Abstract
Emulsion stability (oil separation) in halva is a major problem that affects quality upon storage. Emulsion instability results in toughness, oil separation, and oil contamination on packaging materials. The objective of this study was to evaluate the effectiveness of improving halva quality by incorporating non-hydrogenated palm oil, glycerol, soy protein concentrate, gelatin, lecithin, pectin, gum Arabic, sugar powder, and calcium chloride. Halva was produced by heating sugar (sucrose) solution (65%) containing citric acid (0.65%) and heating to reach 105° C, adding halva root (Saponaria officinalis) extract solution (5.6%) and continuing heating for 60–70 min. This is followed with cooling at room temperature for 15 min and adding tahinia (sesame paste) (1:1) and mixing for 10 min. The additives to improve the quality of halva were incorporated with the sugar solution, during cooking, and with tahinia. The quality of halva was evaluated by measuring the amount of oil separation, microscopic examination, and oil viscosity. Microscopic examination of halva showed a porous non-crystalline sugar melt particles surrounded by a precipitated protein layer originating from tahinia. The oil was found as free non-emulsified fluid, filling in the spaces between solid particles. The saponin (from halva root extract) possibly precipitated the colloidal proteins of tahinia, and contributed to a fragile structure. Soy protein concentrate, gelatin, glycerol, and lecithin incorporation did not improve emulsion stability. However, calcium chloride, sugar powder, gum Arabic, and pectin minimized emulsion instability. Furthermore, 1.0% or 2.5% of non-hydrogenated palm oil increased viscosity of the oil phase and contributed to emulsion stability.
Keywords:
INTRODUCTION
Halva is a product from cooked sugar, tahinia (sesame paste), and Saponaria officinalis (halva roots); nuts, flavors, and additives may be added. It is considered a rich source of carbohydrate, fat, and protein. Addition of nuts and dehydrated fruits enhances flavor and nutritional value.[Citation2] Oil separation in halva during storage leads to a tough texture. The separated oil contaminates the packaging and reduces marketability. A similar problem has been reported in peanut butter.[Citation1] Hinds and others[Citation4] prevented oil separation in peanut butter for one year at room temperature by adding 2.0–2.5% of non-hydrogenated palm oil. Collins and Sanchez[Citation1] added peanut bran to peanut butter that prevented oil separation, and improved firmness and product stability. Woodroof[Citation7] demonstrated glycerol addition prevented oil separation of peanut butter and enhanced smoothness, spreadability, and decreased stickiness of product. The objective of this study was to investigate the improvement of halva quality by incorporating nonhydrogenated palm oil, glycerol, soy protein concentrate, gelatin, lecithin, pectin, gum Arabic, sucrose, and calcium chloride.
MATERIALS AND METHODS
Materials
Commercial Saponaria officinalis (halva roots), nonhydrogenated palm oil, citric acid, sesame paste (tahinia), glycerol, soy protein concentrate (80%), carboxy methyl cellulose (CMC), gelatin, lecithin, pectin, gum Arabic, potato starch, sucrose powder, and calcium chloride were purchased from local markets in Jordan (Amman-Jordan).
Halva Preparation (Control)
Halva was produced by heating sugar solution (65%) containing citric acid (0.65%) and stirring to reach 105° C, adding halva root (Saponaria officinalis) extract solution (5.6%) and continuing heating for 60–70 min. The mixture was allowed to cool to room temperature for 15 min. Sesame paste (tahinia) was added (1:1) and mixed for 10 min. The additives to improve the quality of halva were incorporated with the sugar solution, during cooking, or with tahinia.
Additives to Enhance Emulsion Stability
Commercial gelatin (1.0%), soy protein isolate (1.0%), nonhydrogenated palm oil (1.0% and 2.0%), glycerol (1.0%), sucrose powder (1.0%), pectin (1.0%), potato starch (1.0%), and lecithin (1.0%) were separately added to tahinia and mixed well before adding cooked sugar. Soy protein concentrate (80%) was solubilized at pH 10.0 and added to the sugar mixture during cooking. Gelatin (1.0%), pectin (1.0%), carboxy methyl cellulose (CMC) (0.5%), potato starch (1.0%), gum Arabic, and calcium chloride anhydrate (UK, GCC) at 0.02%, 0.03%, and 0.05% were added separately to the sugar mixture during cooking.
Microscopic Examination of Halva
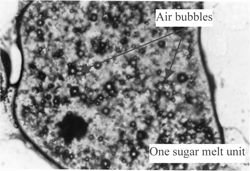
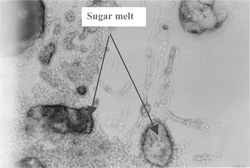
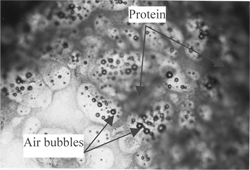
Four treatments were conducted to examine the physical structure and oil separation in halva by a microscope (Olympus Venox-T AH-2 Microscopy 100×). A thin slice (1 cm2 with 1 mm thickness) from halva was treated with a fat-soluble dye (Sudan III, which was prepared by dissolving Sudan III dye in acetone (0.01%) to observe the oil phase in the structure of halva. Another similar thin slice was exposed to nitric acid gas for 4 hours for proteins to nitrate and yellow color development of proteins for examination. A similar third thin slice from halva was washed with petroleum ether to remove oil and to observe noncrystalline sugar melt particles that contains air bubbles. A fourth thin slice was exposed to 150° C for 2 hours to remove distributed air bubbles in molten (cooked) sugar particles.
Measurement of Viscosity of Oil
The viscosity of sesame oil was determined at 25° C and 40° C. Haake Falling Ball-Viscometer (Haake Mess Technik, “Falling Ball Viscometer” Manual, Dieselstr. 6–7500 Karlsuhe 41, Germany)[Citation5] was used to determine the viscosity of sesame oil alone and with saturated palm oil (1% and 2.5%) at 25° C and 40° C. Forty ml from each sample was used to measure the viscosity. The viscosity was expressed as:
Where: viscosity is in Pa·s, A = ball constant, K1 = ball density kg/m3, K2 = sample density kg/m3, and t = time (sec).
Oil Separation Test
First 150 grams of fresh halva were taken in a cup (250 gm capacity). The cup was then covered with perforated aluminum foil. This cup was inverted and placed on a petri dish (weight A) containing three filter papers (Whatman #4) to absorb the oil passing through the perforations in the aluminum foil. The weight of the petri dish with absorbed oil by filter paper was taken during storage to determine the amount of oil separation at 4, 12, 24, and 32 days (weight B). Oil separation from product was evaluated at 25° C and 40° C.
Statistical Analysis
Data were presented as means of three determinations, and analyzed using the general linear model (GLM) procedure with SAS software package.[Citation6] Duncan's multiple range test was used to compare means. Significant differences were defined at p ≤ 0.05.
RESULTS AND DISCUSSION
Microscopic Examination of Halva
to show the microscopic physical structures of halva. shows halva treated with fat-soluble dye (Sudan III). The sugar was found as porous noncrystalline sugar melt particles and the oil as a free (nonemulsified) fluid filling in the spaces between solid particles. shows halva exposed to nitric acid gas for 4 hours. Proteins stained with nitric oxide appeared as brown colored layer on the surface of noncrystalline sugar melt particles (unites). shows halva washed with petroleum ether to remove oil. Air bubbles are between noncrystalline sugar melt particles. shows halva exposed to 150° C for 2 hours to remove distributed air bubbles in molten sugar particles. No air bubbles were seen in noncrystalline sugar melted particles. From the above observations, it is postulated that halva has a porous noncrystalline sugar melt particles surrounded by a precipitated protein layer originating from tahinia (sesame paste). The oil exists as a free (nonemulsified) fluid, filling in the spaces between solid particles. The presence of saponin (from halva roots) in the molten sugar precipitated the colloidal proteins of tahinia.
Figure 1 A microscopic picture (100X) of a thin slice from halva dyed with fat soluble dye (Sudan III).
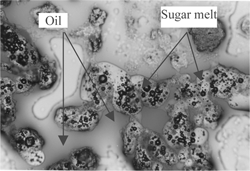
Figure 2 A microscopic picture (100X) a thin slice from halva washed with petroleum ether to remove oil.
Measurement of Viscosity
shows the viscosity of sesame oil with 1.0% and 2.5% non-hydrogenated palm oil at 25° C and 40° C. The viscosity of control (sesame oil alone) was 0.21 (Pa s) at 25° C and increased to 0.25 and 0.30 (Pa s) when 1.0 and 2.5% nonhydrogenated palm oil were incorporated, respectively. Palm oil contributed to the viscosity of oil.
Table 1 The viscosity (Pa s) of sesame oil with and without nonhydrogenated palm oil at 25 and 40° C.Footnote*
Effect of Adding Glycerol, Lecithin, Proteins, and Calcium Chloride on Halva Emulsion
Glycerol, lecithin, soy protein concentrate, and gelatin did not significantly improve halva emulsion (p £ 0.05) at 25° C and 40° C storage (). This was probably due to the influence of other components (sucrose and halva root) on tahinia system that may have interacted with fats and proteins. Glycerol showed no effect on emulsion stability due to precipitation of proteins (lost colloidal properties) on the surface of cooked sugar by saponins (from halva root extract). The precipitation of proteins caused a loss in colloidal properties and its function with glycerol. Addition of lecithin could have contributed to hydrophilicity resulting in increasing emulsion instability in the halva structure. Proteins may not have performed their functional properties due to restricted amount of water (halva contains less than 1% water). However, CaCl2 enhanced emulsion stability, which may be due to calcium cross-linking with proteins.[Citation3]
Table 2 Effect of adding glycerol, lecithin gelatin, soy protein concentrate, and CaCl2 on emulsion stability of halva at 25° C and 40° C storage.
Carbohydrates Addition
shows the effect of adding different types of carbohydrates on emulsion stability of halva at 25° C and 40° C. Addition of carbohydrate minimized emulsion instability. Gum Arabic addition resulted in very smooth sugar particles and a more porous structure in halva that may be related to a delay of solidification of cooked sugar. These additives did not have any significant practical effect on emulsion stability.
Table 3 Effect of adding different types of carbohydrates on emulsion stability of halva at 25° C and 40° C storage.
Non-Hydrogenated Palm Oil
shows the effect of adding nonhydrogenated palm oil on emulsion stability of halva at 25° C and 40° C storage. Addition of 1.0% or 2.5% of non-hydrogenated palm oil prevented oil separation from halva stored at room temperature (25° C), while this addition, as well as other additives, did not prevent the separation of halva stored at 40° C. Addition of 1.0% or 2.5% of nonhydrogenated palm oil (which is solid at room temperature) increased viscosity and prevented oil separation in halva. Also, addition of 1.0% or 2.5% of non-hydrogenated palm oil could be the reason for solidification of halva oil. The mechanism of preventing oil separation seems to be related to an increase in viscosity of the oil phase due to the addition of the solid palm oil, since the measurement of the viscosity of two mixtures of palm oil in sesame oil (at 1.0% and 2.5% level) showed an increase in viscosity at 25° C and no increase at 40° C ().
Table 4 Effect of adding nonhydrogenated palm emulsion stability of halva at 25° C and 40° C storage.
CONCLUSIONS
Microscopic examinations of halva showed a porous noncrystalline sugar melt particles surrounded by a precipitated protein layer originating from tahinia. The oil was found as a free (nonemulsified) fluid, filling in the spaces between solid particles. The presence of halva root extract (saponins) in the molten sugar was responsible for the precipitation of the colloidal proteins of tahinia, and fragile texture of halva. The oil separation is due to the compression of the halva matrix under gravity. Addition of 1.0% or 2.5% of nonhydrogenated palm oil prevented oil separation from halva when stored at room temperature (25° C), while this addition, as well as other additives did not prevent oil separation when halva was stored at 40° C. The mechanism of preventing oil separation seems to be related to an increase in viscosity of the oil phase.
Notes
*Values are average of three determinations.
*:Adding to tahinia before adding cooked sugar.
$:Adding to sugar cooked during cooking.
**:Means followed by different letters in the same column are significantly different (p = 0.05).
$: Adding to tahinia before adding cooked sugar.
*: Adding to sugar cooked during cooking
**: Means followed by different letters in the same column are significantly different (p ≤ 0.05).
*:Adding to tahinia before adding cooked sugar.
**:Means followed by different letters in the same column are significantly different (p ≤ 0.05).
5. Haake Mess Technik Falling Ball Viscometer Manual. Dieseltr. 6–7500 Karlsuhe 41.
REFERENCES
- Collins , J. and Sanchez , J. 1979 . Effect of peanut shell flour on firmness, color and acceptance of peanut butter . J. Food Sci. , 44 ( 4 ) : 944 – 945 .
- El-Kloub , K. , Hamed , A. and Mona , A. 1992 . The nutritive and consumption of sesame product in Egypt . Alex. J. Agric. Res. , 37 ( 3 ) : 12 – 13 .
- Fennema , R. 1985 . Food Chemistry , 2nd , Westport, Connecticut : Marcel Dekker, Inc .
- Hinds , M. , Chinnan , M. and Beuchat , L. 1994 . Un-hydrogenated palm oil as stabilizer for peanut butter . J. Food Sci. , 59 ( 4 ) : 816 – 820 .
- 5. Haake Mess Technik Falling Ball Viscometer Manual. Dieseltr. 6–7500 Karlsuhe 41.
- SAS . 2002 . User's Guide. Release , 8.2 , Cary, NC : SAS Institute Inc .
- Woodroof , J.G. 1973 . Peanuts: Production, Processing Products , 2nd , USA : The Avi Publishing Company .