Abstract
This study was conducted to define the effects of ram horn hydrolysate (RHH) on the pH and color properties of carcasses and dissected products in broilers. Two-hundred and forty male broiler chicks (Ross-308) were fed with basal diets supplied with RHH for 4 wks. Chicks were allocated to four dietary treatments (H0, H1, H2 and H3 groups) in a completely randomized experimental design. Feed and water were offered ad libitum consumption and lightening was continuous throughout the experimental period. H0 group was fed only the basal diet and given normal drinking water. Treatment groups were fed with basal diet plus a 1% (H1), 2% (H2), and 3% (H3)-RHH-added water in place of normal drinking water to meet the daily water requirements of chickens from 1 to 28 days of ages. At the end of the trial all birds were slaughtered, then the ranges of pH and skin color of carcasses were determined at various times during the first 24 hour (1, 3, 7, 12, 17, and 24). After standard dissection of carcasses, breasts and drumsticks were divided into two groups for vacuum and aerobic packaging. Packed breasts and drumsticks were stored at 3 60.58 C, for 12 days, and the color values were determined. The pH values of H3 group were lower than those of H0, H1 and H2 groups (p < 0.05). The lightness (L*), redness (a*) and yellowness (b*) values increased during the 24-h period. The H0 group had the higher b* values than those of RHH-added groups (p < 0.05). The L*, a* and b* values of drumstick meats were higher than those of the breast meats (p < 0.05). The values of b* in aerobic packaged breasts and drumsticks were higher than the vacuum packaged (p < 0.05) treatments. The b* values increased with storage. The a* values of RHH-supplied groups were higher than that of the control (p < 0.05), while b* value of control was higher than those of the RHH-supplemented groups (p < 0.05). While the L* and b* values of drumstick skin were higher than that of drumstick meat, a* values in drumstick meats were higher (p < 0.05). The vacuum packaging increased the a* value during storage. As a result, the use of RHH in broiler diets had a significant effect on the L*, a*, and b* values of carcasses and dissected tissue (p < 0.01).
INTRODUCTION
In recent years, some microbiological cultures and various chemical agents such as probiotics, prebiotics, antibiotics and enzymes have been added to animal diets as feed additives to improve broiler performance and feed efficiency. As known, normal gut microflora in farm animals is important because of its effects on the production of livestock and the quality and safety of livestock products. Taken into consideration native gastrointestinal microflora, avian digestive tract has 90% beneficial microflora such as facultative anaerobic bacteria producing lactic acid (Lactobacillus) and 10% pathogen microorganisms such as E. coli, Enterococcus and Clostridium, etc.[Citation1–3] Supplying the optimal conditions for microbial cultures increases the beneficial bacteria counts and aid nutrient absorption and enhance the microbial balance in the gastrointestinal tract. Therefore, in recent years, one of the important issues is evaluating animal by-products in broiler feeding. During the past several years, various animal-by products are currently being processed and used as feedstuff. Also, intensive production of food animal and animal products has generated an enormous waste disposal problem for the animal industry.
These wastes are largely organic materials and are convertible to useful resources.[Citation4] Ram horns, composed of fibrous protein, are widely produced in the world after slaughtering animals. In Turkey more than 600 tons of horns are produced per year.[Citation5] Increasing concern about pollution that occurres from agriculture and industry wastes has stimulated interest in converting waste materials into commercially valuable product. Ram horns consist of α-keratin protein which has 22% cysteine amino acid. The ram horn protein hydrolysate (RHH) contains high levels of amino acid.[Citation6,Citation7] RHH contains an essential amino acid which is reported by Ozen[Citation8] and Cheeke[Citation9] for broiler diets. To demonstrate the general appeal of such nitrogenous substrates to the biotechnology industry, the effect of RHH on the growth of three types of microorganisms: bacteria (Lactobacillus casei), yeasts (Saccharomyces cerevisiae) and fungi (Aspergillus niger and Penicillum chrysogenum) were investigated in a previous experiments conducted at Ataturk University.[Citation5,Citation7,Citation10] These results showed that the use of RHH at the rates of 3% and 4% supplied optimal microbial biomass yields and stimulated the growth of microorganisms. In addition to the amino acid content, RHH is rich in both organic and inorganic materials. It contains the essential substances required in the microbial media such as sources of carbon, nitrogen and minerals. RHH has been investigated only to a minor extent, and its use in industrial process is still limited. Karaoglu, et al.[Citation11] found that supplemental RHH at different levels (0%, 1%, 2%, and 3%) increased live body weight, daily weight gain and improved feed efficiency and carcass yields. Similarly, Aksu et al.[Citation12] reported that the use of RHH in broiler diets affected the meat quality of broiler carcass parts (breast and drumsticks meats). But there is not any information regarding the effect of RHH on the pH of meat and the color of skin and meat of the broiler carcasses. In this study, RHH has been tested to determine whether it has effect on the pH of meat and the color of skin and meat of the broiler carcasses.
MATERIAL AND METHODS
A study was conducted with 240 male broiler chicks (Ross-308), received from a commercial hatchery (KOY-TUR, Integrated Poultry Co. Erzurum, TURKEY) at 1 day of age. Average 40-g chicks were randomly allocated to four dietary treatments, and were housed in batteries from 1 to 21 days, then in grower broiler pens from 21 to 49 days in the Application and Research Farm of the Agricultural Faculty, Atatürk University. There were 6 replicates pens for each treatment group, each treatment with 10 birds in each pen. Feed and water were offered for ad libitum consumption. All chicks were fed a starter diet from day 1 to 21, and a finisher diet to 49 days. Diets were formulated according to NRC[Citation13] recommendations. Feed composition was analyzed by the AOAC,[Citation14] and composition of the basal diets used in this experiment is shown in . The experimental groups consisting of four dietary treatments: (1) H0 was fed only the basal, (2) H1 was fed the basal diet and given a 1% RHH-added to the drinking water, (3) H2 was fed the basal diet and given a 2% RHH-added to the drinking water, and (4) H3 was fed the basal diet and given a 3% RHH-added to the drinking water for daily water requirements during the experimental period. Hydrolysate composition used in this trial was shown in .
Table 1 Composition of basal diets (%).
Table 2 Composition of RHH (CitationKurbanoglu, 2003).
Preparation of RHH was carried out by the method of Kurbanoglu.[Citation15] RHH-supplemented waters were offered from 1 to 28 day of age to broilers in place of normal drinking water as diluted solutions. Normally, each chick drinks water approximately twice as their daily feed consumption. Therefore, the experimental groups were fed with more amino acids (approximately 0.3%, 0.6%, and 1.0%) by drinking the RHH-supplemented water. All birds were fed same basal diets from 28 to 49 days. At the end of the feeding period, prior to slaughtering, birds were held without food for 8 h, electrically stunned and slaughtered by slitting the throat, then allowed to bleed for 120 s and semiscalded at 54 °C for 30 s before mechanical plucking in a rotary drum plucker for 30 s to remove feathers. The birds were eviscerated manually, washed, and allowed to drain for 10 min.[Citation16] After evisceration, carcasses were stored at 3 ± 0.5 °C for 24 h. Then, the carcasses were dissected according to Pingel et al.[Citation17] The color properties of carcasses and carcass parts (breast fillets and drumstick meats and skin) used as experimental materials were determined as the following three steps.
Carcass pH and Color Values
Skin color and pH values of carcasses were determined at 1st, 3rd, 7th, 12th, 17th and 24th h for all groups. At the end of the feeding period, 50 birds were randomly selected from each treatment group (H0: 50, H1: 50, H2: 50, and H3: 50, for a total of 200 broiler carcasses). Colorimeters readings (L*, a* and b*) were always measured from the same points on carcass surfaces (back, breast, leg) for all carcasses.
Breast and Drumstick Meat Color Values
After dissection, breast and drumstick meats from four different treatment groups (H0, H1, H2 and H3) were divided into two groups and packaged under vacuum or aerobically. Packaged breasts (without skin) and drumsticks (with skin) were stored at 3 ± 0.5 °C for 12 days, and the breast and drumstick meat color values were determined at 0, 2, 4, 6, 8, 10, and 12 days of storage. Color values for a total of 400 breast fillets (200 vacuum and 200 aerobic) and a total of 400 drumstick meats (200 vacuum and 200 aerobic) were measured on the surfaces.
Drumstick Meat and Skin Color Values
In addition the colors of drumstick skin and meat were also determined in the vacuum and aerobic packaged samples (total 400 drumsticks; 200 vacuum and 200 aerobic) during the 12-day storage period.
Vacuum and Aerobic Packaging
The half of the breast fillets and drumsticks were packaged as aerobic. Aerobic packaging was carried out by wrapping with single-stretched film material (LDPE) placed in polystyrene trays. The other half of the breasts and drumsticks were packaged in a bag OPAEVOH/PE (water vapor transmission: 15 g/m2/24 h/38 °C/, 90% RH, 1 atm; O2 transmission: 5 cm3/m2/24 h/23 °C, 50% RH, 1 atm; N2 transmission: 1 cm3/m2/24 h/23 °C, 50% RH, 1 atm; CO2 transmission: 23 cm3/m2/24 h/23 °C, 50% RH, 1 atm) by a Multivac packaging unit (Multivac A 300/16, Sepp Haggenmüller, D 87787 Wolfertschwenden, Germany) as vacuum.
Color values
The color values were measured using a tristimulus colorimeter (Minolta Chroma Meter Measuring Head CR-200, Minolta, Osaka, Japan) and Minolta Model and this was used to objectively measure CIE Lab (where L* measures relative lightness, a* relative redness, and b* relative yellowness). Prior to each measurement, the apparatus was standardized against a white tile. C* and H* color parameters were calculated. Chroma, C* = (a*2 + b*2)0.5 and Hue, H* = tan−1 (a*/b*). The color values were measured on the surface of each carcasses at 1, 3, 7, 10, 12, 17, and 24 hours of chill storage at 3 ± 0.5 °C. Colorimeters readings (L*, a* and b*) were always measured from the same points on carcass surfaces (back, breast, leg) for all the carcasses during post-mortem storage. Also, color values were measured in the breast fillet, drumstick meat and skin during storage.
pH values
The pH value was measured on carcasses by direct probe of pH meter (SCHOTT L 6880, Lab Star pH), thrusting probe into breast and drumstick meats.
Statistic analysis
All data were analyzed by analysis of variance of completely randomized design, using GLM procedure of SPSS/package.[Citation18] The model included RHH levels (H0, H1, H2 and H3), meat type (breast, drumstick), packaging (vacuum, aerobic) and storage period (hours 1, 3, 7, 12, 17, and 24 or days 0, 2, 4, 6, 8, 10, and 12) as main effects and all their interactions, but the only significant interactions were only shown in figures. The differences among means were tested for significance (P < 0.05) by Duncan's multiple range test. The results were presented as mean values ± standard deviation in tables.
RESULTS AND DISCUSSION
pH Values and Skin Color of Carcass
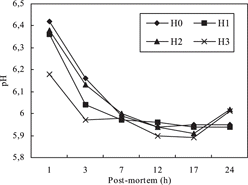
The pH values were affected by the treatment (H0, H1, H2, and H3) and storage (24 h). Among treatment groups the lowest (P < 0.05) pH values were observed in H3 during postmortem (). The pH values of H1, H2, and H3 groups were also lower than that of the control group (H0) after 1 h postmortem (), and decreased gradually for the 17-h storage period (). There was no significant difference between H0 and H1 groups within the 7–24 h, but pH increase was only encountered in the 17–24 h in H2 and H3 groups ().
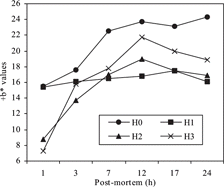
Table 3 The pH and color values of carcass skin (p < 0.05).
The use of RHH in broiler diets had effect on L*, b* and C* values of carcass skin color. But, a* and H* values weren't affected by RHH supplementation. Among the treatment groups the highest b* and C* values were observed in the control group (H0). All color values except for the H* value increased throughout 24 h of storage (). At this period, the most considerable changes were determined in b* values (). The lowest b* values were determined in H2 and H3 groups after 1 h, and then quickly increased by the 12 h of storage. There was no important change in H1 during storage. The quickest increasing in b* value continued by through the 7 h in the control group (H0) ().
The color of skin affects acceptability of the carcass. Broiler skin and meat colors are affected by numerous live production, slaughter, processing factors, handling, and packaging.[Citation19–21] Petracci and Fletcher[Citation22] also indicated that L* and b* values of skin of broiler carcass increased in postmortem (24 h) after slaughtering, but the changing of a* values was no significant. Bilgili et al.[Citation23] reported that color values of broiler breast skin were 73.62–76.14 for L*, 5.62–7.61 for a* and 18.45–21.41 for b* values. The color of meat is highly correlated with meat pH. The darker meat color is associated with higher meat pH, and the lighter meat color is associated with the lower meat pH. In addition, meat pH affects the water binding nature of the proteins and therefore directly affects the physical structure of the meat and its light reflecting properties.[Citation19,Citation24]
Breast and Drumstick Meats Color Values
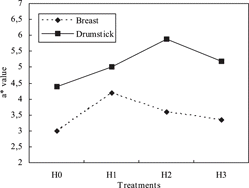
RHH significantly affected the L*, a* b*, H* and C* values in breast and drumstick meats (p < 0.01). The lowest L* value was found in H1. The a* value was lower in H0 than the other groups, and there was no differences (p > 0.05) among treatment groups (). The color values were significant differencet among the meat types (breast and drumstick meats). The L*, a*, b*, and C* values were higher in drumstick meat as compared with the breast meat. Some researchers found similar results in broiler drumstick and breast meats.[Citation25,Citation26] This increasing in redness of drumstick meats was thought to be due to a* high level of myoglobin in the drumstick. Myoglobin is the most abundant pigment in meats and is thought to be responsible for color variations. Qiao et al.[Citation27] reported that L*, a* and b* values of fresh breast fillets were 49.62, 3.25, and 4.93 respectively. In another study, mean color values of breast (L*, a*, and b*) were 62.07 ± 0.54, 4.38 ± 0.22, and 9.68 ± 0.25, respectively.[Citation28] Abeni and Bergoglio[Citation29] observed that L*, a*, b*, C*, and H* values of broiler breast fillets were 56.48–58.98, 6.18–16.40, 14.71–17.62, 16.35–21.79, and 45.05–69.84, respectively. Castellini et al.[Citation26] found that L*, a*, and b* values of breast and drumsticks in fresh broiler carcasses were 59.23, 4.96, 5.16 and 52.86, 5.78, 4.95, respectively. Bianchi and Fletcher[Citation30] reported that L*, a* and b* values in broiler breast meat were determined as 51.8–52.1, 0.1–1.5, and 8.4–8.8 respectively. Some researchers have also indicated that lightness values (L*) to be useful as an indicator of poultry breast meat quality for further processing.[Citation30,Citation31]
Table 4 The color values of breast and drumstick meats (p < 0.05).
The packaging (vacuum and aerobic) had significant effect on a*, b* and H* values in drumstick and breast meats. The a* values of samples vacuum packed and b* values of samples aerobic packed were higher (). Also, shows that RHH supplementation had no effect on L* values of breast and drumstick meats packed. Whereas a* values of drumstick (vacuum and aerobic) and breast (vacuum) were affected with RHH-added diets, treatment had only a higher effect on b* values of drumstick vacuum packed (). On the other hand, Du et al.[Citation32] reported that a* values were not affected by vacuum or aerobic packaged breast fillets at 4 °C for 7 days. During 12-day storage, L* values significantly ranged in breast meat of H2 group. While vacuum packaging affected L* values of breast meats, aerobic packaging had generally decreased on those of drumstick meats ().
Table 5 The effect of RHH levels on the color values of vacuum and aerobic packaged breast and drumstick meats.
Table 6 Effect of RHH supplementation of broilers and packaging way of meat on L values during a positive cold storage.
The a* values of drumstick meat of H1 group increased during storage (). Among color values, the highest change was observed in b* values. The b* values in breast meats of H1 and H3 groups and in drumstick meats of H0, H1, and H2 groups increased (). Depending on the changes in L*, a* and b* values, effect of RHH on Chroma and Hue* values (C and H) was observed ( and ). Yang and Chen[Citation33] found that lightness and redness values of ground breast and thigh meat decreased with storage. It was determined that b* and C* values increased and L* values decreased in drumstick meats and breast fillets samples vacuum and aerobic packed and stored at 3 ± 0.5 °C for 12 days.[Citation25]
Table 7 Effect of RHH supplementation of broilers and packaging way of meat on a* values during a positive cold storage.
Table 8 Effect of RHH supplementation of broilers and packaging way of meat on bFootnote* values during a positive cold storage.
Table 9 Effect of RHH supplementation of broilers and packaging way of meat on HFootnote* values during a positive cold storage.
Table 10 Effect of RHH supplementation of broilers and packaging way of meat on CFootnote* values during a positive cold storage.
Petracci and Fletcher[Citation21] observed that while L* values increased, a* values in drumsticks but decreased in breast fillets stored in plastics bags for 8 days. However the same researchers determined that b* values increased from 2 to 6 days, but decreased later. These results were similar to our findings. Nanke et al.[Citation34] found that a* values of turkey carcasses stored in aerobic conditions at 2 °C for 12 days decreased. In another study, L*, a*, b*, C* and H* values of the meat of breast and leg in chicken stored at 4 °C for 7 days were determined. According to these researchers, while the L* values decreased (mean: 62.24), the b* values increased (mean: 9.21) in breast fillets, and there was no change in a* values (mean: 3.61) during storage. The L*, a*, b*, C*, and H* values of leg meats (0–7 days) were 61.63–58.45, 5.48–5.36, 7.15–7.02, 9.15–8.14, and 51.6–52.9, respectively.[Citation35] Fletcher[Citation20,Citation21] also reported that L* values were between 43.1 and 48.8 in breast fillets. Allen et al.[Citation36,Citation37] showed that the variations in breast meat color was presumably due primarily to pH effect and this significantly affects breast meat shelf life.
Drumstick Meat and Skin Color Values
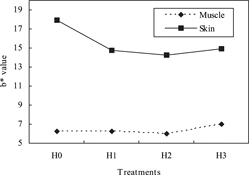
The effect of the use of RHH on a*, b* and H* values of drumstick meat and skin was shown in . The results obtained herein showed that a* value increased but b* values decreased in RHH-treated groups, when compared to the control group. Significant differences were observed between meat and skin color of drumstick. L*, b*, H* and C* values were higher in skin, while a* value was higher in drumstick meat. The interaction of meat type x treatment affected a* and b* values ( and ). The control group (H0) had the lowest a* value among treatments, and the highest a* value was observed in H2 group. presents that when the supplemental hydrolysate increased in diet, a* value of drumstick skin also approached that of the control group; i.e., these values of H1 and H2 were higher than those of H0 and H3. The b* value of the skin decreased in RHH-supplemented groups. On the other hand, there was no difference in b* value in meat of drumstick among RHH-treated groups. As shown in , the difference in b* value between RHH-added groups and control group may have increased due to an increasing b* value of the skin in the control group. In the other words, the highest difference observed in b* value was between skin and meat of drumstick in the control group (). While a* and H* values were affected by packaging, L*, b* and C* values were not. The a* value was higher in samples vacuum packaged than that of aerobic packed. L* and a* values were not affected by storage period, but b* value gradually increased by 4 day of storage (). In the similar study, L*, a* and b* values of meat and skin of drumsticks were 51.58 + 2.14, 3.82 + 1.70, 6.14 + 2.12 and 65.27 + 3.95, 2.33 + 1.59,16.48 + 5.02, respectively.[Citation21] There was no difference among color values of vacuum and aerobic packed drumsticks, stored 3 ± 0.5 °C for 12 days; but the L* value was decreased by storage.
Table 11 The color values of drumstick skin and meat (P < 0.05).
CONCLUSION
The results of this current study showed that the supplemental RHH at different levels affected the color values of carcasses and carcass parts (breast fillets, drumstick meats and skin) packed vacuum or aerobically, during both post-mortem aging time (first 24 h) and storage period (12 d). Since subcutaneous fat accumulation decreased in RHH-added groups according to control group (visual evaluation) the lightness, redness and yellowness values of samples changed. Especially, the level of 1% of RHH improved carcass color traits.
Notes
1Trace mineral mixure provides in milligrams per kg of diet: Mn, 70; Zn, 50; Fe, 30; Cu, 5, Se, 0.3.
2Vitamin mixure provides per kg of diet: vitamin A 8000 IU; cholecalciferol 1000 IU; α-tocopheryl acetate 15 mg/kg; menadione 3 mg/kg; riboflavin 5 mg/kg; niacin 40 mg/kg; thiamin 2 mg/kg; folic acide 0.6 mg/kg; vitamin B12 15 μg/kg.
3Analyzed by AOAC (1984).
aH2SO4 + Mg (OH)2 → MgSO4 + H2O from neutralization step with calculate.
a–dAny two means in the same section of same column having the same letters are not significantly different at p < 0.05.
**p < 0.01,
*: p < 0.05,
a–d: Any two means in the same column having the same letters are not significantly different at p < 0.05.
**p < 0.01,
*p < 0.05, Ns: Non-significant; ±: Standard deviation of samples.
*p < 0.05,
**p < 0.01, Ns: Non-significant,
a–dAny two means in the same row having the same letters are not significantly different at p < 0.05.
*p < 0.05,
**p < 0.01, Ns: Non-significant.
a–g Any two means in the same column having the same letters are not significantly different at p < 0.05.
**p < 0.01, Ns: Non-significant.
a–g Any two means in the same column having the same letters are not significantly different at p < 0.05.
*p < 0.05,
**p < 0.01, Ns: Non-significant.
a–gAny two means in the same column having the same letters are not significantly different at p < 0.05.
*P < 0.05,
**P < 0.01, Ns: Non significant.
a–gAny two means in the same column having the same letters are not significantly different at P < 0.05.
*p < 0.05,
**p < 0.01, Ns: Non-significant.
a–gAny two means in the same column having the same letters are not significantly different at p < 0.05.
a–dAny two means in the same column having the same letters are not significantly different at p < 0.05.
**p < 0.01,
*p < 0.05, Ns: Non-significant; ±: Standard deviation of samples.
REFERENCES
- Fuller , R. 1989 . A review probiotics in man and animals . J. Applied Bact. , 66 : 365 – 378 .
- Kung , L. 1990 . Microbes and enzymes . Feed International , 11 : 10 – 16 .
- Suzuki , K. , Kodama , Y. and Mitsuoka , T. 1989 . Stress and intestinal flora . Bifidobacteria and Microflora , 8 : 23 – 38 .
- Keshavarz , K. and Jackson , M.E. 1992 . Performance of growing pullets and laying hens fed low-protein, amino acid-supplemented diets . Poultry Sci , 71 : 905 – 918 .
- Kurbanoglu , E.B. and Algur , O.F. 2002 . Use of ram horn hydrolysate as peptone for bacterial growth . Turk Journal of Biology , 26 : 115 – 123 .
- Dalev , P. 1990 . An enzyme-alkaline hydrolysis of feather keratin for obtaining a protein concentrate for fodder . Biotechnol. Lett. , 12 : 71 – 72 . [CROSSREF]
- Kurbanoglu , E.B. 2001 . Production of single-cell protein from ram horn hydrolysate . Turk Journal of Biology , 25 : 371 – 377 .
- Ozen , N. Poultry . 1989 . Breeding, Genetics, Nutrition, Diseases, Meat and Egg Technology , 2nd , 157 – 286 . Samsun, , Turkey : Ondokuz Mays University, Publish No: 48 .
- Cheeke , P.R. 1999 . Applied Animal Nutrition. Feeds and Feding , 2nd , 328 Upper Saddle River, New Jersey : Prentice-Hall, Inc. Simon and Schuster/A Viacom Company .
- Kurbanoglu , E.B. and Kurbanoglu , N.I. 2002 . A new process for the utilization as peptone of ram horn waste . J. Biosci. Bioeng. , 94 : 202 – 206 . [CROSSREF]
- Karaoglu , M. , Macit , M. , Aksu , M.I. , Kurbanoglu , E.B. , Esenbuga , N. and Celebi , S. 2005 . Effect of Supplemental Ram Horn Hydrolysate (RHH) at Different Levels on Growth Performance and Slaughter Traits of Broilers . J. Sci. Food Agr. , (in press)
- Aksu , M.I. , Karaoglu , M. , Esenbuga , N. , Kaya , M. and Macit , M. 2005 . Effect of Dietary Ram Horn Hydrolysate on the pH, TBARS and Some Microbiological Properties of Vacuum and Aerobic Packaged Breast and Drumstick Meats of Broilers during Positive Cold Storage . (Food Sci. Technol. Int.) , submitted
- N.R.C. 1984 . National Research Council, Nutrient Requirements of Poultry , 8th rev. , Washington, DC : National Academy Press .
- A.O.A.C. 1984 . Official Methods of Analysis of the Association of Official Analytical Chemist , 14th , Richmond, Virginia : The William Byrd. Press, Inc .
- Kurbanoglu , E.B. 2003 . Enhancement of glycerol production with ram horn hydrolysate by yeast . Energ. Convers. Mgmt. , 44 : 2125 – 2133 . [CROSSREF]
- Yalcin , S. , Ozkan , S. , Acikgoz , Z. and Ozkan , K. 1999 . Effect of dietary methionine on performance, carcass characteristics and breast meat composition of heterozygous naked neck (Na/na+) birds under spring and summer conditions . Brit. Poultry Sci. , 40 : 688 – 694 . [CROSSREF]
- Pingel , H. , Wicke , M. and Lengerken , G.V. 1998 . Gewinnung und Qualitat von Geflügelfleisch , 301 – 334 . Germany : Qualitat von Fleisch und Fleischwaren Band 1. Deutscher Fachverlag .
- S.P.S.S. 1996 . Statistical Packages for the Social Sciences for Windows Release 10.01 , SPSS Inc. .
- Fletcher , D.L. 1989 . Factors influencing pigmentation in poultry . Critical Review of Poultry Biology , 2 : 149 – 170 .
- Fletcher , D.L. 1999 . Color variation in commercially packaged broiler breast fillets . J Appl. Poultry Res. , 8 : 67 – 69 .
- Froning , G.W. 1995 . Color of poultry meat . Poultry Avian Biology Review , 6 : 83 – 93 .
- Petracci , M. and Fletcher , D.L. 2002 . Broiler skin and meat color changes during storage . Poultry Sci , 81 : 1589 – 1597 .
- Bilgili , S.F. , Conner , D. E. , Pinion , J.L. and Tamblyn , K. C. 1998 . Broiler skin color as affected by organic acids: influence of concentration and method of application . Poultry Sci , 77 : 751 – 757 .
- Briskey , E.J. 1964 . Etiological status and associated studies of pale, soft, exudative porcine musculature . Advances in Food Researchers , 13 : 89 – 178 .
- Karaoglu , M. , Aksu , M.I. , Esenbuga , N. , Kaya , M. , Macit , M. and Durdag , H. 2004 . Effect of dietary probiotic on the pH and color characteristics of carcasses, breast fillets and drumsticks of broilers . Animal Sci , 78 : 253 – 259 .
- Castellini , C. , Mugnai , C. and Dal , Basco A. 2002 . Effect of organic production system on broiler carcass and meat quality . Meat Sci , 60 : 219 – 225 . [CROSSREF]
- Qiao , M. , Fletcher , D.L. , Northcutt , J.K. and Smith , D. P. 2002 . The relationship between raw broiler breast meat color and composition . Poultry Sci , 81 : 422 – 427 .
- Qiao , M. , Fletcher , D.L. , Smith , D.P. and Northcutt , J.K. 2002 . Effect of raw broiler breast meat color variation on marination and cooked meat quality . Poultry Sci , 81 : 276 – 380 .
- Abeni , F. and Bergoglio , G. 2001 . Characterization of different strains of broiler chicken by carcass measurements, chemical and physical parameters and NIRS on breast meat . Meat Sci , 57 : 133 – 137 . [CROSSREF]
- Bianchi , M. and Fletcher , D.L. 2002 . Effects of broiler breast meat thickness and background on color measurements . Poultry Sci , 81 : 1766 – 1769 .
- Owens , C.M. , Hirsler , E.M. , Mckee , S.R. , Martinez-Dawson , R. and Sams , A.R. 2000 . The characterization and incidence of pale soft exudative turkey meat in a commercial plant . Poultry Sci , 79 : 553 – 558 .
- Du , M. , Nam , K.C. , Hur , S.J. , Ismail , H and Ahn , D.U. 2002 . Effect of dietary conjugated linoleic acid, irradiation, and packaging conditions on the quality characteristics of raw broiler breast fillets . Meat Sci , 60 : 9 – 15 . [CROSSREF]
- Yang , C.C. and Chen , T.C. 1993 . Effects of refrigerated storage, pH adjustment, and marinate on color of raw and microwave cooked chicken meat . Poultry Sci , 72 : 355 – 362 .
- Nanke , K.E. , Sebranek , J.G. and Olson , D.G. 1999 . Color characteristics of irradiated aerobically packaged pork, beef, and turkey . J. Food Sci. , 64 : 272 – 278 .
- Millar , S.J. , Moss , B.W. and Stevenson , M.H. 2000 . The effect of ionizing radiation on the color of leg and breast of poultry meat . Meat Sci , 55 : 361 – 370 . [CROSSREF]
- Allen , C.D. , Russell , S.M. and Fletcher , D.L. 1997 . The relationship of broiler breast meat color and pH to shelf-life and color development . Poultry Sci , 76 : 1042 – 1046 .
- Allen , C.D. , Fletcher , D.L. , Northcutt , J.K. and Russell , S.M. 1998 . The relationship of broiler breast color to meat quality and shelf-life . Poultry Sci , 77 : 361 – 366 .