobiotics, and bacterial toxins (Broeks et al. Citation1996, Citation1995; Mahajan-Miklos et al. Citation1999; Peng et al. Citation2018). Deletion of pgp-3 enhanced sensitivity to colchicine and chloroquine, and simultaneous knockout of pgp-1 and pgp-3 elevated sensitivity to bacterial toxins, Cd, and arsenite (Broeks et al. Citation1996, Citation1995; Mahajan-Miklos et al. Citation1999). pgp-13 inhibition by RNAi showed that it played a significant role in resistance to imidazolium-based ionic liquids with a suspected similar role for pgp-14 (Peng et al. Citation2018). pgp-12 knockouts are more susceptible to phenazine 14 exposure (Stupp et al. Citation2013). pgp-1 and mrp-1 were both upregulated in ivermectin resistant C. elegans, with greater PGP upregulation at higher resistance levels (James and Davey Citation2009). pgp-12 and pgp-13 are upregulated in ivermectin resistance, with pgp-12 silencing conferring sensitivity (Figueiredo et al. Citation2018). mrp-5 is implicated in embryonic vitamin B12 transport from mother to offspring (Na et al. Citation2018). pgp-5 plays roles in both heavy metal and bacterial infection resistance (Kurz et al. Citation2007). ced-7 promotes the engulfment of apoptotic cells and redistribution of phosphatidylserine (Hamon et al. Citation2000). pgp-2 contributes to lysosome formation and lipid storage within the intestine, as well as communication between intestine and AWA neurons.
SLCs are a family of more than 300 membrane-bound, ATP-independent transporter proteins, including vesicular and mitochondrial transporters, passive transporters, coupled transporters, and exchangers (Hediger et al. Citation2013). Their role in xenobiotic transport is increasingly recognized in humans, with a particular focus in their impact on drug pharmacokinetics (Colas, Man-Un Ung, and Schlessinger Citation2016; Kell Citation2020b). SLCs are responsible for the membrane transport of metformin, antineoplastics such as tyrosine-kinase inhibitors, and angiotensin converting enzyme inhibitors such as captopril and quinaprilat, and might also play a major role in xenobiotic transport (DeGorter et al. Citation2012).
Interestingly, of 17 investigated species across 4 basal eukaryotic branches, C. elegans contained the largest number of conserved SLC families compared to H. sapiens, with 43 of 46 total present (Höglund et al. Citation2011). In the same analysis, 31 SLCs were identified in the C. elegans genome that could not be classified into known human families, highlighting the lack of understanding of the divergent evolution of these key proteins (Höglund et al. Citation2011). In total, the C. elegans genome encodes 348 SLCs, in comparison to 400 in the human genome (Höglund et al. Citation2011).
Functionally, SLCs are understudied in humans and even more so in nematodes (César-Razquin et al. Citation2015; Kell Citation2020a). It is established that SLC subfamilies 8 and 24 (sodium/calcium exchangers), 14 and 4 (chloride transporters), 17 (vesicular transporters), 1, 5, and 6 (neurotransmitter reuptake) play key roles in neuronal function, but these are not well-characterized functionally in worms. The SLC17 subfamily in particular has 51 members in C. elegans, a large expansion compared to the meager nine in humans, but few have been characterized (Hobert Citation2005). SLC17 member eat-4 is involved in glutamatergic neurotransmission, and more recently that vglu-2 may play a role in collagen trafficking in the cuticle (Serrano-Saiz et al. Citation2013, Citation2020). MISC-1, a mitochondrial solute carrier that plays a role in apoptosis (Gallo et al. Citation2011). Slc17.9, a.k.a., droe-5, is upregulated upon dietary restriction (Ludewig, Klapper, and Döring Citation2014). hut-1 maintains endoplasmic reticulum homeostasis and is essential for larval development (Dejima et al. Citation2009). snf-5 assists in sensing in neurons and transport in intestinal cells of L-aspartate and L-glutamate (Metzler et al. Citation2013).
The flux of xenobiotics via SLCs and ABCs, in addition to transporters that are less understood or could not be addressed, often serves as the final step of xenobiotic metabolism prior to excretion from the organism. Though this step is increasingly examined, many transporters remain orphan and uncharacterized, providing ample opportunity for future exploration. Similarly as for phase I and II processes, cell-specific expression of genes and isoforms is also in need of better description.
Transcriptional regulation of stress responses in C. elegans
Given the paramount role of phase I–III detoxification genes, it is not surprising that their transcriptional regulation is subject to myriad of inputs, not all of which are extensively discussed herein. Specifically, the regulation of such genes by metals and metalloids including Cd, iron, zinc, copper or selenium, metal-containing nanoparticles, exposure to reactive oxygen species (ROS) initiating oxidative stress, mitochondrial dysfunction, genetically or pharmacologically induced longevity, advanced glycation end products such as α-dicarbonyls, and/or various diets or dietary components were described elsewhere and are not covered in detail herein (Blackwell et al. Citation2015; Chaudhuri et al. Citation2018; Ferguson and Bridge Citation2019; Gonzalez-Moragas et al. Citation2017b; Hoffmann and Partridge Citation2015; Olsen and Gill Citation2017; Shore and Ruvkun Citation2013; Tejeda-Benitez and Olivero-Verbel Citation2016). The focus of this review is primarily regulation of phase I, II, and III detoxification genes by organic xenobiotic compounds including antihelminthic drugs and compounds used for crop control.
Evolutionary conservation and diversification of transcriptional regulators mediating xenobiotic detoxification
Transcription factors (TFs) and regulatory mechanisms that orchestrate the coordinated induction of a tailor-made detoxification response when an animal encounters a xenobiotic compound are fairly well conserved throughout evolution. Key regulators that control the induction of detoxification genes across species include nuclear hormone receptors (NHRs), the aryl hydrocarbon receptor (AHR; ahr-1 in C. elegans), and the basic leucine zipper (bZIP) protein nuclear factor erythroid 2-related factor 2 (NRF2; skn-1 in C. elegans) (Blackwell et al. Citation2015; Brinkmann et al. Citation2019; Gracida and Eckmann Citation2013; Hoffmann and Partridge Citation2015; Lindblom and Dodd Citation2006; Mackowiak and Wang Citation2016; Tonelli, Chio, and Tuveson Citation2018). In addition, the GATA-type TF ELT-2 is required to express most C. elegans intestinal genes, which includes many phase I, II, and III detoxification genes (McGhee Citation2013); as such, ELT-2 may play a general, permissive role in the expression of many detoxification gene programs. Recently, Herholz et al. (Citation2019) suggested new roles for additional TFs, such as KLF-1, although these are less well understood, especially in their relevance to defense against acute xenobiotic stress. Below, the current understanding of the roles of these regulators, highlighting emerging mechanistic insights as well as conserved and non-conserved functions is described
C. elegans NHRs are involved in xenobiotic detoxification
Based upon their overall architecture, NHRs are grouped into the NR1-NR6 classes (Nuclear Receptors Nomenclature, Committee Citation1999; Weikum, Liu, and Ortlund Citation2018). The NHRs most prominently involved in detoxification gene regulation belong to the NR1J groups in C. elegans and Drosophila melanogaster and to the NR1I and H classes in mammals. The latter group includes several NHRs with important roles in detoxification such as: the pregnane X receptor (PXR; also known as the steroid and xenobiotic sensing nuclear receptor, SXR, NR1I2); constitutive androstane receptor (CAR, NR1I3); liver X receptor (LXR, NR1H3), farnesoid X receptor (FXR, NR1H4); and vitamin D receptor (VDR, NR1I1) (Hoffmann and Partridge Citation2015; Mackowiak and Wang Citation2016; Oladimeji and Chen Citation2018). However, other NHRs also regulate detoxification genes in various situations, including the peroxisome proliferator-activated receptors (PPARs) and the Hepatocyte Nuclear Factor 4 (HNF4) type NHRs (Wallace and Redinbo Citation2013). The latter are especially notable as the C. elegans N2 reference genome features a large group of approximately 265 NHRs that appear to have descended and diversified from an HNF4-like ancestor (Taubert, Ward, and Yamamoto Citation2011). Most of these remain uncharacterized, but recent studies implicated several as putative xenobiotic response regulating NHRs.
Side note on endocrine disruption in C. elegans: lack of evidence for conserved receptors at present time
Although vertebrate endocrine-related receptors are not generally thought of as major regulators of xenobiotic transport and metabolism, a digression on the potential for C. elegans to be used as a model organism for endocrine disruption is warranted. Endocrine disruptors are molecules that interfere with an organism’s intrinsic endocrine systems, which often act by targeting NHRs that regulate endogenous endocrine signals such as estrogen and mammalian NHR estrogen receptor. As such, endocrine disruptors have the potential to disturb the normal physiology and development of an organism. Endocrine disruption is a major concern in environmental toxicology with great relevance for human and wildlife health (Hotchkiss et al. Citation2008; National Academies of Sciences, Engineering, and Medicine Citation2017). There are some investigations reporting results of using C. elegans to study endocrine disruption, and clearly chemicals that are agonists of vertebrate endocrine receptors exert effects in C. elegans (Cao et al. Citation2020; Chen et al. Citation2019; Custodia et al. Citation2001; Fischer et al. Citation2012; Jeong, Kim, and Choi Citation2019; Mimoto et al. Citation2007). However, it needs to be emphasized that conclusions regarding the mechanism by which these effects are mediated be interpreted with great caution. It is far from clear that responses result from presumed receptor agonist or antagonist binding to a worm homolog of the vertebrate receptor. As noted above, sequence comparisons suggest that the NR1I group of classical detoxification NHRs is apparently absent in C. elegans. In addition, the families encoding the classical mammalian steroid/thyroid receptors: NR1A thyroid hormone receptors (TRs), NR3A estrogen receptors (ERs), and NR3C 3-ketosteroid receptors including glucocorticoid receptor (GR), mineralocorticoid receptor (MR), progesterone receptor (PR), and androgen receptor (AR) are also absent (Nuclear Receptors Nomenclature, Committee Citation1999; Taubert, Ward, and Yamamoto Citation2011; Weikum, Liu, and Ortlund Citation2018). Accordingly, to our knowledge, no broad evidence base supports the concept that any C. elegans NHR is (in)activated by (ant)agonists of any of these vertebrate NHR, which would render it and the nematode susceptible to endocrine disruption by the presumed pathway, such as by a xenoestrogens acting on a worm “estrogen receptor.” Therefore, although it is not impossible that functional homologs of vertebrate endocrine receptors exist, one can argue that the burden of proof is on demonstrating such functional homology, which needs to be tested rigorously. On the other hand, it is entirely possible that C. elegans may serve as a useful model for the influence of NHR-activating xenobiotics in invertebrates (Hoss and Weltje Citation2007). Although evidence for such events is currently also lacking, one cannot rule out that endocrine disruption of DAF-12 driven developmental pathways may occur.
C. elegans NR1J group NHRs
DAF-12
In mammals, the NR1I group NHRs (PXR, CAR, VDR) play central roles in detoxification. The apparent absence of NR1I-type NHRs in the C. elegans genome thus suggests that other, perhaps closely related NHRs, might have adopted these roles. The closest C. elegans homologs to NR1I class NHRs are the NR1J group NHRs: DAF-12, NHR-8, and NHR-48. Although little is known regarding NHR-48, the other two have been widely studied for their functions in development and physiology.
DAF-12 is perhaps the best understood NHR in C. elegans, with important roles in development, aging, and metabolism. To date evidence for a direct link between DAF-12 and the detoxification of organic xenobiotics is lacking. This is curious because (i) some detoxification genes, including cyp, gst, and ugt genes, are down-regulated in long-lived daf-12 mutants; (ii) the CYP450, DAF-9, is a key enzyme in the synthesis of the dafachronic acids (DA), a group of molecules that serve as DAF-12 ligands and modulate its activity; and (iii) reduced DA signaling increases the resistance of C. elegans to various types of stress (Fisher and Lithgow Citation2006; Gerisch et al. Citation2001; Hoffmann and Partridge Citation2015; Motola et al. Citation2006). Possibly, the pleiotropic roles of DAF-12 in aging and development accompanied by lack of experiments directly assessing consequences of its loss on xenobiotic sensitivity and induced gene regulation have obscured a role in systemic detoxification for this NHR.
NHR-8
In contrast to DAF-12, NHR-8 has emerged as one of the most important regulators of xenobiotic detoxification in C. elegans, as it controls gene activation in response to xenobiotic exposure and is required for organismal resistance to some compounds. Lindblom, Pierce, and Sluder (Citation2001) first showed that nhr-8 mutation or RNAi rendered worms sensitive to the toxins colchicine and chloroquine. Menez et al. (Citation2019) demonstrated that nhr-8 mutants displayed hypersensitivity to the anthelmintic ivermectin, with nhr-8 silencing in ivermectin-resistant worms enhancing drug efficacy. At the gene regulation level, nhr-8 mutant worms exhibited reduced expression of several phase I, II, and III detoxification genes, including pgp and cyp genes known to impact ivermectin tolerance in C. elegans, with correspondingly diminished ABC transporter-mediated drug efflux activity (Menez et al. Citation2019). Importantly, re-expression of the ABC transporter pgp-6 in nhr-8 mutant worms elevated tolerance to ivermectin, implicating NHR-8 control of ABC drug efflux transporters as a likely mechanism for drug resistance. Support for this model is provided by Guerrero et al. (https://www.biorxiv.org/content/10.1101/823302v1.full), who noted that induction of three pgp phase III detoxification genes by glycosylation inhibitor tunicamycin required nhr-8; that nhr-8 loss-mediated acute tunicamycin sensitivity while NHR-8 overexpression produced tunicamycin resistance; and that chemical inhibition of pgp glycoproteins suppressed tunicamycin resistance. Collectively, evidence suggests that NHR-8 is essential to induce many phase I, II, and III detoxification genes in worms exposed to various toxins, perhaps especially phase III drug efflux transporters. Notably, the role of NHR-8 is xenobiotic-specific, as Lindblom, Pierce, and Sluder (Citation2001) found that loss of nhr-8 rendered worms sensitive to only some, but not all tested xenobiotics. Indeed, NHR-8 likely possesses redundant functions with other transcriptional regulators in regulating xenobiotic detoxification genes, as other studies showed that NHR-8 depletion alone did not broadly abrogate the expression of select phase I and II genes (Chamoli et al. Citation2014; Jones et al. Citation2013). Rather, Chamoli et al. (Citation2014) reported that PHA-4/FoxA, NHR-8, and AHR-1 cooperatively induce various cyp and ugt genes, albeit in a long-lived mutant background, rather than in acute exposure to xenobiotic compounds.
Although clearly important for the response to various xenobiotics, the mechanisms of NHR-8 activation by such compounds is not clear. Chloroquine, colchicine, and several sterol-derived small molecules that bind mammalian LXR failed to activate NHR-8 in a ligand-sensor screen (Magner et al. Citation2013); thus, evidence that NHR-8 may act analogously to PXR and CAR as a xenobiotic sensor remains lacking. An alternative mechanism of NHR-8 activation was suggested at by Verma et al. (Citation2018), who showed that, in phosphatase vhp-1 or flr-4 kinase dead mutants, nhr-8 loss abrogates the expression of cytoprotective genes. As vhp-1 is an important negative regulator of mitogen-activated protein kinase (MAPK) activity, NHR-8 may thus require MAPK signaling to activate phase I, II, and III detoxification genes, although it is not clear whether and how this classical stress sensing kinase signaling pathway is activated by xenobiotic exposure.
C. elegans HNF4-like NHRs
NHR-86
Besides the NR1J group NHRs, several HNF4-related NHRs have recently emerged as regulators of detoxification gene programs in C. elegans. NHR-86 is required to express phase I detoxification in worms exposed to the immunomodulatory toxin RPW24, including four cyp genes, two gst genes, and 14 ugt genes. As in the case of NHR-8, however, the requirement for nhr-86 is not universal, as the highly RPW24-inducible cyp-35A2, cyp-35A3, cyp-35B1, and cyp-35B2 genes remained chemical-responsive in nhr-86 mutants; whether these genes are instead regulated by other NHRs such as NHR-8 which was not tested (Peterson et al. Citation2019). It is noteworthy that Peterson et al. (Citation2019) used chromatin immunoprecipitation followed by sequencing to identify direct NHR-86 targets. The 32 genes whose promoters were bound by this TF and that required its activity for RPW24 induced expression included cyp-35A1 as the most strongly NHR-86 bound gene, as well as three ugt genes and gst-5. This provides first evidence that detoxification regulatory NHRs might directly bind critical response genes in live worms. Further, NHR-86 binding to several promoters increased upon exposure to RPW24. This suggests that NHR-86, and perhaps other NHRs, might display toxin-induced binding to relevant detoxification promoters as a mechanism to enhance their expression on demand.
NHR-114
Another HNF4-like NHR that is required to express detoxification genes is NHR-114 (Gracida and Eckmann Citation2013). In this case, the exposure of nematodes to a bacterial diet containing high levels of the amino acid tryptophan induced a broad detoxification program including cyp, ugt, and gst genes. Notably, some of these genes were nhr-114 dependent, suggesting that adaptation to a specific bacterial diet involves NHR-114-driven transcriptome rewiring to protect the organism from toxic effects. This scenario highlights that NHR (and TF) dependent rewiring of the detoxification pathways is not exclusive to exposure to “foreign”, potentially dangerous, substances, but represents an integrative component of the organism’s response to specific diets and the corresponding (endo-) metabolic modulation that their consumption invokes. Although it is not currently known whether NHR-114 binds any endo- or xenobiotic compound, it is tempting to speculate that it, and perhaps other diet-sensing NHRs, might undertake this to adapt xenobiotic and other metabolic pathways.
Other HNF4-like NHRs
Several other HNF4-like NHRs have also emerged as potential regulators of xenobiotic detoxification. Using high-throughput yeast-one-hybrid assays to study TF-promoter interactions, Fuxman Bass et al. (Citation2016) found a significant overrepresentation of phase I, II, and III detoxification genes, including cyp, gst, ugt, and sdr genes, amongst promoters bound by NHRs. RNAi against 26 NHRs that bound the promoter of at least one detoxification gene in combination with exposure to 16 molecules that are toxic for C. elegans, including some xenobiotics, revealed new roles in xenobiotic resistance for 9 NHRs (NHR-42, −66, −72, −102, −109, −142, −178, −216, and −273). This unbiased study strongly supports the notion that xenobiotic resistance is an important role of HNF4-like NHRs in C. elegans.
Targeted investigations of individual NHRs also support such roles for NHRs. Jones et al. (Citation2013) showed that, despite its role in the response to several toxins, nhr-8 is not universally essential for xenobiotic detoxification (nor were skn-1, ahr-1, hif-1, and mdt-15). Studying chloroquine, dazomet, imidacloprid, and thiabendazole, and performing candidate RNAi screens of 387 TFs, Jones et al. (Citation2013) found 12 genes required for activation of some of 12 phase I, II, and III detoxification genes in response to toxin exposure. All 12 genes were NHRs. Most prominently, nhr-176 depletion enhanced susceptibility to the anthelminthic thiabendazole. A follow-up study demonstrated that NHR-176 directly binds thiabendazole and is required for thiabendazole induced cyp-35 gene transcription (Jones, Flemming, and Urwin Citation2015). In contrast, nhr-176 is dispensable for resistance to 5-hydroxythiabendazole. Thus, Jones, Flemming, and Urwin (Citation2015) proposed that the xenobiotic response of C. elegans is rather specialized and might involve combinatorial actions of multiple regulators.
Several other NHRs also regulate cyp genes. However, these P450 enzymes may not be phase I detoxification genes, but rather participate in lipid synthesis and modification, or act dually in both functions. These functions include the aforementioned regulation of DAF-9 by DAF-12, a feedback loop wherein DAF-9 contributes to the synthesis of the DAF-12 ligands; in this context, DAF-9 is not known to play roles in detoxification. Similarly, the HIF-1 regulated P450, cyp-36A1, is required for the downstream activity of NHR-46 and might contribute NHR-46 ligand synthesis (Pender and Horvitz Citation2018). CYP-36A1 is related to the CYP2 family, which function in both detoxification of xenobiotics and metabolism of endogenous molecules (Nebert, Wikvall, and Miller Citation2013), and as such might also perform detoxification reactions. Similarly, although initially characterized as a regulator of lipid metabolism, NHR-49 also regulates cyp and other detoxification genes in long-lived mutants and when worms experience oxidative stress (Chamoli et al. Citation2014; Goh et al. Citation2018; Hu et al. Citation2018). Whether or not NHR-49 is directly involved in xenobiotic compound sensing and adaptation is not clear.
In sum, phase I, II, and II detoxification genes appear to be common targets for C. elegans NHRs, especially NHR-8 and a growing number of HNF4-related NHRs. These TFs likely act in a combinatorial fashion to induce detoxification gene programs and consequently organismal resistance and tolerance. Underlying mechanisms may involve combinatorial promoter binding, heterodimer formation, or sequential action to evoke a customized response to a toxin(s) an individual worm may encounter. The extraordinarily large number of NHRs in the C. elegans genome, combined with similar, albeit smaller expansion of the cyp and ugt families, suggests that individual NHRs, or NHR dimer pairs, might help C. elegans fine tune its detoxification system in the face of ever changing environmental xenobiotic diversity.
Mechanistic considerations of xenobiotic sensing by C. elegans NHRs
The mammalian NHRs that orchestrate the response to xenobiotics are activated via direct and indirect mechanisms. That is, their activity is increased both by their ability to directly bind xenobiotic compounds that then act as ligand agonists, and also through additional independent mechanisms not involving direct physical contact between the molecule in question and an NHR. Such indirect signaling involving kinases and phosphatases alters NHR activity by inducing nuclear translocation, protein stabilization, and/or interaction with transcriptional coregulators (reviewed in (Mackowiak and Wang Citation2016)).
Unlike mammalian PXR and CAR, C. elegans NHRs have not been studied in as much detail for putative molecular mechanisms underlying their regulation of detoxification gene programs. However, collectively, the studies described above hint that direct and indirect mechanisms might contribute to xenobiotic sensing and signaling by C. elegans NHRs. Specifically, evidence exists suggesting that (i) NHRs can bind some xenobiotic compounds; (ii) their chromatin occupancy in the regulatory regions near detoxification genes increases when xenobiotics are sensed; and (iii) they activate the expression of said genes, thus promoting xenobiotic resistance and tolerance. Together, these studies agree with a model of direct, ligand-like activation of C. elegans NHRs by xenobiotics. Indirect signaling, possibly by the MAPK pathway, could also be required for NHR activation.
MDT-15 – a co-regulator shared between NHRs that control detoxification gene programs
To exert effects on gene regulation, NHRs (and other TFs) need to interact with co-regulators, which help activate and repress gene expression as necessary. An important co-regulator in eukaryotes is the Mediator complex, which is composed of 25–30 subunits, some of which serve as direct docking sites for TFs (Grants, Goh, and Taubert Citation2015). Notably, one such subunit, MDT-15, interacts physically with several of the above noted TFs, including NHR-8, NHR-49, NHR-86, and SKN-1 (see below) (Arda et al. Citation2010; Reece-Hoyes et al. Citation2013; Taubert et al. Citation2006). Accordingly, mdt-15 is required to induce numerous phase I, II, and III detoxification genes in worms exposed to xenobiotic molecules, and its mutation or deletion results in sensitivity to xenobiotics such as fluoranthene and RPW24 (Pukkila-Worley et al. Citation2014; Taubert et al. Citation2008). A model whereby MDT-15 binds NHRs that have been activated in direct or indirect fashion by the presence of a xenobiotic molecule and co-activates the expression of pertinent detoxification genes is thus plausible. As co-regulators might be rate-limiting for expression of inducible gene programs, it would be interesting to further investigate the role of MDT-15 in NHR driven detoxification responses.
AHR-1 is unlikely to perform major xenobiotic detoxification functions in C. elegans
In addition to NHRs, mammalian cyp and ugt genes are regulated by the aryl hydrocarbon receptor (AHR) in response to xenobiotics, especially aromatic (aryl) hydrocarbons including polycyclic aromatic hydrocarbons, dioxins, and polychlorinated biphenyls for which AHR is an important sensor (Bock Citation2014; Nebert et al. Citation2004). The C. elegans genome also encodes an AHR gene, ahr-1, as well as a gene encoding its obligate partner, the AHR nuclear translocator (ARNT) aha-1 (Powell-Coffman, Bradfield, and Wood Citation1998). However, whereas mammalian AHR directly binds several aromatic xenobiotic compounds, C. elegans ahr-1 does not bind the classical AHR ligand, dioxin (Powell-Coffman, Bradfield, and Wood Citation1998). Further, most functional investigations on ahr-1 to date suggested that it is predominantly involved in development, especially of the nervous system (Huang, Powell-Coffman, and Jin Citation2004; Zhang et al. Citation2013). Similarly, transcriptome analyses in two ahr-1 mutants revealed a gene program largely pertinent to neuronal function and development (Aarnio et al. Citation2014), although some genes identified as ahr-1 dependent did feature regulatory elements annotated as xenobiotic responsive elements (XREs). Recently Brinkmann et al. (Citation2019) suggested that ahr-1 may modulate life span in C. elegans, perhaps by modulating the effects of metabolites from commensal bacteria. In any case, the central role of mammalian AHR in the defense against certain aromatic hydrocarbons does not appear to be recapitulated by C. elegans ahr-1, and thus might arise later in evolution.
SKN-1
SKN-1 is a TF that belongs to the basic leucine zipper (bZIP) family of TFs that regulates stress response pathways across species (Blackwell et al. Citation2015). In C. elegans, SKN-1 is especially important for oxidative stress and starvation adaptation and is induced by complex regulatory pathways involving MAPK and insulin signaling as well as negative regulation by the WDR-23 protein, which appears to promote SKN-1 for degradation, an inhibitory response that is alleviated by oxidative stress (Blackwell et al. Citation2015). In addition to its role as an antioxidant regulator, SKN-1 also plays important roles in xenobiotic detoxification. Specifically, skn-1 loss sensitizes worms to the common benzimidazole albendazole, and skn-1 gain-of-function mutations increase tolerance to this drug. skn-1 regulated genes include albendazole induced cyp, gst, and ugt genes, of which ugt-22 loss also enhances albendazole efficacy (Fontaine and Choe Citation2018). Similarly, induction of phase I and II detoxification genes by acrylamide also requires skn-1, at least in part, and unbiased genetic screens for genes involved in stimulation of gst genes by acrylamide identified known components of the SKN-1 pathway, including the negative regulator wdr-23, and the metabolic enzyme alh-6 (Fukushige et al. Citation2017; Hasegawa and Miwa Citation2010). The Skp1 homologs skr-1/2, components of Skp-Cullin-F box ubiquitin ligase (SCF) complexes that regulate SKN-1, are also essential for the SKN-1 detoxification response to acrylamide (Wu et al. Citation2016). The regulation of SKN-1 during xenobiotic stress is thus apparently similar as that seen in oxidative stress, involving derepression from WDR-23 and SKR-1/2 mediated degradation. How xenobiotic molecules act to relieve WDR-23 action on SKN-1 is not known at this time, but the effects of skr-1/2 are notably independent of classical stress activated MAPK signaling, implicating alternative pathways. Indeed, SKN-1 activity is highly regulated, and studies on its activity in oxidative stress conditions revealed numerous novel regulators (Crook-McMahon et al. Citation2014). It would be interesting to test whether any of these factors are necessary for elevated SKN-1 activity in response to xenobiotic exposure.
Conclusions: transcriptional regulation of xenobiotic responsive genes
In sum, the transcriptional response of C. elegans to xenobiotic molecules is both similar and distinct from that found in other animals. Although NHRs and SKN-1 clearly play central roles in toxin resistance and the underlying gene regulation, these NHRs are distinct, belonging to NR1J and HNF4-like groups rather than the mammalian NR1I group, which appears to be absent from the C. elegans genome. Similarly, the role of AHR appears to be marginally conserved with regard to xenobiotic metabolism. Future investigations need to focus on identifying mechanisms pertaining to how xenobiotics activate C. elegans xenobiotic responsive TFs and what specificity or partnerships exist between different TFs to enable tailor-made detoxification and resistance in this worm. Attention also needs to be paid to cell-specific expression to inform our understanding of which cells contribute to xenobiotic metabolism and transport; the existence of publically-available datasets on cell-specific gene expression should facilitate this effort.
Empirical toxicokinetics (TK)
Toxicokinetics(TK) encompass uptake, distribution, metabolism, and excretion of xenobiotics. Understanding the rates at which these processes occur is a critical part of toxicological assessments, because these dictate the actual doses at internal sites of molecular interaction that initiate toxicity. Comparison of internal doses across lifestages, tissues, and species is critical to intraspecies extrapolations (e.g., why does toxicity occur in one cell type and not another?) as well as interspecies extrapolations (e.g., is the toxic effect observed relevant in another species?). Empirical studies of the kinetics of uptake, metabolism, and excretion are an important first step. For example, the absorption rate might be estimated by incubating the animals for different time periods and subsequently measuring the internal doses. Ultimately, TK models are developed, as has been done in many other species, including humans, rats, mice, and zebrafish. To date, a full TK model has not been developed for C. elegans, but some aspects of TK were determined and reported. A variety of methods were used to empirically measure xenobiotic uptake (and in some cases clearance) in C. elegans. The concept of internal dose used here is as defined by the U.S. EPA Exposure Factor Handbook (2011; see https://www.epa.gov/expobox/about-exposure-factors-handbook).
Burns et al. (Citation2010) systematically investigated the uptake of drugs by C. elegans by surveying accumulation of 387 compounds. Unfortunately, Burns et al. (Citation2010) needed to set a high detection limit (19 µM, or roughly half of the external dose of 40 µM), which limited their ability to determine average uptake across the chemical space. However, under these conditions, only 6.7% of the compounds were detected above the detection limit in worm lysates after 6 hr incubation. Burns et al. (Citation2010) went on to construct a structure-activity relationship to predict which physicochemical properties would enable significant accumulation of a compound within C. elegans. Extension of this analysis to additional chemicals and classes of chemicals such as (1) both inorganic and organic, as well as molecules with a wide range of Kow and pKa values would be beneficial. Knowledge of additional lifestages and exposure times would also be of great value to the field.
Unfortunately, despite the thousands of toxicology- and pharmacology-related publications in C. elegans, the number that report internal doses appears to be quite limited. In Supplemental , results from publications are summarized that were identified that report analysis of xenobiotic uptake in C. elegans (Luz et al. Citation2016, Citation2017; Au et al. Citation2009; Yang et al. Citation2014; Maurer et al. Citation2015; Crone et al. Citation2015; Wyatt et al. Citation2016b; Helmcke et al. Citation2009; Allard and Colaiacovo. Citation2010; Chen et al. Citation2016; Zheng et al. Citation2013; Roh, Lee, and Kwon Citation2016; and others). While it may be challenging to draw broad conclusions from these studies because of variability in experimental design such as liquid versus agar plate culture, lifestage, or exposure time course, there are a few general patterns and several important limitations to the current state of the literature can be identified.
Some combination of poor absorption through the cuticle, and perhaps behavioral changes that reduce oral intake, appear to comprise a fairly effective barrier to uptake of many chemicals. For example, Luz et al. (Citation2017) found that uptake of arsenite in 8-day adults was approximately 1% in 24 hr (1 µM internal dose, 100 µM exposure concentration). However, this is not always the case: cisplatin uptake in 2 hr in L4s was higher, approximately 10–15% (Crone et al. Citation2015), and a 48 hr developmental exposure to methylmercury, which biomagnifies in aquatic food webs, was present internally at >100-fold higher concentration compared to the exposure medium (Wyatt et al. Citation2016b). In general, it is probably not surprising that uptake varies significantly by chemical, which underscores the importance of such measurements in future research. It would also be helpful to distinguish, as much as possible, absorption through the gut from cuticular uptake.
Perhaps the more critical question for human health risk assessment is not how the internal dose in C. elegans is compared to the exposure concentration in the medium, but how the measured internal dose in C. elegans is compared to exposure concentrations in other lab models (e.g., cell culture), or internal doses in the human population. Data to date show that for many chemicals, internal doses are reasonable compared to other systems. For example, the internal dose of cisplatin with doses between 150–350 µM result in internal dose less than 1 µM (Crone et al. Citation2015), and patients undergoing cisplatin infusion have peak plasma levels of around 3–5 µM (Hanada et al. Citation2001; Rajkumar et al. Citation2016). Internal doses in C. elegans approximating human blood levels were also been reported for ethanol (Alaimo et al. Citation2012). However, it needs to be emphasized that the literature to date is quite limited. Important areas for future research beyond analysis of additional chemicals include detailed time courses to establish not just internal doses but actual kinetics of uptake and excretion.
A second major limitation even when uptake has been measured is uncertainty regarding target tissue concentration within the worm, and cell-and tissue-specific TK. For example, analytical chemistry techniques are typically quantitative but do not usually provide fine-scale information regarding distribution (i.e., inside the worm versus on the cuticle, and which cells and subcellular organelles have the highest concentrations). Conversely, microscopic or X-ray spectroscopy methods provide more spatial distribution information, but are rarely quantitative. Interestingly, these methods revealed striking spatial variation in concentration, often with higher concentrations in the gut than elsewhere (Brinkhaus et al. Citation2014; Gonzalez-Moragas et al. Citation2017a). On the other hand, Jackson et al. (Citation2005), using synchrotron X-ray spectroscopy, found large differences in the intra-organismal distribution of copper (which was fairly evenly distributed) and lead (which located mostly to the head region). Jackson et al. (Citation2005) were also able to demonstrate that the uptake of lead was almost certainly entirely via ingestion, not across the cuticle. Further, the physicochemical properties of the compound determine how quickly it is taken up into the worm, particularly to peripheral tissues beyond the gut, and how quickly it might be lost after removing the compound. For example, chlorpyrifos reaches peak internal doses and remains stable after approximately one hr exposure, and when the pesticide is removed it is quickly cleared with more than half disappearing within one hr and the remaining chlorpyrifos clearing by 20 hr (Roh, Lee, and Kwon Citation2016). In contrast, ethanol was reported to diffuse readily through the cuticle and might be lost even during wash steps, making accurate estimates of internal doses and TK difficult (Mitchell et al. Citation2007).
Finally, for chemicals that are taken up poorly, even if internal doses comparable to other systems can be achieved with high exposure concentrations, there are practical issues with the low uptake of some chemicals by C. elegans. Higher exposure concentrations require using more chemical, which may be costly, and often is limited by the solubility of the drug or toxicant, even with the use of carriers such as DMSO. A solution that was proposed is to use cuticle-defective mutants such as bus-5, which were demonstrated to display increased permeability to drugs. However, this presents additional challenges including needing this mutation in the background of all other genetic/transgenic strains used, reduced fitness resulting from the compromised cuticle, and possible compensatory changes in gene expression in response to the mutation that may have unintended consequences in the study (Xiong, Pears, and Woollard Citation2017). As a further aid to experimental design, and provide factors that affect uptake of chemicals and nanoparticles in C. elegans, including both aspects of the worm’s biology (lifestage and genetics) and the worm’s environment (type of medium, and presence of food or other organic matter).
Table 2. Factors impacting toxicokinetics in C. elegans
Table 3. Factors impacting nanoparticle toxicokinetics in C. elegans
Genetic diversity: C. elegans as a model for natural population variation in xenobiotic response
Different individuals respond to xenobiotics in diverse ways. The same exposure might lead to extreme toxicity or to mild effects depending upon the genetics of the individual. For example, toxicants that pose high risks to biological systems are ubiquitous in the environment, yet individuals vary greatly in the degree to which these exposures initiate diseases. Knowledge of specific xenobiotic responses is limited to cases with a high known exposure source, which is not typical of a daily exposure scenario. A major missing link between individual responses and exposures comes from a limited understanding of the role of genetic variation in responses to xenobiotics. Toxicity assessment techniques are lacking that properly account for genetic variation in toxicological studies because sample sizes are too limited to accurately reflect true human genetic diversity. Similarly, there is also an absence of methods to correlate genotypes to disease phenotypes in human populations that thoroughly account for heterogeneous environmental exposures because this knowledge is essentially impossible to capture in an informative manner. Using a model system, one can address both weaknesses in xenobiotic assessments ethically and robustly. However, a gap exists in the translation of xenobiotic toxicity mechanisms in most model organisms because most assessments of xenobiotic responses use only one genetic background. This situation is akin to making xenobiotic-induced disease risk assessments from a single subject. Therefore, in order to define population-level risk factors, one requires a discovery platform that determines whether xenobiotic response pathways vary across genetically diverse individuals, and C. elegans is ideally suited for this purpose.
The C. elegans species has genetic diversity similar to humans and genetically distinct individuals are found worldwide (Andersen et al. Citation2012; Lee et al. Citation2020). Recent samples collected from nature have levels of genetic diversity from before the domestication of the lab strain N2 (Cook et al. Citation2017; Crombie et al. Citation2019; Sterken et al. Citation2015). Using these strains, one can begin to characterize the spectrum of xenobiotic responses present in the C. elegans species to make broader conclusions regarding how xenobiotics affect diverse individuals. Quantitative genetics studies correlations between phenotypic differences (e.g. xenobiotic responses) and genotypic differences (e.g. genetic backgrounds) to discover the genes that might underlie population-wide differences. C. elegans offers a unique opportunity to leverage natural genetic variation to identify novel genetic sources of variability in xenobiotic metabolism and transport. Diverse C. elegans populations encounter many xenobiotics in their natural rotting substrates, soil, and groundwater ecosystems. For this reason, subpopulations of C. elegans have likely adapted to either periodic or constant toxicant exposure and retained advantageous genetic differences that produced increased survival rates in these conditions. Alleles that have arisen naturally might be easily translated to human populations because several xenobiotic response pathways are conserved between C. elegans and humans (Ardelli Citation2013; Harlow et al. Citation2016; Jones et al. Citation2013; Shaye and Greenwald Citation2011). Powerful insights into the genetic basis of xenobiotic responses n may therefore be derived from C. elegans as a sentinel of toxic exposure.
Only the beginning of understanding mechanisms of xenobiotic resistance across natural C. elegans populations is known. Although drugs are inarguably essential, chemotherapeutic medications may be classified as xenobiotic compounds with known molecular targets and conserved TK properties (Zdraljevic and Andersen Citation2017). A relatively rare allele of the scb-1 gene induces bleomycin susceptibility in a large recombinant C. elegans population (Brady et al. Citation2019). This allele also confers resistance to several other chemotherapeutic drugs, particularly drugs that produce DNA double-stranded breaks (Evans and Andersen Citation2020; Evans et al. Citation2018). The results are less straightforward with heavy metals and pesticides, two common types of agricultural and industrial xenobiotics. Differential responses to these compounds might be explained by genetic differences among individuals. In particular, three regions of the genome were linked to variation in susceptibility to the pesticides paraquat, chlorpyrifos, and chlorothalonil, as well as the heavy metals silver and copper. However, when one of these regions was isolated through a series of genetic crosses, it alone could only explain variation in one specific trait in response to silver (Evans and Andersen Citation2020; Evans et al. Citation2018). Therefore, resistance to heavy metals and pesticides is a complex trait governed by a set of genes with varying functions. As is the case with the majority of complex traits, individual resistance loci typically exert relatively small effects in isolation and are problematic to characterize. Further, these loci might participate in either antagonistic or synergistic interactions, making individual genetic effects even more difficult to detect and interpret. Nevertheless, some individual genes have outsized influences on xenobiotic resistance, even though the entire set of genes is difficult to infer.
One unique example of the power of C. elegans for translational TK insights is the discovery of a novel pathway to As resistance among natural populations. Genome-wide association (GWA) mapping involves measuring a phenotype of interest among a collection of genetically diverse individuals. The unique advantage of GWA compared to lab crosses is that it leverages past relatedness to detect resistance alleles with potentially low representation in traditional lab strains. In C. elegans, the molecular genetic toolkit is broad and tractable so that causal relationships between genetic variants and phenotypic variation may be established. GWA mapping, followed by a series of metabolomic experiments in edited C. elegans strains and human cell lines, confirmed that one cysteine-to-serine missense variant within the dbt-1 gene enhanced resistance to As by modulating branched chain fatty acid biosynthesis (Zdraljevic et al. Citation2019). Arsenic sensitivity mediated by differential metabolism of branched-chain amino acids is a novel observation that is consistent with other features of As-mediated toxicity in human populations. Most critically, these results demonstrate the unique opportunity for the toxicology community to leverage natural genetic variation in C. elegans to identify novel mechanisms of differential toxicodynamics in the human population and learn how individuals differ in xenobiotic metabolism.
Natural C. elegans populations likely encounter many xenobiotics for which there are poor assessments of human health risk. Nematodes need to find a way to survive in environments contaminated by industrial byproducts such as Cd and Pb. For this reason, C. elegans is sometimes considered a useful environmental indicator (Custodia et al. Citation2001: Hoss et al. Citation2009) and, in turn, lab assessments have identified important metal resistance genes (Broeks et al. Citation1996: Swain et al. Citation2004), including phytochelatins (Vatamaniuk et al. Citation2001), which play a key role in C. elegans but are not expressed in higher eukaryotes. Genetically diverse wild isolates can be sampled from many environments and screened against the battery of toxicants for which detailed human assessments are scant. C. elegans isolates can be collected from polluted sites and their xenobiotic responses compared to more traditional lab strains, offering a unique way to take advantage of natural populations. High-throughput phenotyping platforms (Andersen et al. Citation2015) enabled precise and rapid phenotypic characterization of thousands of individuals. Highly scalable xenobiotic screening efforts enable us to continue to explore C. elegans natural variation and expand the scope of large-scale quantitative genetics analyses (Cook et al. Citation2017). This endeavor might undoubtedly reveal a rich set of genomic regions linked to variation in xenobiotic metabolic and transport capacity (Evans et al. Citation2018), and provide new opportunities to discover drug-initiated cellular targets for xenobiotic-induced disease treatment.
Evolutionary toxicology: applications of inter-species variation to understanding stressor responses
Intraspecific differences in toxicant responses across a single species might serve as a powerful tool to discover new mechanisms of toxicity and toxicity mitigation. This technique can identify natural variation in genes involved in toxin responses, but some genes might not be conserved with other species because C. elegans likely has experienced evolutionary selection by chemical exposure differently. By broadening the number of species where natural history differences in chemical responses are measured, it is possible to increase the likelihood the discovered genes and toxin responses mechanisms might be connected to other species, including humans. The underlying assumption of this hypothesis is that different species have experienced similar evolutionary pressures by chemical exposure, such that mechanisms that were used to adapt are similar. In other words, evolutionary trajectories in different species all lead to the same mechanisms of toxicity mitigation. This hypothesis is likely not universally true, but when it is, the statistical likelihood that genome-wide association studies identify conserved toxin response loci is higher. Thus, some researchers used comparison of toxicological responses of C. elegans to those of other nematode and invertebrate species to infer expected ecotoxicological responses in other invertebrates (Boyd and Williams Citation2003; Haegerbaeumer et al. Citation2018a, Citation2019; Peredney and Williams Citation2000). Similarly, others investigators previously advocated for using evolutionary genetics to inform human health risk assessment (Brady et al. Citation2017; Hahn Citation2019; Leung et al. Citation2017).
Nematodes are incredibly ecologically diverse, either living freely in soils, freshwater, or seawater or as a parasites in plants or animals (De Ley Citation2006). The chemical exposure of nematodes is as diverse as their natural habitats. It includes organic compounds and minerals in soils (Ekschmitt and Korthals Citation2006), pesticides used in agriculture (Rich, Dunn, and Noling Citation2004), as well as defensive compounds secreted by bacteria, fungi, plants, and animals (de Veer, Kemp, and Meeusen Citation2007; Williamson and Kumar Citation2006). Some nematodes have evolved to survive desiccation and high salinity in extreme arid environments such as deserts (Treonis and Wall Citation2005). The panoply of abiotic and biotic stresses has likely contributed to the high abundance and diversity of many of the xenobiotic metabolism and transport-related gene classes discussed above. Even within a single species like C. elegans, the diversity of xenobiotic response genes is far larger than the suite of genes found in the lab-adapted strain N2 (https://www.biorxiv.org/content/10.1101/2020.07.23.218420v1.abstract). The power of C. elegans GWA mapping approaches might be expanded to related self-fertilizing species like Caenorhabditis briggsae and Caenorhabditis tropicalis, when reference genomes are completed and species-wide natural variation has been characterized. Comparative genetics and genomics across these three species will discover conserved Caenorhabditis toxin responses along with species-specific toxin responses. The conserved responses are more likely to be conserved with humans. To further supplement the comparative genetics and genomics approach, natural variation in toxin responses across nematode species beyond Caenorhabditis or even other invertebrate species (e.g., Drosophila melanogaster) need to be studied. The “worm community” has begun in-depth investigations of the biology of multiple related Caenorhabditis species (Elsworth, Wasmuth, and Blaxter Citation2011; Fitch Citation2005; Martin et al. Citation2015). However, the total number of nematode species is estimated at approximately one million (Kiontke and Fitch Citation2013), and only approximately 30,000 species have been described. A limited number of nematode species important in agriculture, biology, and medicine were also examined and provide a basis for comparison, including Ancylostoma duodenale (hookworm), Brugia malayi (Lymphatic filarial nematode), Meloidogyne incognita (root-knot nematode), and Steinernema carpocapsae (entomopathogenic nematode). As xenobiotic response loci are discovered in additional species, comparative approaches might increase in power and enable connections of discovered genes, pathways, and mechanisms to humans.
Finally, although this review focuses on C. elegans as a toxicology model for human health, C. elegans and other nematodes may also serve broader ecological health goals. Nematodes, or communities of nematode species, may serve as useful bioindicator species in ecotoxicology. The species diversity of nematode communities is one measure of ecological integrity, and is sensitive to environmental changes (Franco et al. Citation2019). Morphological identification of nematode species may be time-consuming and requires expertise. However, recent studies demonstrated that metabarcoding samples of nematode communities is similarly efficient to morphological species identification, and may be implemented rapidly (De Ley et al. Citation2005; Schenk et al. Citation2020). Further, given relevance for both human health and ecotoxicology, and the ability to be studied both in the field and in the lab, experiments with C. elegans has strong potential to help bridge ecotoxicological and human health research. Research with nematodes might be used in frameworks that were developed for integrating data from different species from molecular (Mattingly et al. Citation2006), biochemical (Harborne Citation1988), and pathological (Snyder et al. Citation2010) to ecological (Monosson Citation2012) levels, approaches that more recently were incorporated into the EcoHealth and OneHealth concepts (Brooks et al. Citation2020; Haschek et al. Citation2019; Perez and Pierce WiseSr. Citation2018).
Knowledge gaps, opportunities, and future directions
It is hoped that this review will serve to orient C. elegans researchers who are not toxicologists to these important pharmacological and toxicological parameters, and to orient toxicologists and pharmacologists who are new to C. elegans to what is known and not known regarding relevant aspects of C. elegans biology. This review is somewhat narrowly focused on biology relevant to TK, largely excluding some pathways that are nonetheless relevant, including non-enzymatic xenobiotic defenses (metallothioneins, phytochelatins, glutathione, antioxidant molecules), and antioxidant enzyme systems.
It is also hoped that this review will stimulate additional research into some of the less well-understood aspects of TK in this species. Because of the long history of genomic and genetic research in this species, a remarkable understanding of genes involved in TK-related processes exists. Unfortunately, our understanding of the function (e.g., substrate specificity, enzymology, etc.) of the gene products is less complete, and it is recommended more research be done in this area. There is also a limited understanding of which specific cell types are responsible for carrying out many xenobiotic transport and metabolism functions (i.e., which cells act as hepatocytes or renal cells). Similarly, although a handful of careful analyses of internal doses of chemicals in worm exposure studies were carried out, many more are needed. Work to date permits a tentative conclusion that internal doses of C. elegans are often comparable to exposure concentrations in (1) cell culture studies, (2) vertebrate model organisms, and (3) human tissues in exposed individuals. However, the database of chemicals for which internal doses and exposure concentrations have been quantified in C. elegans exposure studies is still far too small to make extrapolations with confidence, or to model likely uptake kinetics based upon chemical characteristics and first principles. Exposure studies need to routinely test internal doses. Finally, there is a remarkable opportunity to tie research in these traditional toxicological areas into more general aspects of the stress response (thermal stress, osmotic stress, hyperoxic and hypoxic stress, or caloric stress). Stress responses, broadly defined, are poorly integrated in any species; i.e., how do multiple different stress-response pathways integrate in the context of combined stressors, chemical and otherwise? Such exposures are the reality for individuals and species that one attempts to protect with toxicological studies, and are therefore critical to study. The opportunity to do combination studies and examine the role of multiple stress response pathways is great in C. elegans, in which the non-xenobiotic stress response pathways are exceptionally well-studied and tractable.
Better toxicological knowledge will be critical in permitting rigorous use of C. elegans for toxicological research that might be used to protect human and environmental health. This is true for in-depth, mechanistic toxicological research, such as on the role of specific enzymes in mediating toxicity of specific chemicals (Harris et al. Citation2020) and the ability to extrapolate worm studies to other species. It will also permit adoption of this powerful genetic model to “functional toxicology” studies (Gaytan and Vulpe Citation2014). Finally, it will be crucial to the adoption of the C. elegans model for New Approach Methodologies that seek to reduce time, cost, and vertebrate animal use in chemical safety regulation and risk assessment. The Frank R. Lautenberg Chemical Safety for the 21st Century Act (2016) mandates the U.S. Environmental Protection Agency (U.S. EPA) to explore the use of non-vertebrate models for toxicity testing (U.S. Congress Citation2016). In 2019, the U.S. EPA Administrator Andrew Wheeler signed a directive to reduce funding for animal research 30% by 2025 and eliminate it by 2035 (U.S. Environmental Protection Agency Environmental Protection Citation2019). C. elegans has attracted interest of a number of regulatory agencies and governmental labs (Boyd et al. Citation2016; Gong et al. Citation2018; Hunt et al. Citation2018). Yet, at the time of this publication, only two standardized testing documents were developed (International Standard Organization (ISO) Citation2020, (ASTM), American Society of Testing and Materials. Citation2001), and none by the U.S. EPA exist. Closing these knowledge gaps and developing standardized protocols will support the use of C. elegans model to address key knowledge gaps in risk assessment, such as developmental neurotoxicity (Hunt et al. Citation2018), neurodegeneration (Sammi, Agim, and Cannon Citation2018), and mixture effects (Wittkowski et al. Citation2019).
Supplemental Material
Download MS Excel (22.3 KB)Acknowledgements
We thank Steven Nadler, Christopher Pagan, and Leona Scanlan for their help, and Wormbase for providing online resources. This work was also supported by the National Institutes of Health (T32ES021432 supported KSM; R01ES028218 and P42ES010356 to JNM; K99ES029552 to JHH; NSF GRFP DGE-1644868 to DEK; R01ES029930 to ECA), the Canadian Institutes of Health Research (CIHR; PJT-153199 to ST; http://www.cihr-irsc.gc.ca), and the Natural Sciences and Engineering Research Council of Canada (NSERC; RGPIN-2018-05133 to ST; http://www.nserc-crsng.gc.ca). No potential competing interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Aarnio, V., L. Heikkinen, J. Peltonen, G. Goldsteins, M. Lakso, and G. Wong. 2014. Transcriptional profiling reveals differential expression of a neuropeptide-like protein and pseudogenes in aryl hydrocarbon receptor-1 mutant Caenorhabditis elegans. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics 9:40–48. doi: 10.1016/j.cbd.2013.12.001.
- Abbass, M., Y. Chen, V. M. Arlt, and S. R. Sturzenbaum. 2021. Benzo[a]pyrene and Caenorhabditis elegans: Defining the genotoxic potential in an organism lacking the classical CYP1A1 pathway. Archives of Toxicology. doi: 10.1007/s00204-020-02968-z.
- Ahn, J.-M., H.-J. Eom, X. Yang, J. N. Meyer, and J. Choi. 2014. Comparative toxicity of silver nanoparticles on oxidative stress and DNA damage in the nematode, Caenorhabditis elegans. Chemosphere 108:343–52. doi: 10.1016/j.chemosphere.2014.01.078.
- Ahn, T., and C.-H. Yun. 2010. Molecular Mechanisms Regulating the Mitochondrial Targeting of Microsomal Cytochrome P450 EnzymesMolecular Mechanisms Regulating the Mitochondrial Targeting of Microsomal Cytochrome P450 Enzymes. <![CDATA[Current Drug Metabolism]]> 11:830–38 doi: 10.2174/138920010794479655. 10
- Alaimo, J. T., S. J. Davis, S. S. Song, C. R. Burnette, M. Grotewiel, K. L. Shelton, J. T. Pierce-Shimomura, A. G. Davies, and J. C. Bettinger. 2012. Ethanol Metabolism and Osmolarity Modify Behavioral Responses to Ethanol inC. elegans Alcoholism: Clinical and Experimental Research 36: 1840–50 doi: 10.1111/j.1530-0277.2012.01799.x. 11
- Allard, P., and M. P. Colaiacovo. 2010. Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. Proceedings of the National Academy of Sciences 107: 20405–10. doi: 10.1073/pnas.1010386107. 47
- Allard, P., N. C. Kleinstreuer, T. B. Knudsen, and M. P. Colaiacovo. 2013. A C. elegans screening platform for the rapid assessment of chemical disruption of germline function Environ. Health. Perspect. 121: 717–24. doi: 10.1289/ehp.1206301.
- Altun, Z. F., and D. H. Hall 2009a. Alimentary System, Pharynx. In WormAtlas.
- Altun, Z. F., and D. H. Hall 2009b. Excretory system. In WormAtlas.
- Anbalagan, C., I. Lafayette, M. Antoniou-Kourounioti, C. Gutierrez, J. R. Martin, D. K. Chowdhuri, and D. I. De Pomerai. 2013. Use of transgenic GFP reporter strains of the nematode Caenorhabditis elegans to investigate the patterns of stress responses induced by pesticides and by organic extracts from agricultural soils. Ecotoxicology. 22:72–85. doi: 10.1007/s10646-012-1004-2.
- Andersen, E. C., J. P. Gerke, J. A. Shapiro, J. R. Crissman, R. Ghosh, J. S. Bloom, M. A. Felix, and L. Kruglyak. 2012 Chromosome-scale selective sweeps shape Caenorhabditis elegans genomic diversity. Nat. Genet. 44:285–90. doi: 10.1038/ng.1050.
- Andersen, E. C., T. C. Shimko, J. R. Crissman, R. Ghosh, J. S. Bloom, H. S. Seidel, J. P. Gerke, and L. Kruglyak 2015. A powerful new quantitative genetics platform, combining Caenorhabditis elegans high-throughput fitness assays with a large collection of recombinant strains. G3. (Bethesda). 5:911–20 doi: 10.1534/g3.115.017178.
- Arda, H. E., S. Taubert, L. T. MacNeil, C. C. Conine, B. Tsuda, M. Van Gilst, R. Sequerra, L. Doucette-Stamm, K. R. Yamamoto, and A. J. Walhout. 2010. Functional modularity of nuclear hormone receptors in a Caenorhabditis elegans metabolic gene regulatory network. Mol. Syst. Biol. 6:367. doi: 10.1038/msb.2010.23.
- Ardelli, B. F. 2013. Transport proteins of the ABC systems superfamily and their role in drug action and resistance in nematodes. Parasitol. Int. 62:639–46. doi: 10.1016/j.parint.2013.02.008.
- Ashrafi, K., F. Y. Chang, J. L. Watts, A. G. Fraser, R. S. Kamath, J. Ahringer, and G. Ruvkun. 2003. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 421:268–72.
- (ASTM), American Society of Testing and Materials. 2001. Conducting Laboratory Soil Toxicity Tests with the Nematode Caenorhabditis elegans.
- Au, C., A. Benedetto, J. Anderson, A. Labrousse, K. Erikson, J. J. Ewbank, and M. Aschner. 2009. SMF-1, SMF-2 and SMF-3 DMT1 orthologues regulate and are regulated differentially by manganese levels in C. Elegans. PLoS One 4:e7792. doi: 10.1371/journal.pone.0007792.
- Avdeef, A. 2001. Physicochemical profiling (solubility, permeability and charge state). Curr. Top. Med. Chem. 1:277–351. doi: 10.2174/1568026013395100.
- Avery, L., and Y. J. You . 2012. C. elegans feeding (May 21, 2012), ed. In WormBook. The C. elegans Research Community
- Baba, T., T. Ara, M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. A. Datsenko, M. Tomita, B. L. Wanner, and H. Mori. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2: 2006 0008. doi: 10.1038/msb4100050.
- Bargmann, C. I. 2006. Chemosensation in C. elegans. WormBook: Pasadena, CA, United States. 1–29.
- Baugh, L. R. 2013. To grow or not to grow: Nutritional control of development during Caenorhabditis elegans L1 arrest. Genetics. 194: 539–55. doi: 10.1534/genetics.113.150847.
- Baumeister, R., E. Schaffitzel, and M. Hertweck. 2006. Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. J. Endocrinol. 190:191–202. doi: 10.1677/joe.1.06856.
- Behl, M., J. H. Hsieh, T. J. Shafer, W. R. Mundy, J. R. Rice, W. A. Boyd, J. H. Freedman, E. S. Hunter 3rd, K. A. Jarema, S. Padilla, et al. 2015. Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol. Teratol. 52:181–93. doi: 10.1016/j.ntt.2015.09.003.
- Benz, R., K. Janko, and P. Läuger. 1980. Pore formation by the matrix protein (porin) of Escherichia coli in planar bilayer membranes. Ann. N. Y. Acad. Sci. 358:13–24. doi: 10.1111/j.1749-6632.1980.tb15382.x.
- Berg, M., B. Stenuit, J. Ho, A. Wang, C. Parke, M. Knight, L. Alvarez-Cohen, and M. Shapira. 2016. Assembly of the Caenorhabditis elegans gut microbiota from diverse soil microbial environments. Isme J 10:1998–2009. doi: 10.1038/ismej.2015.253.
- Blackwell, T. K., M. J. Steinbaugh, J. M. Hourihan, C. Y. Ewald, and M. Isik. 2015. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 88: 290–301. doi: 10.1016/j.freeradbiomed.2015.06.008.
- Blum, J., and I. Fridovich. 1983. Superoxide, hydrogen peroxide, and oxygen toxicity in two free-living nematode species. Arch. Biochem. Biophys. 222:35–43.
- Bock, K. W. 2014. Homeostatic control of xeno- and endobiotics in the drug-metabolizing enzyme system. Biochem. Pharmacol. 90:1–6. doi: 10.1016/j.bcp.2014.04.009.
- Boyd, W. A., M. V. Smith, C. A. Co, J. R. Pirone, J. R. Rice, K. R. Shockley, and J. H. Freedman. 2015. Developmental effects of the ToxCast Phase I and II chemicals in and corresponding responses in zebrafish, rats, and rabbits. Environ. Health. Perspect. doi: 10.1289/ehp.1409645.
- Boyd, W. A., M. V. Smith, C. A. Co, J. R. Pirone, J. R. Rice, K. R. Shockley, and J. H. Freedman. 2016. Developmental effects of the ToxCast™ Phase I and Phase II chemicals in Caenorhabditis elegans and corresponding responses in zebrafish, rats, and rabbits. Environ. Health. Perspect. 124:586–93. doi: 10.1289/ehp.1409645.
- Boyd, W. A., M. V. Smith, G. E. Kissling, and J. H. Freedman. 2009. Medium- and high-throughput screening of neurotoxicants using C. Elegans. Neurotoxicol Teratol.
- Boyd, W. A., and P. L. Williams. 2003. Comparison of the sensitivity of three nematode species to copper and their utility in aquatic and soil toxicity tests. Environ. Toxicol. Chem. 22: 2768–74.
- Boyd, W. A., S. J. McBride, and J. H. Freedman. 2007. Effects of genetic mutations and chemical exposures on Caenorhabditis elegans feeding: Evaluation of a novel, high-throughput screening assay. PLoS .ONE. 2:e1259.
- Boyd, W. A., S. J. McBride, J. R. Rice, D. W. Snyder, and J. H. Freedman. 2010. A high-throughput method for assessing chemical toxicity using a Caenorhabditis elegans reproduction assay. Toxicol. Appl. Pharmacol. 245: 153–59.
- Brady, S. C., S. Zdraljevic, K. W. Bisaga, R. E. Tanny, D. E. Cook, D. Lee, Y. Wang, and E. C. Andersen. 2019. A novel gene underlies bleomycin-response variation in Caenorhabditis elegans. Genetics. 212:1453–68. doi: 10.1534/genetics.119.302286.
- Brady, S. P., E. Monosson, C. W. Matson, and J. W. Bickham. 2017. Evolutionary toxicology: Toward a unified understanding of life’s response to toxic chemicals. Evol. Appl 10:745–51. doi: 10.1111/eva.12519.
- Brinkhaus, S. G., J. Bornhorst, S. Chakraborty, C. A. Wehe, R. Niehaus, O. Reifschneider, M. Aschner, and U. Karst. 2014. Elemental bioimaging of manganese uptake in C. Elegans. Metallomics. 6:617–21. doi: 10.1039/c3mt00334e..
- Brinkmann, V., N. Ale-Agha, J. Haendeler, and N. Ventura. 2019. The aryl hydrocarbon receptor (AhR) in the aging process: Another puzzling role for this highly conserved transcription factor. Front Physiol. 10:1561. doi: 10.3389/fphys.2019.01561.
- Broeks, A., B. Gerrard, R. Allikmets, M. Dean, and R. H. Plasterk. 1996. Homologues of the human multidrug resistance genes MRP and MDR contribute to heavy metal resistance in the soil nematode Caenorhabditis. Elegans. EMBO. J. 15 6132–43.
- Broeks, A., H. W. Janssen, J. Calafat, and R. H. Plasterk. 1995. A P-glycoprotein protects Caenorhabditis elegans against natural toxins. Embo. J. 14:1858–66.
- Brooks, B. W., T. Sabo-Attwood, K. Choi, S. Kim, J. Kostal, C. A. LaLone, L. M. Langan, L. Margiotta-Casaluci, J. You, and X. Zhang. 2020. Toxicology advances for 21st century chemical pollution. OneEarth. 2:312–16.
- Burchell, B., C.H. Brierley, G. Monaghan, and D.J. Clarke. 1998. „The structure and function of the UDP-glucuronosyltransferase gene family.„ Adv Pharmacol 42:335-8. doi: 10.1016/s1054-3589(08)60758-9.
- Burns, A. R., I. M. Wallace, J. Wildenhain, M. Tyers, G. Giaever, G. D. Bader, C. Nislow, S. R. Cutler, and P. J. Roy. 2010. A predictive model for drug bioaccumulation and bioactivity in Caenorhabditis elegans. Nat. Chem. Biol. 6:5 49–557. doi: 10.1038/nchembio.380.
- Cabreiro, F., C. Au, K. Y. Leung, N. Vergara-Irigaray, H. M. Cocheme, T. Noori, D. Weinkove, E. Schuster, N. D. Greene, and D. Gems. 2013. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 153:228–39. doi: 10.1016/j.cell.2013.02.035.
- Cabreiro, F., and D. Gems. 2013. Worms need microbes too: Microbiota, health and aging in Caenorhabditis elegans. EMBO. Mol. Med. 5:1300–10. doi: 10.1002/emmm.201100972.
- Cao, X., C. Yan, X. Wu, L. Zhou, and G. Xiu. 2020. Nonylphenol induced individual and population fluctuation of Caenorhabditis elegans: Disturbances on developmental and reproductive system. Environ. Res. 186:109486. doi: 10.1016/j.envres.2020.109486.
- Carroll, B. T., G. R. Dubyak, M. M. Sedensky, and P. G. Morgan. 2006. Sulfated signal from ASJ sensory neurons modulates stomatin-dependent coordination in Caenorhabditis elegans. J. Biol. Chem. 281:35989–96. doi: 10.1074/jbc.M606086200.
- Casarett, L. J., J. Doull, and C. D. Klaassen. 2008. Casarett and Doull’s Toxicology: The Basic Science of Poisons. 7th ed. New York: McGraw-Hill.
- Cassada, R. C., and R. L. Russell. 1975. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 46:326–42. doi: 10.1016/0012-1606(75)90109-8.
- César-Razquin, A., B. Snijder, T. Frappier-Brinton, R. Isserlin, G. Gyimesi, X. Bai, R. A. Reithmeier, D. Hepworth, M. A. Hediger, A. M. Edwards, et al. 2015. A call for systematic research on solute carriers. Cell. 162: 478–87. doi: 10.1016/j.cell.2015.07.022.
- Chakrapani, B. P., S. Kumar, and J. R. Subramaniam. 2008. Development and evaluation of an in vivo assay in Caenorhabditis elegans for screening of compounds for their effect on cytochrome P450 expression. J. Biosci. 33: 269–77.
- Chamoli, M., A. Singh, Y. Malik, and A. Mukhopadhyay. 2014. A novel kinase regulates dietary restriction-mediated longevity in Caenorhabditis elegans. Aging.Cell. 13:641–55. doi: 10.1111/acel.12218.
- Chang, T. K., J. Chen, V. Pillay, J. Y. Ho, and S. M. Bandiera. 2003. Real-time polymerase chain reaction analysis of CYP1B1 gene expression in human liver. Toxicol. Sci. 71: 11–19. doi: 10.1093/toxsci/71.1.11.
- Chaudhuri, J., Y. Bains, S. Guha, A. Kahn, D. Hall, N. Bose, A. Gugliucci, and P. Kapahi. 2018. The role of advanced glycation end products in aging and metabolic diseases: Bridging association and causality. Cell Metab. 28:337–52. doi: 10.1016/j.cmet.2018.08.014.
- Chen, C., T. K. Samuel, M. Krause, H. A. Dailey, and I. Hamza. 2012. Heme utilization in the Caenorhabditis elegans hypodermal cells is facilitated by heme-responsive gene-2. J. Biol. Chem. 287:9601–12. doi: 10.1074/jbc.M111.307694.
- Chen, H., C. Wang, H. Li, R. Ma, Z. Yu, L. Li, M. Xiang, X. Chen, X. Hua, and Y. Yu. 2019. A review of toxicity induced by persistent organic pollutants (POPs) and endocrine-disrupting chemicals (EDCs) in the nematode Caenorhabditis elegans.J. Environ. Manage. 237:519–25. doi: 10.1016/j.jenvman.2019.02.102.
- Chen, Y., L. Shu, Z. Qiu, D. Y. Lee, S. J. Settle, S. Que Hee, D. Telesca, X. Yang, and P. Allard. 2016. Exposure to the BPA-substitute Bisphenol S causes unique alterations of germline function. PLoS Genet. 12:e1006223. doi: 10.1371/journal.pgen.1006223.
- Choi, J., O. V. Tsyusko, J. M. Unrine, N. Chatterjee, J. M. Ahn, X. Y. Yang, B. L. Thornton, I. T. Ryde, D. Starnes, and J. N. Meyer. 2014. A micro-sized model for the in vivo study of nanoparticle toxicity: What has Caenorhabditis elegans taught us? Environ. Chem. 11: 227–46. doi: 10.1071/En13187.
- Claus, S. P., H. Guillou, and S. Ellero-Simatos. 2016. The gut microbiota: A major player in the toxicity of environmental pollutants? NPJ. Biofilms. Microbiomes. 2:16003. doi: 10.1038/npjbiofilms.2016.3.
- Clavijo, A., M. F. Kronberg, A. Rossen, A. Moya, D. Calvo, S. E. Salatino, E. A. Pagano, J. A. Morabito, and E. R. Munarriz. 2016. The nematode Caenorhabditis elegans as an integrated toxicological tool to assess water quality and pollution. Sci. Total Environ. 569-570: 252–61. doi: 10.1016/j.scitotenv.2016.06.057.
- Colas, C., P. Man-Un Ung, and A. Schlessinger. 2016. SLC transporters: Structure, function, and drug discovery. Med. Chem. Comm. 7:1069–81. doi: 10.1039/C6MD00005C.
- Collin, B., E. Oostveen, O. V. Tsyusko, and J. M. Unrine. 2014. Influence of natural organic matter and surface charge on the toxicity and bioaccumulation of functionalized ceria nanoparticles in Caenorhabditis elegans. Environ. Sci. Technol. 48: 1280–89. doi: 10.1021/es404503c.
- Congress, U. S. 2016. Frank R. Lautenberg Chemical Safety for the 21st Century Act. In 130 Stat. 448. Washington, DC: U.S. Government Publishing Office.
- Cook, D. E., S. Zdraljevic, J. P. Roberts, and E. C. Andersen. 2017. CeNDR, the Caenorhabditis elegans natural diversity resource. Nucl. Acids. Res. 45: D650–D657. doi: 10.1093/nar/gkw893.
- Cooper, J. F., and J. M. Van Raamsdonk. 2018. Modeling Parkinson’s disease in C. Elegans. J. Parkinsons. Dis. 8:17–32. doi: 10.3233/JPD-171258.
- Cox, G. N., M. Kusch, and R. S. Edgar. 1981a. Cuticle of Caenorhabditis elegans: Its isolation and partial characterization. J. Cell Biol. 90:7–17. doi: 10.1083/jcb.90.1.7.
- Cox, G. N., S. Staprans, and R. S. Edgar. 1981b. The cuticle of Caenorhabditis elegans: II. Stage-specific changes in ultrastructure and protein composition during postembryonic development. Dev. Biol. 86:456–70. doi: 10.1016/0012-1606(81)90204-9.
- Crombie, T. A., S. Zdraljevic, D. E. Cook, R. E. Tanny, S. C. Brady, Y. Wang, K. S. Evans, S. Hahnel, D. Lee, B. C. Rodriguez, et al. 2019. Deep sampling of Hawaiian Caenorhabditis elegans reveals high genetic diversity and admixture with global populations. Elife. 8. doi: 10.7554/eLife.50465.
- Crone, B., M. Aschner, T. Schwerdtle, U. Karst, and J. Bornhorst. 2015. Elemental bioimaging of cisplatin in Caenorhabditis elegans by LA-ICP-MS. Metallomics. 7:1189–95. doi: 10.1039/c5mt00096c.
- Crook-McMahon, H. M., M. Olahova, E. L. Button, J. J. Winter, and E. A. Veal. 2014. Genome-wide screening identifies new genes required for stress-induced phase 2 detoxification gene expression in animals. BMC Biol. 12:64. doi: 10.1186/s12915-014-0064-6.
- Cui, Y. X., S. J. McBride, W. A. Boyd, S. Alper, and J. H. Freedman. 2007. Toxicogenomic analysis of Caenorhabditis elegans reveals novel genes and pathways involved in the resistance to cadmium toxicity. Genome Biol. 8. doi: 10.1186/gb-2007-8-6-r122. 1465–6914.
- Custodia, N., S. J. Won, A. Novillo, M. Wieland, C. Li, and I. P. Callard. 2001. Caenorhabditis elegans as an environmental monitor using DNA microarray analysis. Ann. N. Y. Acad. Sci. 948: 32–42. doi: 10.1111/j.1749-6632.2001.tb03984.x.
- Cvilink, V., L. Skálová, B. Szotáková, J. Lamka, R. Kostiainen, and R. A. Ketola. 2008. LC-MS-MS identification of albendazole and flubendazole metabolites formed ex vivo by Haemonchus contortus. Anal Bioanal Chem 391:337–43. doi: 10.1007/s00216-008-1863-9.
- Dasgupta, M., M. Shashikanth, A. Gupta, A. Sandhu, A. De, S. Javed, and V. Singh. 2020. “NHR-49 transcription factor regulates immunometabolic response and survival of Caenorhabditis elegans during Enterococcus faecalis infection. Infect. Immun. 88. doi: 10.1128/iai.00130-20.
- Davies, A. G., J. T. Pierce-Shimomura, H. Kim, M. K. VanHoven, T. R. Thiele, A. Bonci, C. I. Bargmann, and S. L. McIntire. 2003. A central role of the BK potassium channel in behavioral responses to ethanol in C. Elegans. Cell. 115: 655–66. doi: 10.1016/s0092-8674(03)00979-6.
- De Ley, P. 2006. A quick tour of nematode diversity and the backbone of nematode phylogeny WormBook:1–8. doi: 10.1895/wormbook.1.41.1.
- De Ley, P., I. T. De Ley, K. Morris, E. Abebe, M. Mundo-Ocampo, M. Yoder, J. Heras, D. Waumann, A. Rocha-Olivares, A. H. Jay Burr, et al. 2005. An integrated approach to fast and informative morphological vouchering of nematodes for applications in molecular barcoding. Phil.Trans. R.Soc .Lond B. Biol. Sci. 360: 1945–58. doi: 10.1098/rstb.2005.1726.
- de Veer, M. J., J. M. Kemp, and E. N. Meeusen. 2007. The innate host defence against nematode parasites. Parasite. Immunol. 29:1–9. doi: 10.1111/j.1365-3024.2006.00910.x.
- DeGorter, M. K., C. Q. Xia, J. J. Yang, and R. B. Kim. 2012. Drug transporters in drug efficacy and toxicity. Annu. Rev. Pharmacol. Toxicol. 52:249–73. doi: 10.1146/annurev-pharmtox-010611-134529.
- Dejima, K., D. Murata, S. Mizuguchi, K. H. Nomura, K. Gengyo-Ando, S. Mitani, S. Kamiyama, S. Nishihara, and K. Nomura. 2009. The ortholog of human solute carrier family 35 member B1 (UDP-galactose transporter-related protein 1) is involved in maintenance of ER homeostasis and essential for larval development in Caenorhabditis. Elegans. FASEB. J. 23: 2215–25. doi: 10.1096/fj.08-123737.
- Deline, M., J. Keller, M. Rothe, W. H. Schunck, R. Menzel, and J. L. Watts. 2015. Epoxides derived from dietary dihomo-gamma-linolenic acid induce germ cell death in C. elegans. Sci. Rep. 5:15417. doi: 10.1038/srep15417.
- 1996 Interconnections Between Human and Ecosystem Health. Edited by R. T. Di Giulio, and E. Monosson: Springer.
- Di, R., H. Zhang, and M. A. Lawton. 2018. Transcriptome analysis of C. elegans reveals novel targets for DON cytotoxicity. Toxins. 10. doi: 10.3390/toxins10070262.
- Dietrich, N., C. H. Tan, C. Cubillas, B. J. Earley, and K. Kornfeld. 2016. Insights into zinc and cadmium biology in the nematode Caenorhabditis elegans. Arch. Biochem. Biophys. 611:120–33. doi: 10.1016/j.abb.2016.05.021.
- Dirksen, P., A. Assie, J. Zimmermann, F. Zhang, A. M. Tietje, S. A. Marsh, M. A. Felix, M. Shapira, C. Kaleta, H. Schulenburg, et al. 2020. CeMbio - The Caenorhabditis elegans microbiome resource. G3. (Bethesda). doi: 10.1534/g3.120.401309.
- Dirksen, P., S. A. Marsh, I. Braker, N. Heitland, S. Wagner, R. Nakad, S. Mader, C. Petersen, V. Kowallik, P. Rosenstiel, et al. 2016. The native microbiome of the nematode Caenorhabditis elegans: Gateway to a new host-microbiome model. BMC Biol. 14:38. doi: 10.1186/s12915-016-0258-1.
- Ekschmitt, K., and G. W. Korthals. 2006. Nematodes as sentinels of heavy metals and organic toxicants in the soil. J. Nematol. 38:13–19.
- Elsworth, B., J. Wasmuth, and M. Blaxter. 2011. NEMBASE4: The nematode transcriptome resource. Int. J. Parasitol. 41:881–94. doi: 10.1016/j.ijpara.2011.03.009.
- Emmons, S. W. 2005. Male development (November 10, 2005), WormBook, ed. In WormBook. The C. elegans Research Community.
- Environmental Protection, U. S. Agency (US EPA). 2019. Directive to Prioritize Efforts to Reduce Animal Testing. Washington, DC: U.S. EPA
- Erkut, C., S. Penkov, H. Khesbak, D. Vorkel, J.-M. Verbavatz, K. Fahmy, and T. V. Kurzchalia. 2011. Trehalose renders the dauer larva of Caenorhabditis elegans resistant to extreme desiccation. Curr. Biol. 21:1331–36. doi: 10.1016/j.cub.2011.06.064.
- Evans, K. S., and E. C. Andersen. 2020. The gene scb-1 underlies variation in Caenorhabditis elegans chemotherapeutic responses. G3. (Bethesda). 10: 2353–64 doi: 10.1534/g3.120.401310.
- Evans, K. S., S. C. Brady, J. S. Bloom, R. E. Tanny, D. E. Cook, S. E. Giuliani, S. W. Hippleheuser, M. Zamanian, and E. C. Andersen. 2018. Shared genomic regions underlie natural variation in diverse toxin responses. Genetics. 210:1509–25. doi: 10.1534/genetics.118.301311.
- Ferguson, G. D., and W. J. Bridge. 2019. The glutathione system and the related thiol network in Caenorhabditis elegans. Redox. Biol. 24:101171. doi: 10.1016/j.redox.2019.101171.
- Ferreira, D. W., and P. Allard. 2015. Models of germ cell development and their application for toxicity studies. Environ. Mol. Mutagen. 56:637–49. doi: 10.1002/em.21946.
- Fielenbach, N., and A. Antebi. 2008. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 22:2149–65. doi: 10.1101/gad.1701508.
- Figueiredo, L. A., T. F. Rebouças, S. R. Ferreira, G. F. Rodrigues-Luiz, R. C. Miranda, R. N. Araujo, and R. T. Fujiwara. 2018. Dominance of P-glycoprotein 12 in phenotypic resistance conversion against ivermectin in Caenorhabditis elegans. PloS. One. 13: e0192995–e0192995. doi: 10.1371/journal.pone.0192995.
- Fischer, M., C. Regitz, M. Kahl, M. Werthebach, M. Boll, and U. Wenzel. 2012. Phytoestrogens genistein and daidzein affect immunity in the nematode Caenorhabditis elegans via alterations of vitellogenin expression. Mol. Nutr. Food. Res. 56:957–65. doi: 10.1002/mnfr.201200006.
- Fisher, A. L., and G. J. Lithgow. 2006. The nuclear hormone receptor DAF-12 has opposing effects on Caenorhabditis elegans lifespan and regulates genes repressed in multiple long-lived worms. Aging.Cell. 5:127–38. doi: 10.1111/j.1474-9726.2006.00203.x.
- Fitch, D. H. 2005. Introduction to nematode evolution and ecology. WormBook:1–8. doi: 10.1895/wormbook.1.19.1.
- Fitsanakis, V. A., R. Negga, and H. E. Hatfield. 2014. Caenorhabditis elegans as a model for biomarkers of diseases and toxicities. Biomarker. Toxicol.: 113–28. doi: 10.1016/B978-0-12-404630-6.00006-3.
- Fontaine, P., and K. Choe. 2018. The transcription factor SKN-1 and detoxification gene ugt-22 alter albendazole efficacy in Caenorhabditis elegans. Int J Parasitol Drugs Drug Resist 8: 312–19. doi: 10.1016/j.ijpddr.2018.04.006.
- Franco, A. L. C., L. A. Gherardi, C. M. de Tomasel, W. S. Andriuzzi, K. E. Ankrom, E. A. Shaw, E. M. Bach, O. E. Sala, and D. H. Wall. 2019. Drought suppresses soil predators and promotes root herbivores in mesic, but not in xeric grasslands. Proc. Natl. Acad. Sci. U.S.A. 116:12883–88. doi: 10.1073/pnas.1900572116.
- Freedman, J. H., L. W. Slice, D. Dixon, A. Fire, and C. S. Rubin. 1993. The novel metallothionein genes of Caenorhabditis elegans. Structural organization and inducible, cell-specific expression. J. Biol. Chem. 268:2554–64.
- Fukushige, T., H. E. Smith, J. Miwa, M. W. Krause, and J. A. Hanover. 2017. A genetic analysis of the Caenorhabditis elegans detoxification response. Genetics. 206: 939–52. doi: 10.1534/genetics.117.202515.
- Fuxman Bass, J. I., C. Pons, L. Kozlowski, J. S. Reece-Hoyes, S. Shrestha, A. D. Holdorf, A. Mori, C. L. Myers, and A. J. Walhout. 2016. A gene-centered C. elegans protein-DNA interaction network provides a framework for functional predictions. Mol. Syst. Biol. 12: 884. doi: 10.15252/msb.20167131.
- Gallo, M., D. Park, D. S. Luciani, K. Kida, F. Palmieri, O. E. Blacque, J. D. Johnson, and D. L. Riddle. 2011. MISC-1/OGC links mitochondrial metabolism, Apoptosis and insulin secretion. PLoS .ONE. 6:e17827
- Garcia-Gonzalez, A. P., A. D. Ritter, S. Shrestha, E. C. Andersen, L. S. Yilmaz, and A. J. M. Walhout. 2017. Bacterial metabolism affects the C. elegans response to cancer chemotherapeutics. Cell. 169:431–441 e8. doi: 10.1016/j.cell.2017.03.046
- Gaytan, B. D., and C. D. Vulpe. 2014. Functional toxicology: Tools to advance the future of toxicity testing. Front Genet 5:110. doi: 10.3389/fgene.2014.00110.
- Gerbaba, T. K., L. Green-Harrison, and A. G. Buret. 2017. Modeling host-microbiome interactions In. Caenorhabditis Elegans. J Nematol 49: 348–56.
- Gerisch, B., C. Weitzel, C. Kober-Eisermann, V. Rottiers, and A. Antebi. 2001. A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Dev. Cell 1:841–51. doi: 10.1016/s1534-5807(01)00085-5.
- Gill, M. S., J. M. Held, A. L. Fisher, B. W. Gibson, and G. J. Lithgow. 2004. Lipophilic regulator of a developmental switch in Caenorhabditis elegans. Aging.Cell. 3:413–21. doi: 10.1111/j.1474-9728.2004.00126.x.
- Glavinas, H., P. Krajcsi, J. Cserepes, and B. Sarkadi. 2004. The role of ABC transporters in drug resistance, metabolism and toxicity. Curr. Drug. Deliv. 1: 27–42. doi: 10.2174/1567201043480036.
- Goh, G. Y. S., J. J. Winter, F. Bhanshali, K. R. S. Doering, R. Lai, K. Lee, E. A. Veal, and S. Taubert. 2018. NHR-49/HNF4 integrates regulation of fatty acid metabolism with a protective transcriptional response to oxidative stress and fasting. Aging.Cell. 17:e12743. doi: 10.1111/acel.12743.
- Gong, P., K. B. Donohue, A. M. Mayo, Y. Wang, H. Hong, M. S. Wilbanks, N. D. Barker, X. Guan, and K. A. Gust. 2018. Comparative toxicogenomics of three insensitive munitions constituents 2,4-dinitroanisole, nitroguanidine and nitrotriazolone in the soil nematode Caenorhabditis elegans. BMC .Syst .Biol 12 (Suppl 7): 92–92. doi: 10.1186/s12918-018-0636-0.
- Gonzalez-Moragas, L., L. L. Maurer, V. M. Harms, J. N. Meyer, A. Laromaine, and A. Roig. 2017b. Materials and toxicological approaches to study metal and metal-oxide nanoparticles in the model organism Caenorhabditis elegans.” Mater. Horiz. 4:719–46. doi: 10.1039/C7MH00166E.
- Gonzalez-Moragas, L., P. Berto, C. Vilches, R. Quidant, A. Kolovou, R. Santarella-Mellwig, Y. Schwab, S. Stürzenbaum, A. Roig, and A. Laromaine. 2017a. In vivo testing of gold nanoparticles using the Caenorhabditis elegans model organism. Acta Biomater 53:598–609. doi: 10.1016/j.actbio.2017.01.080.
- Govindan, J. A., E. Jayamani, X. Zhang, E. Mylonakis, and G. Ruvkun. 2015. Dialogue between E. coli free radical pathways and the mitochondria of C. elegans. Proc. Natl. Acad. Sci. U.S.A. 112:12456–61. doi: 10.1073/pnas.1517448112.
- Gracida, X., and C. R. Eckmann. 2013. Fertility and germline stem cell maintenance under different diets requires NHR-114/HNF4 in C. Elegans. Curr. Biol. 23: 607–13. doi: 10.1016/j.cub.2013.02.034.
- Grants, J. M., G. Y. Goh, and S. Taubert. 2015. The mediator complex of Caenorhabditis elegans: Insights into the developmental and physiological roles of a conserved transcriptional coregulator.” Nucl. Acids. Res. 43:2442–53. doi: 10.1093/nar/gkv037.
- Gu, Q. L., Y. Zhang, X. M. Fu, Z. L. Lu, Y. Yu, G. Chen, R. Ma, W. Kou, and Y. M. Lan. 2020. Toxicity and metabolism of 3-bromopyruvate in Caenorhabditis elegans. J. Zhejiang. Univ. Sci. B. 21:77–86. doi: 10.1631/jzus.B1900370.
- Guengerich, F. P. 2006. Cytochrome P450s and other enzymes in drug metabolism and toxicity. Aaps J 8:E101–E111. doi: 10.1208/aapsj080112.
- Guengerich, F. P. 2008. Cytochrome p450 and chemical toxicology. Chem. Res. Toxicol. 21: 70–83. doi: 10.1021/tx700079z.
- Gujar, M. R., A. M. Stricker, and E. A. Lundquist. 2017. Flavin monooxygenases regulate Caenorhabditis elegans axon guidance and growth cone protrusion with UNC-6/Netrin signaling and Rac GTPases. PLoS Genet. 13:e1006998. doi: 10.1371/journal.pgen.1006998.
- Haegerbaeumer, A., R. Raschke, N. Reiff, W. Traunspurger, and S. Hoss. 2019. Comparing the effects of fludioxonil on non-target soil invertebrates using ecotoxicological methods from single-species bioassays to model ecosystems. Ecotoxicol. Environ. Saf. 183:109596. doi: 10.1016/j.ecoenv.2019.109596.
- Haegerbaeumer, A., S. Hoss, P. Heininger, and W. Traunspurger. 2018a. Is Caenorhabditis elegans representative of freshwater nematode species in toxicity testing? Environ. Sci. Pollut. Res. Int. 25:2879–88. doi: 10.1007/s11356-017-0714-7.
- Haegerbaeumer, A., S. Hoss, P. Heininger, and W. Traunspurger. 2018b. Response of nematode communities to metals and PAHs in freshwater microcosms. Ecotoxicol. Environ. Saf. 148:244–53. doi: 10.1016/j.ecoenv.2017.10.030.
- Hagerbaumer, A., S. Hoss, P. Heininger, and W. Traunspurger. 2015. Experimental studies with nematodes in ecotoxicology: An overview. J. Nematol. 47:11–27.
- Hahn, M. E. 2019. Evolutionary concepts can benefit both fundamental research and applied research in toxicology (A comment on Brady et al. 2017). Evol. Appl 12:350–52. doi: 10.1111/eva.12695.
- Haitzer, M., B. K. Burnison, S. Höss, W. Traunspurger, and C. E. W. Steinberg. 1999. Effects of quantity, quality, and contact time of dissolved organic matter on bioconcentration of benzo[a]pyrene in the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 18: 459–65. doi: 10.1002/etc.5620180314.
- Hamon, Y., C. Broccardo, O. Chambenoit, M.-F. Luciani, F. Toti, S. Chaslin, J.-M. Freyssinet, P. F. Devaux, J. McNeish, D. Marguet, et al. 2000. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat. Cell Biol. 2: 399–406. doi: 10.1038/35017029.
- Hanada, K., K. Nishijima, H. Ogata, S. Atagi, and M. Kawahara. 2001. Population pharmacokinetic analysis of cisplatin and its metabolites in cancer patients: Possible misinterpretation of covariates for pharmacokinetic parameters calculated from the concentrations of unchanged cisplatin, ultrafiltered platinum and total platinum. Jap. J. Clin. Oncol. 31: 179–84. doi: 10.1093/jjco/hye040.
- Harborne, J. B. 1988. Introduction to Ecological Biochemistry. 3rd ed. London; San Diego: Academic Press.
- Harlow, P. H., S. J. Perry, A. J. Stevens, and A. J. Flemming. 2018. Comparative metabolism of xenobiotic chemicals by cytochrome P450s in the nematode Caenorhabditis elegans. Rep. 8:13333. doi: 10.1038/s41598-018-31215-w.
- Harlow, P. H., S. J. Perry, S. Widdison, S. Daniels, E. Bondo, C. Lamberth, R. A. Currie, and A. J. Flemming. 2016. The nematode Caenorhabditis elegans as a tool to predict chemical activity on mammalian development and identify mechanisms influencing toxicological outcome. Sci. Rep. 6:22965. doi: 10.1038/srep22965.
- Harrington, A. J., S. Hamamichi, G. A. Caldwell, and K. A. Caldwell. 2010. C. elegans as a model organism to investigate molecular pathways involved with Parkinson’s disease. Dev.Dynamics. 239: 1282–95 doi: 10.1002/Dvdy.22231.
- Harris, J. B., J. H. Hartman, A. L. Luz, J. Y. Wilson, A. Dinyari, and J. N. Meyer. 2020. Zebrafish CYP1A expression in transgenic Caenorhabditis elegans protects from exposures to benzo[a]pyrene and a complex polycyclic aromatic hydrocarbon mixture Toxicology. 440: 152473. doi: 10.1016/j.tox.2020.152473.
- Harris, T. R., P. A. Aronov, P. D. Jones, H. Tanaka, M. Arand, and B. D. Hammock. 2008. Identification of two epoxide hydrolases in Caenorhabditis elegans that metabolize mammalian lipid signaling molecules. Arch. Biochem. Biophys. 472:139–49. doi: 10.1016/j.abb.2008.01.016.
- Hartman, P. S., and R. K. Herman. 1982. Radiation-sensitive mutants of Caenorhabditis elegans. Genetics. 102:159–78.
- Harvey, S. C., and H. E. Orbidans. 2011. All eggs are not equal: The maternal environment affects progeny reproduction and developmental fate in Caenorhabditis elegans. PLoS. One. 6:e25840. doi: 10.1371/journal.pone.0025840.
- Haschek, W. M., M. Berenbaum, D. E. Hinton, M. Cora, N. Chernoff, G. Travlos, C. W. Liu, K. Lu, and M. Law. 2019. Pathology in ecological research with implications for one health: Session summary. Toxicol. Pathol. 47:1072–75. doi: 10.1177/0192623319880530.
- Hasegawa, K., and J. Miwa. 2010. Genetic and cellular characterization of Caenorhabditis elegans mutants abnormal in the regulation of many phase II enzymes. PLoS. One. 5: e11194. doi: 10.1371/journal.pone.0011194.
- Hasegawa, K., S. Miwa, K. Isomura, K. Tsutsumiuchi, H. Taniguchi, and J. Miwa. 2007. Acrylamide-responsive genes in the nematode Caenorhabditis elegans. Toxicol. Sci. 101: 215–25. doi: 10.1093/toxsci/kfm276.
- Hasegawa, K., S. Miwa, K. Tsutsumiuchi, and J. Miwa. 2010. Allyl isothiocyanate that induces GST and UGT expression confers oxidative stress resistance on C. elegans, as demonstrated by nematode biosensor. Plos. One. 5:e9267. doi: 10.1371/journal.pone.0009267.
- Hattori, K., M. Inoue, T. Inoue, H. Arai, and H. Tamura. 2006. A novel sulfotransferase abundantly expressed in the dauer larvae of Caenorhabditis elegans. J. Biochem. 139: 355–62. doi: 10.1093/jb/mvj041.
- Hediger, M. A., B. Clémençon, R. E. Burrier, and E. A. Bruford. 2013. The ABCs of membrane transporters in health and disease (SLC series): Introduction. Mol. Aspects Med. 34: 95–107. doi: 10.1016/j.mam.2012.12.009.
- Helmcke, K. J., D. S. Avila, and M. Aschner. 2010. Utility of Caenorhabditis elegans in high throughput neurotoxicological research. Neurotoxicol. Teratol. 32: 62–67. doi: 10.1016/j.ntt.2008.11.005.
- Helmcke, K. J., T. Syversen, D. M. Miller 3rd, and M. Aschner. 2009. Characterization of the effects of methylmercury on Caenorhabditis elegans. Toxicol. Appl. Pharmacol. 240: 265–72.
- Hengartner, M. O., and H. R. Horvitz. 1994. Programmed cell death in Caenorhabditis elegans. Curr. Opin. Genet. Dev. 4: 581–86.
- Herholz, M., E. Cepeda, L. Baumann, A. Kukat, J. Hermeling, S. Maciej, K. Szczepanowska, V. Pavlenko, P. Frommolt, and A. Trifunovic. 2019. KLF-1 orchestrates a xenobiotic detoxification program essential for longevity of mitochondrial mutants. Nat. Commun. 10: 3323. doi: 10.1038/s41467-019-11275-w.
- Herndon, L. A., P. J. Schmeer, J. M. Dudaronek, P. A. Brown, K. M. Listner, Y. Sakano, M. C. Paupard, D. H. Hall, and M. Driscoll. 2002. Stochastic and genetic factors influence tissue-specific decline in ageing C. Elegans. Nature. 419: 808–14.
- Hirani, N., M. Westenberg, P. T. Seed, M. I. R. Petalcorin, and C. T. Dolphin. 2016. C. elegans flavin-containing monooxygenase-4 is essential for osmoregulation in hypotonic stress. Biol. Open. 5: 537–49. doi: 10.1242/bio.017400.
- Hobert, O. 2005. Specification of the nervous system. In WormBook, edited by The C. elegans Research Community. Internet: Wormbook.
- Hoffmann, J. M., and L. Partridge. 2015. Nuclear hormone receptors: Roles of xenobiotic detoxification and sterol homeostasis in healthy aging. Crit. Rev. Biochem. Mol. Biol. 50: 380–92. doi: 10.3109/10409238.2015.1067186.
- Höglund, P. J., K. J. V. Nordström, H. B. Schiöth, and R. Fredriksson. 2011. The solute carrier families have a remarkably long evolutionary history with the majority of the human families present before divergence of Bilaterian species. Mol. Biol. Evol. 28:1531–41. doi: 10.1093/molbev/msq350.
- Honnen, S. 2017. Caenorhabditis elegans as a powerful alternative model organism to promote research in genetic toxicology and biomedicine. Arch. Toxicol. 91:2029–44. doi: 10.1007/s00204-017-1944-7.
- Horsman, J. W., and D. L. Miller. 2016. Mitochondrial sulfide quinone oxidoreductase prevents activation of the unfolded protein response in hydrogen sulfide. J. Biol. Chem. 291: 5320–25. doi: 10.1074/jbc.M115.697102.
- Höss, S., K. Schlottmann, and W. Traunspurger. 2011. Toxicity of ingested cadmium to the nematode Caenorhabditis elegans. Environ. Sci. Technol. 45:10219–25. doi: 10.1021/es2027136.
- Hoss, S., and L. Weltje. 2007. Endocrine disruption in nematodes: Effects and mechanisms. Ecotoxicology. 16:15–28. doi: 10.1007/s10646-006-0108-y.
- Hoss, S., M. Haitzer, W. Traunspurger, and C. E. W. Steinberg. 1999. Growth and fertility of Caenorhabditis elegans (Nematoda) in unpolluted freshwater sediments: Response to particle size distribution and organic content. Environ. Toxicol. Chem. 18:2921–25.
- Hoss, S., M. HAitzer, W. Traunspurger, H. Gratzer, W. Ahlf, and C. Steinberg. 1997. Influence of particle size distribution and content of organic matter on the toxicity of copper in sediment bioassays using Caenorhabditis elegans (nematoda). Water. Air. Soi.l Pollut. 99: 689–95.
- Hoss, S., S. Jansch, T. Moser, T. Junker, and J. Rombke. 2009. Assessing the toxicity of contaminated soils using the nematode Caenorhabditis elegans as test organism. Ecotoxicol. Environ. Saf. 72:1811–18. doi: 10.1016/j.ecoenv.2009.07.003.
- Hotchkiss, A. K., C. V. Rider, C. R. Blystone, V. S. Wilson, P. C. Hartig, G. T. Ankley, P. M. Foster, C. L. Gray, and L. E. Gray. 2008. Fifteen years after “Wingspread”–environmental endocrine disrupters and human and wildlife health: Where we are today and where we need to go. Toxicol. Sci. 105:235–59. doi: 10.1093/toxsci/kfn030.
- Hu, Q., D. R. D’Amora, L. T. MacNeil, A. J. M. Walhout, and T. J. Kubiseski. 2018. The Caenorhabditis elegans oxidative stress response requires the NHR-49 transcription factor. G3. (Bethesda). 8: 3857–63 doi: 10.1534/g3.118.200727.
- Huang, X., J. A. Powell-Coffman, and Y. Jin. 2004. The AHR-1 aryl hydrocarbon receptor and its co-factor the AHA-1 aryl hydrocarbon receptor nuclear translocator specify GABAergic neuron cell fate in C. Elegans. Development. 131:819–28. doi: 10.1242/dev.00959.
- Hunt, P. R. 2017. The C. elegans model in toxicity testing. J. Appl. Toxicol. 37:50–59. doi: 10.1002/jat.3357.
- Hunt, P. R., N. Olejnik, K. D. Bailey, C. A. Vaught, and R. L. Sprando. 2018. C. elegans development and activity test detects mammalian developmental neurotoxins. Food Chem. Toxicol. 121:583–92. doi: 10.1016/j.fct.2018.09.061.
- International Standard Organization (ISO). 2020. ISO 10872 - Water and soil quality - Determination of the toxic effect of sediment and soil samples on growth, fertility and reproduction of Caenorhabditis elegans (Nematoda). Switzerland: ISO.
- Ioannides, C. 2001. Xenobiotic metabolism: An overview. In Enzyme Systems that Metabolise Drugs and Other Xenobiotics, pp. 1–32.
- Jackson, B. P., P. L. Williams, A. Lanzirotti, and P. M. Bertsch. 2005. Evidence for biogenic pyromorphite formation by the nematode Caenorhabditis elegans. Environ. Sci. Technol. 39:5620–25. doi: 10.1021/es050154k.
- Jacobs, M. A., A. Alwood, I. Thaipisuttikul, D. Spencer, E. Haugen, S. Ernst, O. Will, R. Kaul, C. Raymond, R. Levy, et al. 2003. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 100:14339–44. doi: 10.1073/pnas.2036282100.
- James, C. E., and M. W. Davey. 2009. Increased expression of ABC transport proteins is associated with ivermectin resistance in the model nematode Caenorhabditis elegans. Int. J. Parasitol. 39:213–20. doi: 10.1016/j.ijpara.2008.06.009.
- Jeong, J., H. Kim, and J. Choi. 2019. In silico molecular docking and in vivo validation with Caenorhabditis elegans to discover molecular initiating events in adverse outcome pathway framework: Case study on endocrine-disrupting chemicals with estrogen and androgen receptors. Int. J. Mol. Sci. 20. doi: 10.3390/ijms20051209.
- Jia, K., P. S. Albert, and D. L. Riddle. 2002. DAF-9, a cytochrome P450 regulating C. elegans larval development and adult longevity. Development. 129: 221–31.
- Jin, M. S., M. L. Oldham, Q. Zhang, and J. Chen. 2012. Crystal structure of the multidrug transporter P-glycoprotein from Caenorhabditis elegans. Nature. 490: 566–69. doi: 10.1038/nature11448.
- Johnston, W. L., and J. W. Dennis. 2012. The eggshell in the C. elegans oocyte-to-embryo transition. Genesis. 50:333–49. doi: 10.1002/dvg.20823.
- Johnstone, I. L. 1994. The cuticle of the nematode Caenorhabditis elegans: A complex collagen structure. Bioessays. 16:171–78. doi: 10.1002/bies.950160307.
- Johnstone, I. L. 2000. Cuticle collagen genes: Expression in Caenorhabditis elegans. Trends. Genet. 16:21–27. doi: 10.1016/S0168-9525(99)01857-0.
- Jones, L. M., A. J. Flemming, and P. E. Urwin. 2015. NHR-176 regulates CYP-35d1 to control hydroxylation-dependent metabolism of thiabendazole in Caenorhabditis elegans. Biochem. J. 466: 37–44. doi: 10.1042/BJ20141296.
- Jones, L. M., S. J. Rayson, A. J. Flemming, and P. E. Urwin. 2013. Adaptive and specialised transcriptional responses to xenobiotic stress in Caenorhabditis elegans are regulated by nuclear hormone receptors. PLoS. One. 8: e69956. doi: 10.1371/journal.pone.0069956.
- Kamath, R. S., A. G. Fraser, Y. Dong, G. Poulin, R. Durbin, M. Gotta, A. Kanapin, N. Le Bot, S. Moreno, M. Sohrmann, et al. 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 421: 231–37.
- Kapitonov, D., and R. K. Yu. 1999. Conserved domains of glycosyltransferases. Glycobiology. 9:961–78. doi: 10.1093/glycob/9.10.961.
- Kell, D. 2020a. How drugs really get into cells: Why passive bilayer diffusion is a myth.
- Kell, D. B. 2020b. Hitchhiking into the cell. Nat. Chem. Biol. 16:367–68. doi: 10.1038/s41589-020-0489-x.
- Kelly, W. G. 2014. Transgenerational epigenetics in the germline cycle of Caenorhabditis elegans. Epigenet. Chromatin. 7: 6. doi: 10.1186/1756-8935-7-6.
- Kiontke, K., and D. H. Fitch. 2013. Nematodes. Curr. Biol. 23:R862–R864. doi: 10.1016/j.cub.2013.08.009.
- Klaassen, C. D. 2019. Casarett and Doull’s Toxicology, 9th ed., 159–169. Blacklick, USA, United States: McGraw-Hill Professional Publishing.
- Koontz, J. M., B. C. R. Dancy, C. L. Horton, J. D. Stallings, V. T. DiVito, and J. A. Lewis. 2019. The role of the human microbiome in chemical toxicity. Int. J. Toxicol. 38: 251–64. doi: 10.1177/1091581819849833.
- Koppel, N., V. Maini Rekdal, and E. P. Balskus. 2017. Chemical transformation of xenobiotics by the human gut microbiota. Science 356. doi: 10.1126/science.aag2770.
- Kulas, J., C. Schmidt, M. Rothe, W. H. Schunck, and R. Menzel. 2008. Cytochrome P450-dependent metabolism of eicosapentaenoic acid in the nematode Caenorhabditis Elegans. Arch. Biochem. Biophys. 472:65–75.
- Kurz, C. L., M. Shapira, K. Chen, D. L. Baillie, and M.-W. Tan. 2007. Caenorhabditis elegans pgp-5 is involved in resistance to bacterial infection and heavy metal and its regulation requires TIR-1 and a p38 map kinase cascade. Biochem. Biophys. Res. Commun. 363: 438–43. doi: 10.1016/j.bbrc.2007.08.190.
- Laing, S. T., A. Ivens, R. Laing, S. Ravikumar, V. Butler, D. J. Woods, and J. S. Gilleard. 2010. Characterization of the xenobiotic response of Caenorhabditis elegans to the anthelmintic drug albendazole and the identification of novel drug glucoside metabolites. Biochem. J. 432: 505–16. doi: 10.1042/BJ20101346.
- Lažetić, V., and D. S. Fay. 2017. Molting in C. elegans. Worm. 6:e1330246–e1330246. doi: 10.1080/21624054.2017.1330246.
- Lee, J. H., Y. G. Kim, M. Kim, E. Kim, H. Choi, Y. Kim, and J. Lee. 2017. Indole-associated predator-prey interactions between the nematode Caenorhabditis elegans and bacteria. Environ. Microbiol. 19:1776–90. doi: 10.1111/1462-2920.13649.
- Lee, S., Y. Kim, and J. Choi. 2020. Effect of soil microbial feeding on gut microbiome and cadmium toxicity in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 187:109777. doi: 10.1016/j.ecoenv.2019.109777.
- Leiser, S. F., H. Miller, R. Rossner, M. Fletcher, A. Leonard, M. Primitivo, N. Rintala, F. J. Ramos, D. L. Miller, and M. Kaeberlein. 2015. Cell nonautonomous activation of flavin-containing monooxygenase promotes longevity and health span. Science 350:1375–78. doi: 10.1126/science.aac9257.
- Lenz, K. A., C. Pattison, and H. Ma. 2017. Triclosan (TCS) and triclocarban (TCC) induce systemic toxic effects in a model organism the nematode Caenorhabditis elegans. Environ. Pollut. 231: 462–70 doi: 10.1016/j.envpol.2017.08.036.
- Leung, M. C., J. V. Goldstone, W. A. Boyd, J. H. Freedman, and J. N. Meyer. 2010. Caenorhabditis elegans generates biologically relevant levels of genotoxic metabolites from aflatoxin B1 but not benzo[a]pyrene in vivo. Toxicol. Sci. 118:444–53. doi: 10.1093/toxsci/kfq295.
- Leung, M. C., P. L. Williams, A. Benedetto, C. Au, K. J. Helmcke, M. Aschner, and J. N. Meyer. 2008. Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology. Toxicol. Sci. 106:5–28. doi: 10.1093/toxsci/kfn121.
- Leung, M. C. K., A. C. Procter, J. V. Goldstone, J. Foox, R. DeSalle, C. J. Mattingly, M. E. Siddall, and A. R. Timme-Laragy. 2017. Applying evolutionary genetics to developmental toxicology and risk assessment. Reprod. Toxicol. 69:174–86. doi: 10.1016/j.reprotox.2017.03.003.
- Liao, V. H., and C. W. Yu. 2005. Caenorhabditis elegans gcs-1 confers resistance to arsenic-induced oxidative stress. Biometals 18:519–28.
- Lincke, C. R., A. Broeks, I. The, R. H. Plasterk, and P. Borst. 1993. The expression of two P-glycoprotein (pgp) genes in transgenic Caenorhabditis elegans is confined to intestinal cells. Embo. J. 12:1615–20.
- Lindblom, T. H., and A. K. Dodd. 2006. Xenobiotic detoxification in the nematode Caenorhabditis elegans. J. Exp. Zoolog. Part A Comp. Exp. Biol. 305:720–30. doi: 10.1002/jez.a.324.
- Lindblom, T. H., G. J. Pierce, and A. E. Sluder. 2001. A C. elegans orphan nuclear receptor contributes to xenobiotic resistance. Curr. Biol. 11: 864–68. doi: 10.1016/s0960-9822(01)00236-6.
- Lints, R., and D. H. Hall 2009. The cuticle. In WormAtlas. doi:10.3908/wormatlas.1.12.
- Liu, F., Y. Zhang, M. Zhang, Q. Luo, X. Cao, C. Cui, K. Lin, and K. Huang. 2020. Toxicological assessment and underlying mechanisms of tetrabromobisphenol A exposure on the soil nematode Caenorhabditis elegans. Chemosphere 242: 125078. doi: 10.1016/j.chemosphere.2019.125078.
- Ludewig, A. H., M. Klapper, and F. Döring. 2014. Identifying evolutionarily conserved genes in the dietary restriction response using bioinformatics and subsequent testing in Caenorhabditis elegans. Genes Nutr 9:363–363. doi: 10.1007/s12263-013-0363-5.
- Luz, A. L., T. R. Godebo, D. P. Bhatt, O. R. Ilkayeva, L. L. Maurer, M. D. Hirschey, and J. N. Meyer. 2016. From the over: Arsenite uncouples mitochondrial respiration and induces a Warburg-like effect in Caenorhabditis elegans. Toxicol. Sci. 152:349–62. doi: 10.1093/toxsci/kfw093.
- Luz, A. L., T. R. Godebo, L. L. Smith, T. C. Leuthner, L. L. Maurer, and J. N. Meyer. 2017. Deficiencies in mitochondrial dynamics sensitize Caenorhabditis elegans to arsenite and other mitochondrial toxicants by reducing mitochondrial adaptability. Toxicology. doi: 10.1016/j.tox.2017.05.018.
- Mackowiak, B., and H. Wang. 2016. Mechanisms of xenobiotic receptor activation: Direct vs. indirect. Biochim. Biophys. Acta 1859:1130–40. doi: 10.1016/j.bbagrm.2016.02.006.
- Magner, D. B., J. Wollam, Y. Shen, C. Hoppe, D. Li, C. Latza, V. Rottiers, H. Hutter, and A. Antebi. 2013. The NHR-8 nuclear receptor regulates cholesterol and bile acid homeostasis in C. Elegans. Cell. Metab. 18:212–24. doi: 10.1016/j.cmet.2013.07.007.
- Mahajan-Miklos, S., M. W. Tan, L. G. Rahme, and F. M. Ausubel. 1999. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell. 96:47–56.
- Mak, H. Y., and G. Ruvkun. 2004. Intercellular signaling of reproductive development by the C. elegans DAF-9 cytochrome P450. Development. 131:1777–86. doi: 10.1242/dev.01069.
- Manallack, D. T., R. J. Prankerd, E. Yuriev, T. I. Oprea, and D. K. Chalmers. 2013. The significance of acid/base properties in drug discovery. Chem. Soc. Rev. 42:485–96. doi: 10.1039/c2cs35348b.
- Martin, J., B. A. Rosa, P. Ozersky, K. Hallsworth-Pepin, X. Zhang, V. Bhonagiri-Palsikar, R. Tyagi, Q. Wang, Y. J. Choi, X. Gao, et al. 2015. Helminth.net: Expansions to nematode.net and an introduction to trematode.net. Nucl. Acids. Res. 43 ( Database issue): D698–D06. doi: 10.1093/nar/gku1128.
- Mattingly, C. J., M. C. Rosenstein, A. P. Davis, G. T. Colby, J. N. Forrest Jr., and J. L. Boyer. 2006. The comparative toxicogenomics database: A cross-species resource for building chemical-gene interaction networks. Toxicol. Sci. 92:587–95. doi: 10.1093/toxsci/kfl008.
- Maupas, E. 1900. Modes et formes de reproduction des nématodes. Arch. Zool. Exp. 8:463–624.
- Maurer, L. L., A. L. Luz, and J. N. Meyer. 2018. Detection of mitochondrial toxicity of environmental pollutants using Caenorhabditis elegans. In Drug-Induced Mitochondrial Dysfunction, edited by J. A. Dykens, and Y. Will, 655-689. Hoboken, NJ, USA: Wiley.
- Maurer, L. L., X. Yang, A. J. Schindler, R. K. Taggart, C. Jiang, H. Hsu-Kim, D. R. Sherwood, and J. N. Meyer. 2015. Intracellular trafficking pathways in silver nanoparticle uptake and toxicity in Caenorhabditis Elegans. Nanotoxicology:1–5. doi: 10.3109/17435390.2015.1110759.
- Maurer, L. L., X. Yang, A. J. Schindler, R. K. Taggart, C. Jiang, H. Hsu-Kim, D. R. Sherwood, and J. N. Meyer. 2016. Intracellular trafficking pathways in silver nanoparticle uptake and toxicity in Caenorhabditis elegans. Nanotoxicology 10:831–35. doi: 10.3109/17435390.2015.1110759.
- McDonnell, A. M., and C. H. Dang. 2013. Basic review of the cytochrome p450 system. J. Adv. Practitioner. Oncol. 4:263–68. doi: 10.6004/jadpro.2013.4.4.7.
- McGhee, J. D. 2013. The Caenorhabditis elegans intestine. Wiley.Interdiscip. Rev. Dev. Biol. 2: 347–67. doi: 10.1002/wdev.93.
- Meech, R., D. G. Hu, R. A. McKinnon, S. N. Mubarokah, A. Z. Haines, P. C. Nair, A. Rowland, and P. I. Mackenzie. 2019. The UDP-glycosyltransferase (UGT) superfamily: New members, new functions, and novel paradigms. Physiol. Rev. 99:1153–222. doi: 10.1152/physrev.00058.2017.
- Melendez, A., and B. Levine. 2009. Autophagy in C. elegans. WormBook:1–26
- Menez, C., M. Alberich, E. Courtot, F. Guegnard, A. Blanchard, H. Aguilaniu, and A. Lespine. 2019. The transcription factor NHR-8: A new target to increase ivermectin efficacy in nematodes. PLoS Pathog. 15:e1007598. doi: 10.1371/journal.ppat.1007598.
- Menzel, R., S. Sturzenbaum, A. Barenwaldt, J. Kulas, and C. E. Steinberg. 2005. Humic material induces behavioral and global transcriptional responses in the nematode Caenorhabditis. Elegans. Environ. Sci. Technol. 39:8324–32.
- Menzel, R., T. Bogaert, and R. Achazi. 2001. A systematic gene expression screen of Caenorhabditis elegans cytochrome P450 genes reveals CYP35 as strongly xenobiotic inducible. Arch. Biochem. Biophys. 395:158–68.
- Mesnage, R., M. N. Antoniou, D. Tsoukalas, G. N. Goulielmos, and A. Tsatsakis. 2018. Gut microbiome metagenomics to understand how xenobiotics impact human health Curr. Opin. Toxicol. 11-12:51–58.
- Metzler, R., E. A. Meleshkevitch, J. Fox, H. Kim, and D. Y. Boudko. 2013. An SLC6 transporter of the novel B0,− system aids in absorption and detection of nutrient amino acids in Caenorhabditis elegans. J. Exp. Biol. 216:2843–57. doi: 10.1242/jeb.081497.
- Meyer, D., and P. L. Williams. 2014. Toxicity testing of neurotoxic pesticides in Caenorhabditis elegans. J. Toxicol. Environ. Health B. 17: 284–306. doi: 10.1080/10937404.2014.933722.
- Meyer, J. N., C. A. Lord, X. Y. Yang, E. A. Turner, A. R. Badireddy, S. M. Marinakos, A. Chilkoti, M. R. Wiesner, and M. Auffan. 2010. Intracellular uptake and associated toxicity of silver nanoparticles in Caenorhabditis elegans. Aquat. Toxicol. 100:140–50. doi: 10.1016/j.aquatox.2010.07.016.
- Mimoto, A., M. Fujii, M. Usami, M. Shimamura, N. Hirabayashi, T. Kaneko, N. Sasagawa, and S. Ishiura. 2007. Identification of an estrogenic hormone receptor in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 364:883–88. doi: 10.1016/j.bbrc.2007.10.089.
- Mitchell, P. H., K. Bull, S. Glautier, N. A. Hopper, L. Holden-Dye, and V. O’Connor. 2007. The concentration-dependent effects of ethanol on Caenorhabditis elegans behaviour. Pharmacogenomics J. 7:411–17. doi: 10.1038/sj.tpj.6500440.
- Miwa, J., E. Schierenberg, S. Miwa, and G. von Ehrenstein. 1980. Genetics and mode of expression of temperature-sensitive mutations arresting embryonic development in Caenorhabditis elegans. Dev. Biol. 76:160–74. doi: 10.1016/0012-1606(80)90369-3.
- Monosson, E. 2012. Evolution in a Toxic World: How Life Responds to Chemical Threats. Washington, D.C: Island Press.
- Monteverdi, G. H., and R. T. Di Giulio. 2000. In vitro and in vivo association of 2,3,7,8-tetrachlorodibenzo-p-dioxin and benzo[a]pyrene with the yolk-precursor protein vitellogenin. Environ. Toxicol. Chem. 19: 2502–11. doi: 10.1002/etc.5620191016.
- Motola, D. L., C. L. Cummins, V. Rottiers, K. K. Sharma, T. Li, Y. Li, K. Suino-Powell, H. E. Xu, R. J. Auchus, A. Antebi, et al. 2006. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. Elegans. Cell. 12: 1209–23. doi: 10.1016/j.cell.2006.01.037.
- Murphy, C. T. 2006. The search for DAF-16/FOXO transcriptional targets: Approaches and discoveries. Exp. Gerontol. 41:910–21. doi: 10.1016/j.exger.2006.06.040.
- Na, H., O. Ponomarova, G. E. Giese, and A. J. M. Walhout. 2018. C. elegans MRP-5 exports vitamin B12 from mother to offspring to support embryonic development. Cell. Rep. 22: 3126–33. doi: 10.1016/j.celrep.2018.02.100.
- Nass, R., D. H. Hall, D. M. Miller, and R. D. Blakely. 2002. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc. Nat. Acad. Sc.i USA. 99:3264–69.
- Nass, R., D. M. Miller, and R. D. Blakely. 2001. C-elegans: A novel pharmacogenetic model to study Parkinson’s disease. Parkinsonism. Related. Disorders. 7:185–91.
- Nass, R., and I. Hamza. 2007. The nematode C. elegans as an animal model to explore toxicology in vivo: Solid and axenic growth culture conditions and compound exposure parameters. Curr. Protoc. Toxicol. Chapter. 1: Unit1 9. doi: 10.1002/0471140856.tx0109s31.
- National Academies of Sciences, Engineering, and Medicine. 2017. Application of Systematic Review Methods in an Overall Strategy for Evaluating Low-Dose Toxicity from Endocrine Active Chemicals. Washington, DC: The National Academies Press.
- National Academies of Sciences, Engineering, and Medicine. 2018. Environmental Chemicals, the Human Microbiome, and Health Risk: A Research Strategy. Washington, DC: The National Academies Press.
- Nebert, D. W., K. Wikvall, and W. L. Miller. 2013. Human cytochromes P450 in health and disease. Phil.Trans. R.Soc .Lond B. Biol. Sci. 368: 20120431. doi: 10.1098/rstb.2012.0431.
- Nebert, D. W., T. P. Dalton, A. B. Okey, and F. J. Gonzalez. 2004. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J. Biol. Chem. 279:23847–50. doi: 10.1074/jbc.R400004200.
- Negi, H., S. K. Saikia, R. Kanaujia, S. Jaiswal, and R. Pandey. 2017. 3β-Hydroxy-urs-12-en-28-oic acid confers protection against ZnONPs induced adversity in Caenorhabditis elegans. Environ. Toxicol. Pharmacol. 53:105–10. doi: 10.1016/j.etap.2017.05.004.
- Nelson, F. K., and D. L. Riddle. 1984. Functional study of the Caenorhabditis elegans secretory-excretory system using laser microsurgery. J. Exp. Zool. 231:45–56. doi: 10.1002/jez.1402310107.
- Nelson, F. K., P. S. Albert, and D. L. Riddle. 1983. Fine structure of the Caenorhabditis elegans secretory-excretory system. J. Ultrastruct. Res. 82:156–1571. doi: 10.1016/s0022-5320(83)90050-3.
- Nigon, V. M., and M. A. Felix. 2017. History of research on C. elegans and other free-living nematodes as model organisms. WormBook: 1–91. doi: 10.1895/wormbook.1.181.1.
- Nikoletopoulou, V., and N. Tavernarakis. 2014. Necrotic cell death in Caenorhabditis elegans. Meth. Enzymol. 545:127–55. doi: 10.1016/B978-0-12-801430-1.00006-8.
- Nuclear Receptors Nomenclature, Committee. 1999. “A unified nomenclature system for the nuclear receptor superfamily.” Cell. 97 (2):161–63. doi: 10.1016/s0092-8674(00)80726-6.
- Offermann, K., A. Matthäi, and W. Ahlf. 2009. Assessing the importance of dietborne cadmium and particle characteristics on bioavailability and bioaccumulation in the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 28:1149–58. doi: 10.1897/08-272.1.
- Oladimeji, P. O., and T. Chen. 2018. PXR: More than just a master xenobiotic receptor. Mol. Pharmacol. 93:119–27. doi: 10.1124/mol.117.110155.
- Olsen, A., and M. Gill. 2017. Ageing: Lessons from C. elegans.. Cham, Switzerland: Springer.
- Olson, S. K., G. Greenan, A. Desai, T. Müller-Reichert, and K. Oegema. 2012. Hierarchical assembly of the eggshell and permeability barrier in C. Elegans. J.Cell. Biology. 198: 731–48. doi: 10.1083/jcb.201206008.
- Page, A. P., and I. L. Johnstone 2007. The cuticle (March 19, 2007), WormBook, ed. In WormBook. The C. elegans Research Community. doi/10.1895/ wormbook.1.138.1, http://www.wormbook.org.
- Pender, C. L., and H. R. Horvitz. 2018. Hypoxia-inducible factor cell non-autonomously regulates C. elegans stress responses and behavior via a nuclear receptor. Elife. 7. doi: 10.7554/eLife.36828.
- Peng, Y., Z.-H. Tong, H.-J. Chong, and X.-Y. Shao. 2018. Toxic effects of prolonged exposure to [C14mim]Br on Caenorhabditis elegans. Chemosphere 208:226–32. doi: 10.1016/j.chemosphere.2018.05.176.
- Perally, S., E. J. LaCourse, A. M. Campbell, and P. M. Brophy. 2008. Heme transport and detoxification in nematodes: Subproteomics evidence of differential role of glutathione transferases. J. Proteome Res. 7:4557–65. doi: 10.1021/pr800395x.
- Peredney, C. L., and P. L. Williams. 2000. Utility of Caenorhabditis elegans for assessing heavy metal contamination in artificial soil. Arch. Environ. Contam. Toxicol. 39:113–18. doi: 10.1007/s002440010086.
- Perez, A., and J. Pierce WiseSr., 2018. One environmental health: An emerging perspective in toxicology. F1000Res 7. doi: 10.12688/f1000research.14233.1.
- Perkins, L. A., E. M. Hedgecock, J. N. Thomson, and J. G. Culotti. 1986. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117:456–87. doi: 10.1016/0012-1606(86)90314-3.
- Petalcorin, M. I., G. W. Joshua, P. M. Agapow, and C. T. Dolphin. 2005. The FMO genes of Caenorhabditis elegans and C. briggsae: Characterisation, gene expression and comparative genomic analysis. Gene. 346:83–96. doi: 10.1016/j.gene.2004.09.021.
- Peterson, N. D., H. K. Cheesman, P. Liu, S. M. Anderson, K. J. Foster, R. Chhaya, P. Perrat, J. Thekkiniath, Q. Yang, C. M. Haynes, et al. 2019. The nuclear hormone receptor NHR-86 controls anti-pathogen responses in C.elegans. PLoS Genet. 15:e1007935. doi: 10.1371/journal.pgen.1007935.
- Powell, J. R., and F. M. Ausubel. 2008. Models of Caenorhabditis elegans infection by bacterial and fungal pathogens. Meth.Mol. Biol. 415:403–27. doi: 10.1007/978-1-59745-570-1_24.
- Powell-Coffman, J. A., C. A. Bradfield, and W. B. Wood. 1998. Caenorhabditis elegans orthologs of the aryl hydrocarbon receptor and its heterodimerization partner the aryl hydrocarbon receptor nuclear translocator. Proc. Natl. Acad. Sci. U.S.A. 95:2844–49. doi: 10.1073/pnas.95.6.2844.
- Prahlad, V., and R. I. Morimoto. 2009. Integrating the stress response: Lessons for neurodegenerative diseases from C. elegans. Trends Cell Biol. 19:52–61. doi: 10.1016/j.tcb.2008.11.002.
- Pryor, R., P. Norvaisas, G. Marinos, L. Best, L. B. Thingholm, L. M. Quintaneiro, W. De Haes, D. Esser, S. Waschina, C. Lujan, et al. 2019. Host-microbe-drug-nutrient screen identifies bacterial effectors of metformin therapy. Cell. 178: 1299–1312 e29. doi: 10.1016/j.cell.2019.08.003.
- Pukkila-Worley, R., R. L. Feinbaum, D. L. McEwan, A. L. Conery, and F. M. Ausubel. 2014. The evolutionarily conserved mediator subunit MDT-15/MED15 links protective innate immune responses and xenobiotic detoxification. PLoS Pathog. 10:e1004143. doi: 10.1371/journal.ppat.1004143.
- Queiros, L., J. L. Pereira, F. J. M. Goncalves, M. Pacheco, M. Aschner, and P. Pereira. 2019. Caenorhabditis elegans as a tool for environmental risk assessment: Emerging and promising applications for a “nobelized worm”. Crit. Rev. Toxicol. 49:411–29. doi: 10.1080/10408444.2019.1626801.
- Rajkumar, P., B. S. Mathew, S. Das, R. Isaiah, S. John, R. Prabha, and D. H. Fleming. 2016. Cisplatin concentrations in long and short duration infusion: Implications for the optimal time of radiation delivery. J .Clin. Diagnostic. Res. 10: XC01–XC04. doi: 10.7860/JCDR/2016/18181.8126.
- Rappleye, C. A., A. Tagawa, N. Le Bot, J. Ahringer, and R. V. Aroian. 2003. Involvement of fatty acid pathways and cortical interaction of the pronuclear complex in Caenorhabditis elegans embryonic polarity. BMC Dev. Biol. 3:8.
- Reece-Hoyes, J. S., C. Pons, A. Diallo, A. Mori, S. Shrestha, S. Kadreppa, J. Nelson, S. Diprima, A. Dricot, B. R. Lajoie, et al. 2013. Extensive rewiring and complex evolutionary dynamics in a C. elegans multiparameter transcription factor network. Mol. Cell. 5:116–27. doi: 10.1016/j.molcel.2013.05.018.
- Rees, D. C., E. Johnson, and O. Lewinson. 2009. ABC transporters: The power to change. Nat. Rev. Mol. Cell Biol. 10:218–27. doi: 10.1038/nrm2646.
- Rich, J. R., R. A. Dunn, and J. W. Noling. 2004. Nematicides: Past and present uses. In Nematology: Advances and Perspectives, edited by Z. X. Chen, S. Y. Chen, and D. W. Dickson. Cambridge, MA: CABI Publishing. pp. 1179–200.
- Rieckher, M., A. Bujarrabal, M. A. Doll, N. Soltanmohammadi, and B. Schumacher. 2018. A simple answer to complex questions: Caenorhabditis elegans as an experimental model for examining the DNA damage response and disease genes. J. Cell. Physiol. 233: 2781–90. doi: 10.1002/jcp.25979.
- Ristow, M., and S. Schmeisser. 2011. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 51:327–36. doi: 10.1016/j.freeradbiomed.2011.05.010.
- Rodriguez, M., L. B. Snoek, M. De Bono, and J. E. Kammenga. 2013. Worms under stress: C. elegans stress response and its relevance to complex human disease and aging. Trends. Genet. 29:367–74. doi: 10.1016/j.tig.2013.01.010.
- Roh, J. Y., H. J. Lee, and J. H. Kwon. 2016. Internal concentration and time are important modifiers of toxicity: The case of chlorpyrifos on Caenorhabditis elegans. Environ. Sci. Technol. 50:9689–96. doi: 10.1021/acs.est.6b02751.
- Rutter, J. W., T. Ozdemir, E. R. Galimov, L. M. Quintaneiro, L. Rosa, G. M. Thomas, F. Cabreiro, and C. P. Barnes. 2019. Detecting changes in the Caenorhabditis elegans intestinal environment using an engineered bacterial biosensor. A. CS. Synth. Biol. 8: 2620–28. doi: 10.1021/acssynbio.9b00166.
- Sammi, S. R., Z. S. Agim, and J. R. Cannon. 2018. From the cover: Harmane-induced selective dopaminergic neurotoxicity in Caenorhabditis elegans. Toxicol. Sci. 161:335–48. doi: 10.1093/toxsci/kfx223.
- Schafer, P., M. Muller, A. Kruger, C. E. Steinberg, and R. Menzel. 2009. Cytochrome P450-dependent metabolism of PCB52 in the nematode Caenorhabditis elegans. Arch. Biochem. Biophys. 488:60–68.
- Schenk, J., S. Hoss, M. Brinke, N. Kleinbolting, H. Bruchner-Huttemann, and W. Traunspurger. 2020. Nematodes as bioindicators of polluted sediments using metabarcoding and microscopic taxonomy. Environ Int 143:105922. doi: 10.1016/j.envint.2020.105922.
- Schmeisser, K., J. Mansfeld, D. Kuhlow, S. Weimer, S. Priebe, I. Heiland, M. Birringer, M. Groth, A. Segref, Y. Kanfi, et al. 2013. Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide Nat. Chem. Biol. 9: 693–700. doi: 10.1038/nchembio.1352.
- Schmid, T., L. B. Snoek, E. Fröhli, M. Leontien van der Bent, J. Kammenga, and A. Hajnal. 2015. Systemic regulation of RAS/MAPK signaling by the serotonin metabolite 5-HIAA. PLoS. Genet. 11: e1005236. doi: 10.1371/journal.pgen.1005236.
- Schwartz, M. S., J. L. Benci, D. S. Selote, A. K. Sharma, A. G. Chen, H. Dang, H. Fares, and O. K. Vatamaniuk. 2010. Detoxification of multiple heavy metals by a half-molecule ABC transporter, HMT-1, and coelomocytes of Caenorhabditis elegans. PLoS. One. 5: e9564. doi: 10.1371/journal.pone.0009564.
- Scott, T. A., L. M. Quintaneiro, P. Norvaisas, P. P. Lui, M. P. Wilson, K. Y. Leung, L. Herrera-Dominguez, S. Sudiwala, A. Pessia, P. T. Clayton, et al. 2017. Host-microbe Co-metabolism dictates cancer drug efficacy in C. elegans. Cell. 169:442–456.e18. doi: 10.1016/j.cell.2017.03.040.
- Scotti, E., S. Boué, G. Lo Sasso, F. Zanetti, V. Belcastro, C. Poussin, N. Sierro, J. Battey, A. Gimalac, N. V. Ivanov, et al. 2017. Exploring the microbiome in health and disease: Implicationsfor toxicology. Toxicol. Res. Appl 1:2397847317741884. doi: 10.1177/2397847317741884.
- Sender, R., S. Fuchs, and R. Milo. 2016. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 14:e1002533. doi: 10.1371/journal.pbio.1002533.
- Sengupta, P. 2007. Generation and modulation of chemosensory behaviors in C. Elegans. Pflügers.Archiv - Eur. J Physiol. 454:721. doi: 10.1007/s00424-006-0196-9.
- Serrano-Saiz, E., M. C. Vogt, S. Levy, Y. Wang, K. K. Kaczmarczyk, X. Mei, G. Bai, A. Singson, B. D. Grant, and O. Hobert. 2020. „SLC17A6/7/8 Vesicular Glutamate Transporter Homologs in Nematodes.„Genetics 214 (1):163–178. doi:10.1534/genetics.119.302855
- Serrano-Saiz, E., R. J. Poole, T. Felton, F. Zhang, E. D. De La Cruz, and O. Hobert. 2013. Modular control of glutamatergic neuronal identity in C. elegans by distinct homeodomain proteins. Cell. 155:659–73. doi: 10.1016/j.cell.2013.09.052.
- Shaye, D. D., and I. Greenwald. 2011. OrthoList: A compendium of C. elegans genes with human orthologs. PLoS. One. 6: e20085. doi: 10.1371/journal.pone.0020085.
- Shen, C., D. Nettleton, M. Jiang, S. K. Kim, and J. A. Powell-Coffman. 2005. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans. J. Biol. Chem. 280:20580–88. doi: 10.1074/jbc.M501894200.
- Shen, P., R. Zhang, D. J. McClements, and Y. Park. 2019. Nanoemulsion-based delivery systems for testing nutraceutical efficacy using Caenorhabditis elegans: Demonstration of curcumin bioaccumulation and body-fat reduction. Food. Res. Int. 120:157–66. doi: 10.1016/j.foodres.2019.02.036.
- Sheps, J. A., S. Ralph, Z. Zhao, D. L. Baillie, and V. Ling. 2004. The ABC transporter gene family of Caenorhabditis elegans has implications for the evolutionary dynamics of multidrug resistance in eukaryotes. Genome Biol. 5:R15. doi: 10.1186/gb-2004-5-3-r15.
- Shiizaki, K., M. Kawanishi, and T. Yagi. 2017. Modulation of benzo[a]pyrene-DNA adduct formation by CYP1 inducer and inhibitor. Genes.Environ. 39:14. doi: 10.1186/s41021-017-0076-x.
- Shomer, N., A. Z. Kadhim, J. M. Grants, X. Cheng, D. Alhusari, F. Bhanshali, A. F. Poon, M. Y. Y. Lee, A. Muhuri, J. I. Park, et al. 2019. Mediator subunit MDT-15/MED15 and nuclear receptor HIZR-1/HNF4 cooperate to regulate toxic metal stress responses in Caenorhabditis elegans. PLoS Genet. 15: e1008508. doi: 10.1371/journal.pgen.1008508.
- Shore, D. E., and G. Ruvkun. 2013. A cytoprotective perspective on longevity regulation. Trends Cell Biol. 23:409–20. doi: 10.1016/j.tcb.2013.04.007.
- Simmer, F., C. Moorman, A. M. van der Linden, E. Kuijk, P. V. van den Berghe, R. S. Kamath, A. G. Fraser, J. Ahringer, and R. H. Plasterk. 2003. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol. 1:e12.
- Sinclair, J., and I. Hamza. 2015. Lessons from bloodless worms: Heme homeostasis in C. elegans. Biometals 28:481–89. doi: 10.1007/s10534-015-9841-0.
- Singh, R. N., and J. E. Sulston. 1978. Some observations on moulting in Caenorhabditis elegans. Nematologica. 24:63. doi: 10.1163/187529278X00074.
- Slice, L. W., J. H. Freedman, and C. S. Rubin. 1990. Purification, characterization, and cDNA cloning of a novel metallothionein-like, cadmium-binding protein from Caenorhabditis. Elegans. J. Biol. Chem. 265: 256–63.
- Snyder, P. W., P. Mann, B. Bolon, S. Elmore, W. Haschek-Hock, K. McDorman, D. M. Morton, R. Ochoa, and A. M. Ryan. 2010. The Society of Toxicologic Pathology and the “One Health” initiative Toxicol. Pathol. 38:521.
- Song, B. J., H. V. Gelboin, S. S. Park, C. S. Yang, and F. J. Gonzalez. 1986. Complementary DNA and protein sequences of ethanol-inducible rat and human cytochrome P-450s. Transcriptional and post-transcriptional regulation of the rat enzyme. J. Biol. Chem. 261: 16689–97.
- Spann, N., W. Goedkoop, and W. Traunspurger. 2015. Phenanthrene bioaccumulation in the nematode Caenorhabditis elegans. Environ. Sci. Technol. 49:1842–50. doi: 10.1021/es504553t.
- Sprando, R. L., N. Olejnik, H. N. Cinar, and M. Ferguson. 2009. A method to rank order water soluble compounds according to their toxicity using Caenorhabditis elegans, a complex object parametric analyzer and sorter, and axenic liquid media. Food Chem. Toxicol. 47: 722–28. doi: 10.1016/j.fct.2009.01.007.
- Starnes, D. L., J. M. Unrine, C. P. Starnes, B. E. Collin, E. K. Oostveen, R. Ma, G. V. Lowry, P. M. Bertsch, and O. V. Tsyusko. 2015. Impact of sulfidation on the bioavailability and toxicity of silver nanoparticles to Caenorhabditis elegans. Environ. Pollut. 196:239–46. doi: 10.1016/j.envpol.2014.10.009.
- Stein, K. K., and A. Golden 2015. The C. elegans eggshell. (December 30, 2015), WormBook, ed In WormBook. The C. elegans Research Community.
- Steinberg, C. E., S. R. Sturzenbaum, and R. Menzel. 2008. Genes and environment - striking the fine balance between sophisticated biomonitoring and true functional environmental genomics. Sci. Total Environ. 400:142–61. doi: 10.1016/j.scitotenv.2008.07.023.
- Sterken, M. G., L. B. Snoek, J. E. Kammenga, and E. C. Andersen. 2015. The laboratory domestication of Caenorhabditis elegans. Trends. Genet. 31:224–31. doi: 10.1016/j.tig.2015.02.009.
- Stone, C. E., D. H. Hall, and M. V. Sundaram. 2009. Lipocalin signaling controls unicellular tube development in the Caenorhabditis elegans excretory system. Dev. Biol. 329:201–11. doi: 10.1016/j.ydbio.2009.02.030.
- Stupp, G. S., S. H. von Reuss, Y. Izrayelit, R. Ajredini, F. C. Schroeder, and A. S. Edison. 2013. Chemical detoxification of small molecules by Caenorhabditis elegans ACS. Chem. Biol. 8: 309–13. doi: 10.1021/cb300520u.
- Sulston, J. E., D. G. Albertson, and J. N. Thomson. 1980. The Caenorhabditis elegans male: Postembryonic development of nongonadal structures. Dev. Biol. 78:542–76. doi: 10.1016/0012-1606(80)90352-8.
- Sumida, A., K. Kinoshita, T. Fukuda, H. Matsuda, I. Yamamoto, T. Inaba, and J. Azuma. 1999. Relationship between mRNA levels quantified by reverse transcription-competitive PCR and metabolic activity of CYP3A4 and CYP2E1 in human liver. Biochem. Biophys. Res. Commun. 262:499–503. doi: 10.1006/bbrc.1999.1233.
- Sundaram, M. V., and M. Buechner. 2016. The Caenorhabditis elegans excretory system: A model for tubulogenesis, cell fate specification, and plasticity. Genetics. 203:35. doi: 10.1534/genetics.116.189357.
- Swain, S. C., K. Keusekotten, R. Baumeister, and S. R. Sturzenbaum. 2004. C. elegans metallothioneins: New insights into the phenotypic effects of cadmium toxicosis. J. Mol. Biol. 341:951–59. doi: 10.1016/j.jmb.2004.06.050.
- Tang, B., P. L. Williams, K. S. Xue, J. S. Wang, and L. Tang. 2020. Detoxification mechanisms of nickel sulfate in nematode Caenorhabditis elegans. Chemosphere 260:127627. doi: 10.1016/j.chemosphere.2020.127627.
- Taubert, S., J. D. Ward, and K. R. Yamamoto. 2011. Nuclear hormone receptors in nematodes: Evolution and function. Mol. Cell. Endocrinol. 334:49–55. doi: 10.1016/j.mce.2010.04.021.
- Taubert, S., M. Hansen, M. R. Van Gilst, S. B. Cooper, and K. R. Yamamoto. 2008. The mediator subunit MDT-15 confers metabolic adaptation to ingested material. PLoS Genet. 4: e1000021. doi: 10.1371/journal.pgen.1000021.
- Taubert, S., M. R. Van Gilst, M. Hansen, and K. R. Yamamoto. 2006. A mediator subunit, MDT-15, integrates regulation of fatty acid metabolism by NHR-49-dependent and -independent pathways in C. Elegans. Genes. Dev. 20:1137–49. doi: 10.1101/gad.1395406.
- Tejeda-Benitez, L., and J. Olivero-Verbel. 2016. Caenorhabditis elegans, a biological model for research in toxicology. Rev Environ Contam Toxicol 237:1–35. doi: 10.1007/978-3-319-23573-8_1.
- Tonelli, C., I. I. C. Chio, and D. A. Tuveson. 2018. Transcriptional regulation by Nrf2. Antioxid. Redox Signal. 29:1727–45. doi: 10.1089/ars.2017.7342.
- Traunspurger, W., M. Haitzer, S. Hoss, S. Beier, W. Ahlf, and C. Steinberg. 1997. Ecotoxicological assessment of aquatic sediments with Caenorhabditis elegans (nematoda) - A method for testing liquid medium and whole-sediment samples. Environ. Toxicol. Chem. 16:245–50.
- Treonis, A. M., and D. H. Wall. 2005. Soil nematodes and desiccation survival in the extreme arid environment of the antarctic dry valleys. Integr. Comp. Biol. 45:741–50. doi: 10.1093/icb/45.5.741.
- Tsiaoussis, J., M. N. Antoniou, I. Koliarakis, R. Mesnage, C. I. Vardavas, B. N. Izotov, A. Psaroulaki, and A. Tsatsakis. 2019. Effects of single and combined toxic exposures on the gut microbiome: Current knowledge and future directions. Toxicol. Lett. 312:72–97. doi: 10.1016/j.toxlet.2019.04.014.
- Vatamaniuk, O. K., E. A. Bucher, J. T. Ward, and P. A. Rea. 2001. A new pathway for heavy metal detoxification in animals. Phytochelatin synthase is required for cadmium tolerance in Caenorhabditis elegans. J. Biol. Chem. 276: 20817–20.
- Verma, S., U. Jagtap, A. Goyala, and A. Mukhopadhyay. 2018. A novel gene-diet pair modulates C. elegans aging. PLoS Genet. 14: e1007608. doi: 10.1371/journal.pgen.1007608.
- Viñuela, A., L. B. Snoek, J. A. G. Riksen, and J. E. Kammenga. 2010. Genome-wide gene expression analysis in response to organophosphorus pesticide chlorpyrifos and diazinon in C. Elegans. PloS. One. 5:e12145–e12145. doi: 10.1371/journal.pone.0012145.
- Wallace, B. D., and M. R. Redinbo. 2013. Xenobiotic-sensing nuclear receptors involved in drug metabolism: A structural perspective. Drug Metab. Rev. 45: 79–100. doi: 10.3109/03602532.2012.740049.
- Wang, D. 2019a. Molecular Toxicology in Caenorhabditis elegans: Springer.
- Wang, D. 2019b. Target organ toxicology in Caenorhabditis elegans: Springer.
- Wang, D. 2020. Exposure Toxicology in Caenorhabditis elegans: Springer.
- Wang, J., J. Luo, D. K. Aryal, W. C. Wetsel, R. Nass, and J. L. Benovic. 2017. G protein-coupled receptor kinase-2 (GRK-2) regulates serotonin metabolism through the monoamine oxidase AMX-2 in Caenorhabditis elegans. J. Biol. Chem. 292:5943–56. doi: 10.1074/jbc.M116.760850.
- Wani, K. A., D. Goswamy, S. Taubert, R. Ratnappan, A. Ghazi, and J. E. Irazoqui. 2020. NHR-49/PPAR-α and HLH-30/TFEB promote C. elegans host defense via a flavin-containing monooxygenase. Bio.Rxiv: 2020.9.03.282087. doi: 10.1101/2020.09.03.282087.
- Ward, S., N. Thomson, J. G. White, and S. Brenner. 1975. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans.?2UU. J. Comp. Neurol. 160: 313–37. doi: 10.1002/cne.901600305.
- Ware, R. W., D. Clark, K. Crossland, and R. L. Russell. 1975. The nerve ring of the nematode Caenorhabditis elegans: Sensory input and motor output. J. Comp. Neurol. 162:71–110. doi: 10.1002/cne.901620106.
- Watson, E., L. T. MacNeil, A. D. Ritter, L. S. Yilmaz, A. P. Rosebrock, A. A. Caudy, and A. J. M. Walhout. 2014. Interspecies systems biology uncovers metabolites affecting C. elegans gene expression and life history traits. Cell. 156:1336–37. doi: 10.1016/j.cell.2014.02.036.
- Weikum, E. R., X. Liu, and E. A. Ortlund. 2018. The nuclear receptor superfamily: A structural perspective. Protein. Sci. 27:1876–92. doi: 10.1002/pro.3496.
- Weinhouse, C., L. Truong, J. N. Meyer, and P. Allard. 2018. Caenorhabditis elegans as an emerging model system in environmental epigenetics. Environ. Mol. Mutagen. 59:560–75. doi: 10.1002/em.22203.
- Williams, P. L., and D. B. Dusenbery. 1987. Screening test for neurotoxins using Caenorhabditis elegans. Prog. Clin. Biol. Res. 253:163–70.
- Williams, P. L., and D. B. Dusenbery. 1988. Using the nematode Caenorhabditis elegans to predict mammalian acute lethality to metallic salts. Toxicol. Ind. Health. 4: 469–78.
- Williams, P. L., and D. B. Dusenbery. 1990a. A promising indicator of neurobehavioral toxicity using the nematode Caenorhabditis elegans and computer tracking. Toxicol Ind Health 6: 425–40.
- Williams, P. L., and D. B. Dusenbery. 1990b. Aquatic toxicity testing using the nematode, Caenorhabditis elegans. Environ. Toxicol. Chem. 9:1285–90.
- Williamson, V. M., and A. Kumar. 2006. Nematode resistance in plants: The battle underground. Trends. Genet. 22: 396–403. doi: 10.1016/j.tig.2006.05.003.
- Williamson, V. M., M. Long, and G. Theodoris. 1991. Isolation of Caenorhabditis elegans mutants lacking alcohol dehydrogenase activity. Biochem. Genet. 29: 313–23. doi: 10.1007/BF00554139.
- Wittkowski, P., P. Marx-Stoelting, N. Violet, V. Fetz, F. Schwarz, M. Oelgeschläger, G. Schönfelder, and S. Vogl. 2019. Caenorhabditis elegans as a promising alternative model for environmental chemical mixture effect assessment-A comparative study. Environ. Sci. Technol. 53: 12725–33. doi: 10.1021/acs.est.9b03266.
- Wolkow, C. A., and D. H. Hall 2011. The dauer cuticle.” In WormAtlas.
- Woodruff, G. C., C. M. Knauss, T. K. Maugel, and E. S. Haag. 2014. Mating damages the cuticle of C. elegans hermaphrodites. PloS. One. 9: e104456–e104456. doi: 10.1371/journal.pone.0104456.
- Wu, C. W., A. Deonarine, A. Przybysz, K. Strange, and K. P. Choe. 2016. The Skp1 homologs SKR-1/2 are required for the Caenorhabditis elegans SKN-1 antioxidant/detoxification response independently of p38 MAPK. PLoS Genet. 12: e1006361. doi: 10.1371/journal.pgen.1006361.
- Wu, H., C. Huang, F. A. Taki, Y. Zhang, D. L. Dobbins, L. Li, H. Yan, and X. Pan. 2015. Benzo-α-pyrene induced oxidative stress in Caenorhabditis elegans and the potential involvements of microRNA. Chemosphere 139:496–503. doi: 10.1016/j.chemosphere.2015.08.031.
- Wyatt, L. H., S. E. Diringer, L. A. Rogers, H. Hsu-Kim, W. K. Pan, and J. N. Meyer. 2016. Antagonistic growth effects of mercury and selenium in Caenorhabditis elegans are chemical-species-dependent and do not depend on internal Hg/Se ratios. Environ. Sci. Technol. doi: 10.1021/acs.est.5b06044.
- Xia, M., R. Huang, Q. Shi, W. A. Boyd, J. Zhao, N. Sun, J. R. Rice, P. E. Dunlap, A. J. Hackstadt, M. F. Bridge, et al. 2018. Comprehensive analyses and prioritization of Tox21 10K chemicals affecting mitochondrial function by in-depth mechanistic studies. Environ. Health. Perspect. 126:077010. doi: 10.1289/EHP2589.
- Xiong, H., C. Pears, and A. Woollard. 2017. An enhanced C. elegans based platform for toxicity assessment. Sci. Rep. 7: 9839. doi: 10.1038/s41598-017-10454-3.
- Xu, C., C. Y. Li, and A. N. Kong. 2005. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 28:249–68. doi: 10.1007/bf02977789.
- Yang, X. Y., C. J. Jiang, H. Hsu-Kim, A. R. Badireddy, M. Dykstra, M. Wiesner, D. E. Hinton, and J. N. Meyer. 2014. Silver nanoparticle behavior, uptake, and toxicity in Caenorhabditis elegans: Effects of natural organic matter. Environ. Sci. Technol. 48: 3486–95. doi:10.1021/Es404444n.
- Yang, Y. F., Y. J. Lin, and C. M. Liao. 2017. Toxicity-based toxicokinetic/toxicodynamic assessment of bioaccumulation and nanotoxicity of zerovalent iron nanoparticles in Caenorhabditis elegans. Int. J. Nanomed. 12: 4607–21. doi: 10.2147/ijn.S138790.
- Zdraljevic, S., B. W. Fox, C. Strand, O. Panda, F. J. Tenjo, S. C. Brady, T. A. Crombie, J. G. Doench, F. C. Schroeder, and E. C. Andersen. 2019. Natural variation in C. elegans arsenic toxicity is explained by differences in branched chain amino acid metabolism. Elife. 8. doi: 10.7554/eLife.40260.
- Zdraljevic, S., and E. C. Andersen. 2017. Natural diversity facilitates the discovery of conserved chemotherapeutic response mechanisms. Curr. Opin. Genet. Dev. 47:41–47. doi: 10.1016/j.gde.2017.08.002.
- Zhang, F., M. Berg, K. Dierking, M. A. Felix, M. Shapira, B. S. Samuel, and H. Schulenburg. 2017. Caenorhabditis elegans as a model for microbiome research. Front. Microbiol. 8:485. doi: 10.3389/fmicb.2017.00485.
- Zhang, J., A. D. Holdorf, and A. J. Walhout. 2017. C. elegans and its bacterial diet as a model for systems-level understanding of host-microbiota interactions. Curr. Opin. Biotechnol. 46:74–80. doi: 10.1016/j.copbio.2017.01.008.
- Zhang, J., X. Li, A. R. Jevince, L. Guan, J. Wang, D. H. Hall, X. Huang, and M. Ding. 2013. Neuronal target identification requires AHA-1-mediated fine-tuning of Wnt signaling in C. elegans. PLoS Genet. 9: e1003618. doi: 10.1371/journal.pgen.1003618.
- Zhang, X., H. Q. Zhong, Z. W. Chu, X. Zuo, L. Wang, X. L. Ren, H. Ma, R. Y. Du, J. J. Ju, X. L. Ye, et al. 2020. Arsenic induces transgenerational behavior disorders in Caenorhabditis elegans and its underlying mechanisms. Chemosphere 252:126510. doi: 10.1016/j.chemosphere.2020.126510.
- Zhao, Y. L., Q. L. Wu, Y. P. Li, and D. Y. Wang. 2013. Translocation, transfer, and in vivo safety evaluation of engineered nanomaterials in the non-mammalian alternative toxicity assay model of nematode Caenorhabditis elegans. RSC. Adv 3:5741–57. doi: 10.1039/c2ra22798c.
- Zhao, Z., J. A. Sheps, V. Ling, L. L. Fang, and D. L. Baillie. 2004. Expression Analysis of ABC transporters reveals differential functions of tandemly duplicated genes in Caenorhabditis elegans. J. Mol. Biol. 344: 409–17. doi: 10.1016/j.jmb.2004.09.052.
- Zheng, S.-Q., A.-J. Ding, G.-P. Li, G.-S. Wu, and H.-R. Luo. 2013. Drug absorption efficiency in Caenorhbditis elegans delivered by different methods. PloS. One. 8:e56877–e56877. doi: 10.1371/journal.pone.0056877.
- Zhou, G. W., X. R. Yang, F. Zheng, Z. X. Zhang, B. X. Zheng, Y. G. Zhu, and X. M. Xue. 2020. Arsenic transformation mediated by gut microbiota affects the fecundity of Caenorhabditis elegans. Environ. Pollut. 260: 113991. doi: 10.1016/j.envpol.2020.113991.