Abstract
Controlled low-temperature vacuum dehydration (CLTVD) and tunnel drying (TD) processes were applied to mashed potatoes and the quality of the dried products was compared for both processes. Slabs of different thickness (0.26, 0.52 and 0.78 mm) were prepared. CLTVD was carried out at a low temperature avoiding freezing the product, at absolute pressure of 0.67, 1.00 and 1.33 kPa. For the TD experiments air temperatures were 40, 50 and 60° C. CLTVD and TD processes were compared using engineering and quality parameters (bulk density, water adsorption and total color difference). During the constant-rate drying period 40 to 70% of moisture was removed. The best quality product was generated at 0.26 mm, 0.67 kPa for CLTVD and 0.26 mm, 40° C for TD. For these treatments, the flux of mass was 7.8 3 1025 and 3.3 3 1024 kg m22s21 for CLTVD and TD, respectively. The mass transfer coefficient was 6.2 3 1026 kg m22s21 ΔY21 for CLTVD and 3.6 3 1022 kg m22s21 ΔY21 for TD. The falling-rate drying period was divided into three sub-periods. The effective diffusivity coefficient ranged from 1.1 3 10211 to 4.8 3 10211 m2/s and from 4.2 3 10211 to 9.6 3 10211 m2/s for CLTVD and TD, respectively. A change in water transport mechanism was achieved and both processes were controlled by mass transfer; internal and external mass transfer resistances were higher for CLTVD than for TD. CLTVD yields a product with lower shrinkage and a color closer to the white standard than the products obtained by TD. CLTVD may be considered as a potential alternative for drying of temperature-sensitive materials.
Introduction
Drying is one of the most widely used methods for food preservation. During drying, water activity is reduced to levels that limit the growing of microorganisms and chemical and enzymatic deteriorative reactions. Knowledge of the basic principles involved during drying has allowed the development of different approaches for obtaining high quality food products in terms of sensorial, physicochemical, functional and nutritional features. From an engineering point of view, drying procedures tend to become more efficient as drying times and equipment design could be optimized to obtain a given product.[Citation1,Citation2] This requires the evaluation of drying parameters, such as mass transfer (k Y) and effective diffusivity (D eff) coefficients as they indicate a quantitative factor for the process quality.[Citation3–5] During the drying process food systems undergo several physical and structural modifications that affect the quality of the final product.[Citation2]
Convective hot-air drying is widely used in food dehydration probably due to its low cost. Freeze-drying process generates food products of high quality, sometimes with a practically unchanged internal structure.[Citation6,Citation7] However, it is an energy-intensive and time-consuming process, and therefore more expensive. On the other hand, vacuum drying takes less time, but water removal could induce unfavorable phenomena such as hardening and shrinkage, that may reduce the quality of the product.[Citation8,Citation9]
Derived from controlled freezing-point food storage, a controlled low-temperature vacuum dehydration approach (CLTVD) was proposed[Citation10] to achieve food products with better quality than those obtained by vacuum drying, and with less drying times and operational costs than those produced by freeze-drying. A clam paste and a gelatin-microcrystalline cellulose model food system were used. In this process, drying was carried out at a temperature as low as possible without freezing the product at about −3° C to +5° C, while heat and vacuum were simultaneously applied in order to promote the evaporation of water. Using a response surface methodology, optimum conditions for CLTVD of a gelatin-microcrystalline cellulose model food system was determined.[Citation11] CLTVD not only reduced the drying time but also increased the survival of Lactobacillus acidophilus with the avoidance of freeze-injury.[Citation12] A kinetic analysis for CLTVD and freeze-drying was carried out where CLTVD was described as a uniformly retreating ice front and a sublimation-vaporization model was used to simulate the drying process.[Citation13] CLTVD produced a dried breadfruit of a quality equivalent to that achieved by freeze-drying, and of a quality better than that obtained by vacuum and cross flow drying.[Citation14] In a study of the effect of protectants on Lactobacillus acidophilus, the injured survival in CLTVD was lower than that in freeze-drying.[Citation6] Although a higher death ratio was caused by CLTVD, residual bacteria were less injured. The rates of myoglobin degradation and lipid oxidation in dried beef and pork were lower for CLTVD than those for freeze-drying.[Citation15] Chlorophyll degradation in spinach dried by CLTVD was less significant than that in samples dried by freeze-drying.[Citation16]
Controlled low-temperature vacuum dehydration has demonstrated certain advantages over vacuum drying and freeze-drying as well as applicability for selected products.[Citation10] The objective of this study was to compare the controlled low-temperature vacuum dehydration process with the tunnel drying process through the evaluation of engineering and quality parameters such as bulk density, water adsorption and total color difference, using mashed potato as a test material.
MATERIALS AND METHODS
Statistical Analysis
A completely random design was used for both dehydration processes. Tunnel drying factors were air temperature (40, 50 and 60° C) and slab thickness (0.26, 0.52 and 0.78 mm). CLTVD factors were absolute pressure (0.67, 1.00 and 1.33 kPa) and slab thickness (0.26, 0.52 and 0.78 mm). Three replicates were taken for each treatment. Differences between mean values were obtained using the LSD multiple range tests.
Preparation of Mashed Potato Slabs
Potato (Solanum tuberosum) cv. Alpha was canned for homogenization and preservation purposes. Potatoes were washed (sodium hypochlorite 200 ppm), peeled manually and cut in cubes of 2.5 cm. Cubes were canned (303 × 406) using a 2% brine and a closing temperature of 85° C. Closed cans were thermally processed at 121.1° C for 15 min,[Citation17] cooled to 45° C in water (sodium hypochlorite 7 ppm) and stored at 10° C. For each treatment, one can was opened, drained for 1 min and cubes were mashed using a perforated bean grinder. Slabs with nominal thickness of 0.26, 0.52 and 0.78 mm were prepared placing 2, 4 or 6 masking tapes in the parallel extremes of a 0.019 m2 support. Mashed potato was spread evenly on the support using a Teflon-coated spoon.
Tunnel Drying
A laboratory tunnel dryer with square section was used for drying mashed potato. A variable speed electric motor drove a centrifugal fan. Air temperature was 40, 50 and 60° C. Air velocity was fixed at 2 m/s and the flow was parallel to the sample. Moisture content was measured according to AOAC.[Citation18] Sample mass was registered each 2 min using an electronic balance (Sartorius, PT1200; Gottingen, Germany); wet and dry bulb temperature were registered each 15 min using a sling psychrometer (Bacharach Instruments, Pittsburgh, PA). Drying curves were drawn using dimensionless moisture contents, X/Xi where Xi was the initial moisture content of the sample.
Controlled Low-Temperature Vacuum Dehydration (CLTVD)
Samples were dried at a low temperature without freezing (−1° C to −9° C) using vacuum to enhance the drying rate. All tests were carried out in a Freeze-dryer (25SL, Virtis; Gardiner, NY) at pressures (0.67, 1.00 and 1.33 kPa) close to that of the triple point of water but higher than it to prevent freezing the product. An electronic balance was installed inside the drying chamber to register sample mass variations during dehydration. All samples were dried until no detectable mass change was observed. Moisture content was measured at the beginning and at the end of the process using an infrared moisture balance (AD-4713; A&D Mercury, Japan) at 90° C for 2 h.
Evaluation of Critical Moisture Content
Moisture content, X as a function of time (t) was assumed to follow a quadratic equation:
A multiple linear regression analysis was performed to estimate the β coefficients. First, all moisture content and time data in a treatment were considered in the regression analysis. Then, final data were removed one by one and the significance of the β‘s was evaluated every time a data point (X, t) was removed. When the coefficient of the quadratic term, β 2 was no significant (α > 0.05), the most suitable model for adjusting the remainder experimental data was a straight line, as expected for data corresponding to the constant-rate drying period. Critical moisture content corresponded to the moisture content at the end of the constant-rate drying period. Besides, surface temperature was registered during drying.
Constant-Rate Drying Period
The mass transfer coefficient, k Y was calculated from:
where N is drying rate or flux of mass, YS represents absolute humidity at the adiabatic saturated temperature and Y is absolute humidity of air stream. For tunnel drying, absolute humidity of air was calculated using the dry and wet bulb temperatures at an absolute pressure of 77.81 kPa and absolute humidity of saturated air was calculated assuming an adiabatic saturation process. For CLTVD, absolute humidity of saturated air and absolute humidity of air were calculated using the mean values of surface temperature at absolute pressures of 0.67, 1.00 and 1.33 kPa.
Falling-Rate Drying Period
For this stage, the average moisture content of a 2L-thick slab, where L is half the thickness, is supplied by the following infinite series:[Citation19]
where (X – X eq)/(X c– X eq) is the unaccomplished moisture fraction, D eff is the effective diffusivity coefficient and Fom = Defft/L 2 is the mass transfer Fourier number. As the Fom number increases, the first term of the series solution becomes the dominant term. Only the first term of this series solution will be taken.
Because the subsequent terms in the expansion are neglected, the model is only valid for Fom numbers in excess of 0.02, with an error of less than 1.4% in the unaccomplished moisture fraction. A Ln [(X – Xeq )/(Xc – Xeq )] against time curve was drawn. When the curve was not a straight line several falling rate drying stages were considered, each of them characterized by an effective diffusivity coefficient. These falling-rate drying sub-periods were determined using a quadratic equation for time in a similar way to that used for calculating the critical moisture content. For every sub-period, a regression analysis was performed to obtain the most suitable value of the slope which was used to calculate the effective diffusivity coefficient corresponding to each falling-rate drying sub-period.
Quality Parameters of the Product
Bulk density of the dried product was measured by a combination of an electronic balance and a graduated cylinder with an accuracy of 0.2 mL. Samples (5 g) with a particle size ≤ 0.38 mm (mesh 40) were used in every individual measurement. Water adsorption (WA) of the dried product was measured using a modification of the method reported by Lin et al.[Citation20] Samples (0.5 g) with a particle size ≤ 0.38 mm were placed in a centrifugal tube (14 3 19 mm) adding 5 mL of distilled water. Mixture was shaken in a vortex (M16715, Maxi-Mix I, Thermolyne; Dubuque, Iowa) for 5 min and centrifuged (HN-SII, Damon IEC, Needham Heights, MA) at 1610 × g for 25 min. The supernatant was removed and WA was determined by mass difference. With the intention of having an estimation of color, total color difference, ΔE between the dried product and a white standard was measured[Citation21] using a reflectance colorimeter (Color mate colorimeter, Milton Roy, Rochester, NY) with 10° observation (CIE) and illuminant D65. Mashed potato samples (5 g) with a particle size between 0.28 mm (mesh 50) and 0.38 mm (mesh 40) were used to avoid variability in readings due to particle size.[Citation22] Three replicates were taken for each property.
Selection of a Treatment for Each Drying Process
To compare the advantages of the CLTVD process with the tunnel drying process the treatment that generated the best quality product was selected in each drying process. Selection criteria were the lowest density as it indicates a lower shrinkage of the product,[Citation10] the highest water adsorption as it indicates a higher rehydration capacity and quality of the product and the lowest total color difference as it indicates the nearest color to the white standard[Citation21] and less browning. Five replicates were taken for each of these two treatments.
RESULTS AND DISCUSSION
Drying Curves
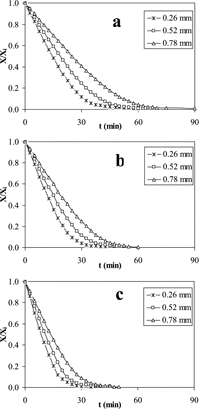
Tunnel Drying (TD)
Drying curves for mashed potato obtained by tunnel drying are shown in . Drying was faster at lower thickness and higher temperature; these differences were observed all along the drying curves, but the differences were larger at low moisture contents. Both drying periods were influenced by air temperature and slab thickness; this behavior is in agreement with that reported by Ganjyal et al.[Citation23]
Controlled Low-Temperature Vacuum Dehydration (CLTVD)
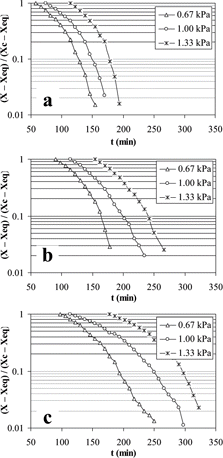
Drying curves for mashed potato obtained by CLTVD at 1.33, 1.00 and 0.67 kPa for slabs of 0.26, 0.52 and 0.78 mm are shown in . For all treatments there was a short initial lag period; here water evaporation was increased since chamber pressure was lower than water vapor pressure in the food. This rise in moisture migration induced an abrupt decrease of sample temperature as it took the vaporization heat from itself. This reduction in temperature generated a drop in water vapor pressure affecting the driving force for drying. After a short period, the quantity of evaporated moisture was not enough to keep the sample temperature; therefore, the slab temperature increased slowly to reach the chamber temperature. Mashed potato showed smooth drying curves and drying rate was larger at lower thickness and lower pressure. However, drying rate was higher for tunnel drying than for CLTVD, which represents a longer process time for CLTVD (). The effect of thickness was nearly the same for both processes. During the constant-rate drying period, the driving force for evaporation is the difference between water vapor pressure at the surface sample temperature and water partial pressure at the chamber temperature, and when this difference was increased the drying rate was also increased.
Figure 2 Controlled low-temperature vacuum dehydration curves at different thickness of mashed potato slabs for (a) 1.33 kPa, (b) 1.00 kPa and (c) 0.67 kPa.
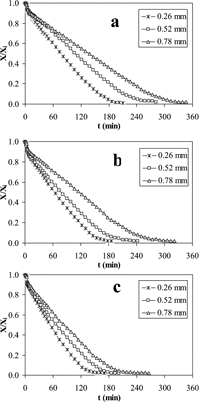
Table 1 Process drying time, critical moisture content (Xc), flux (N) and mass transfer coefficient (kY) for tunnel drying (TD) and controlled low-temperature vacuum dehydration (CLTVD) processes.
Critical moisture content for both dehydration processes is shown in . For tunnel drying, critical moisture content values were higher than those reported by May et al.[Citation1] for constant controlled drying conditions of raw potato using an automatic thermo-gravimetric analyzer. These differences could be explained by the different structure of the samples and conditions of the processes used in this work as compared with those used by these researchers. Critical moisture content of mashed potato dried by tunnel drying was significantly affected by temperature (p < 0.05) and thickness (p < 0.05). As temperature increased, the critical moisture content decreased; this could be attributed to a higher mobility of water as temperature rises, allowing a higher evaporation before the change of drying periods. Mean critical moisture content was different for slabs of 0.78 mm indicating that surface saturation was faster lost than for the other thicknesses, probably due to the fact that inner water mobility was not enough to keep surface saturation. For CLTVD, mean critical moisture content was affected by pressure (p < 0.05) ranging from 1.38 to 1.93 kg/kg dry solids, and by thickness of the slab ranging from 1.56 to 1.91 kg/kg dry solids. At 1.33 kPa, the mean drying rate was very low allowing a higher quantity of water to be transferred from inside the sample to the surface before the sample lost its saturation.
Mashed potato remains in the constant-rate drying period about a quarter or a third of the total drying time for tunnel drying and about a third or a half of the total drying time for CLTVD. The flux (N) and mass transfer coefficient (kY ) for the laboratory tunnel drying and controlled low-temperature vacuum dehydration processes are shown in . For tunnel drying, as temperature increased at a constant thickness, the flux increased and the mass transfer coefficient did not roughly change. This behavior could be explained based on the increment of the driving force due to the temperature increase. A similar behavior was observed for CLTVD. For both drying processes, the difference in flux was about one order of magnitude, while the difference in mass transfer coefficient was about four orders of magnitude; this indicates that mass transfer resistance, given as the reciprocal of k Y, was higher for CLTVD.
Mass transfer coefficients may be useful in determining water fluxes at conditions other than those used in this work for the constant rate period. Specifically, in the case of CLTVD, other temperatures could be explored and for tunnel drying, both, airflow and temperature may be changed in the calculations. This could be carried out by means of the usage of mass transfer equations for slabs, based on dimensionless relationships, considering the limits of application of such equations.[Citation24, Citation25]
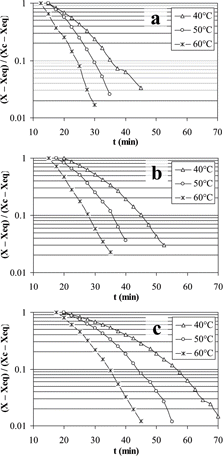
The unaccomplished moisture fraction for the tunnel drying process of mashed potato slabs is shown in . For treatments of 60° C, 0.26 mm and 60° C, 0.52 mm, a straight line could be adjusted but for the others there were changes in the slope. The latter behavior has been reported by Karathanos and Saravacos[Citation26] for starch with increments in the slope of the curve. The absolute value of the slope was increased as temperature increased and thickness decreased; indicating that the effective diffusivity coefficient increased as drying proceeded. The corresponding unaccomplished moisture fraction for CLTVD is shown in . In this case, the slope changed for all treatments as pressure and thickness changed. It was observed that the curvature of the graph could generate several falling rate drying sub-periods, each of them characterized by an effective diffusivity coefficient.
Effective Diffusivity
The effective diffusivity coefficient ranged from 3.78 × 10−11 m2/s to 7.22 × 10−10 m2/s for tunnel drying and from 1.01 × 10−11 m2/s to 2.16 × 10−10 m2/s for CLTVD. Effective diffusivity increased as moisture content decreased and three or four sub-periods could be present during the process depending on the drying conditions. The effective diffusivity coefficient increased as moisture content decreased in the range of 1.0 to 0.18 kg/kg dry solids for sponge cake, a porous product.Citation27 Values of the effective diffusivity coefficient for the tunnel drying process are comparable with those reported by Sablani et al.[Citation7] for potato. Based on the fact that starch represents about 75% of potato dry solids[Citation28] and considering the values of the effective diffusivity coefficient obtained in this study, it can be inferred that during the falling-rate drying period there was a change in the transport mechanism for water. The increase in effective diffusivity for starch, as moisture content is decreased, could be ascribed to an increment in porosity. Therefore, at a first stage the controlling transport mechanism was liquid diffusion and, as drying proceeded, a porosity structure was produced and capillarity and vapor diffusion phenomena should have been the controlling mechanisms for the process.
Quality Parameters
Mean values of bulk density (BD), water adsorption (WA) and total color difference (ΔE) of mashed potato for tunnel drying and controlled low-temperature vacuum dehydration processes are shown in . For tunnel drying, bulk density of mashed potato was significantly (p < 0.05) affected by thickness and the lowest BD was obtained for slabs of 0.26 mm. BD was not significantly affected by temperature. For CLTVD, bulk density was affected by thickness (p < 0.05) and pressure (p < 0.05); the lowest BD of mashed potato was obtained for slabs of 0.26 mm and at pressures of 0.67 and 1.00 kPa. For tunnel drying, water adsorption of mashed potato was significantly (p < 0.05) affected by thickness but not by temperature; the highest WA was obtained for slabs of 0.26 mm. For CLTVD, water adsorption was affected by thickness (p < 0.05) and pressure (p < 0.05); the highest WA was obtained at a pressure of 0.67 kPa and thickness of 0.26 and 0.52 mm. For both processes, ΔE of mashed potato was not affected by thickness, temperature or pressure.
Table 2 Bulk density, water adsorption and total color difference, ΔE of mashed potato slabs for tunnel drying (TD) and controlled low-temperature vacuum dehydration (CLTVD) processes.
For tunnel drying, slabs of 0.26 mm showed the lowest bulk density and the highest water adsorption. Bulk density, water adsorption and total color difference were not affected by temperature at the levels studied. Therefore, the treatment of 0.26 mm, 40° C was selected in the laboratory tunnel dehydration process to compare with the CLTVD process. For CLTVD, slabs of 0.26 mm and chamber absolute pressure of 0.67 kPa showed the lowest bulk density and the highest water adsorption. Total color difference was not affected by pressure or thickness at the levels studied. The treatment of 0.26 mm, 0.67 kPa was chosen in the controlled low-temperature vacuum dehydration process to compare with the laboratory tunnel dehydration process.
Comparison Based on Engineering Parameters
Means of five replications of flux, mass transfer and effective diffusivity coefficients in the falling-rate drying period corresponding to the selected treatments are shown in . Flux for CLTVD was significantly (p < 0.05) lower than that for tunnel drying; the difference was about one order of magnitude. Transfer rate for CLTVD was about four times lower than that for tunnel drying. The mass transfer coefficient for CLTVD was significantly (p < 0.05) lower about four orders of magnitude than that for tunnel drying. Therefore, there was a much higher mass transfer resistance for CLTVD. This could be due mainly to a decrease in the external resistance to mass transfer caused by the turbulence of air in the laboratory tunnel and to the higher temperatures used in this process. It can be observed that effective diffusivity for CLTVD was about three times lower than that obtained for the tunnel drying process. This could be explained by the influence of air temperature during the tunnel drying process and by the influence of air as a heat conducting agent compared with the vacuum dehydration process.
Table 3 Quality and engineering parameters of dry mashed potato for the selected treatments. Tunnel drying (TD) process (0.26 mm, 40° C). Controlled low-temperature vacuum dehydration (CLTVD) process (0.26 mm, 0.67 kPa).
Comparison Based on Quality Parameters
Means of five replications of bulk density, water absorption and total color difference corresponding to the selected treatments for each dehydration process are shown in . Mashed potato dehydrated by CLTVD showed a significant (p < 0.05) lower bulk density than that dried by the tunnel drying process. Based on this, CLTVD induced a lower shrinkage of the product. There were not significant differences for water adsorption between both dehydration processes. Mashed potato dehydrated by CLTVD showed a significant (p < 0.05) lower total color difference; it indicates that the controlled low-temperature vacuum dehydration process generated a product with a color closer to the white standard.
CONCLUSIONS
Parameters that controlled CLTVD were chamber pressure, consequently sample temperature, and mashed potato slab thickness. The constant-rate drying period represented about a quarter to a third of the total time for tunnel drying and about a third to a half the amount of time for CLTVD, removing from 40% to 70% of moisture, although a higher time interval was achieved by the falling-rate drying period. In both dehydration processes, the falling-rate drying period was divided in two, three or four sub-periods depending on drying conditions. The number of sub-periods increased as the thickness and process time increased. A change in water transport mechanism was accomplished; water was first diffused as liquid, later as a mixture of liquid and vapor and finally as vapor. Capillarity also had an effect on water transport mechanism. Both drying processes were controlled by mass transfer; internal and external mass transfer resistances were higher for CLTVD than for tunnel drying as could be observed by the kY and Deff values. Thickness of the slab affected bulk density and water adsorption of mashed potato and the studied temperature interval did not exerted a significant influence on quality parameters for the tunnel drying process. For CLTVD, slab thickness and pressure affected bulk density and water adsorption of mashed potato. CLTVD yields a product with lower bulk density and total color difference than tunnel drying. There was not difference in water adsorption for products dehydrated by both processes. For CLTVD, the lowest bulk density and the highest water adsorption of products was obtained for slabs of 0.26 mm and 0.67 kPa of absolute pressure. Controlled low-temperature vacuum dehydration represents an alternative for drying of temperature-sensitive products. Studies on CLTVD, especially on equipment design, transfer phenomena and the use of other food products could be conducted along with an evaluation of the costs of the operation.
REFERENCES
- May , B.K. , Sinclair , A.J. , Halmos , A.L. and Tran , V.N. 1999 . Quantitative analysis of drying behavior of fruits and vegetables . Drying Technol , 17 ( 7/8 ) : 1441 – 1448 .
- Karel , M. 1974 . “ Fundamentals of dehydration processes ” . In Advances in preconcentration and dehydration of foods , Edited by: Spicer , A. 45 – 94 . London : Applied Science Publishers Ltd .
- Krokida , M.K. , Zogzas , N.P. and Maroulis , Z.B. 2002 . Mass transfer coefficient in food processing: compilation of literature data . International Journal of Food Properties, , 4 ( 3 ) : 372 – 282 .
- Maroulis , Z.B. , Saravacos , G.D. , Panagiotou , N.M. and Krokida , M.K. 2001 . Moisture diffusivity data compilation for foodstuffs: effect of material moisture content and temperature . International Journal of Food Properties , 4 ( 2 ) : 225 – 237 . [CROSSREF]
- Panagiotou , N.M. , Krokida , M.K. , Maroulis , Z.B. and Saravacos , G.D. 2004 . Moisture Diffusivity: Literature Data Compilation for Foodstuffs . International Journal of Food Properties, , 7 ( 2 ) : 273 – 299 . [CROSSREF]
- King , V.A.E. and Lin , H.J. 1995 . Studies on the effect of protectants on Lactobacillus acidophilus strain dehydrated under controlled low-temperature vacuum dehydration and freeze-drying by using response surface methodolog . J. Sci. Food Agric. , 68 : 191 – 196 .
- Sablani , S.S. , Rahman , M.S. and Al-Habsi , N. 2003 . Effect of convection air-drying on apparent density, porosity and moisture diffusivity of potato and apple . J. Food Sci. Technol. , 40 ( 6 ) : 592 – 596 .
- Labuza , T.P. and Hyman , C.R. 1998 . Moisture migration and control in multi-domain foods . Trends in Foods Science & Technology , 9 : 47 – 55 . [INFOTRIEVE] [CROSSREF]
- Aguilera , J. M. and Stanley , D. W. 1999 . “ Simultaneous heat and mass transfer: Dehydration ” . In Microestructural Principles of Food Processing and Engineering , 2nd , 373 – 411 . Gaithersburg Aspen Publishers, Inc. .
- King , V.A.E. , Zall , R.R. and Ludington , D.C. 1989 . Controlled low-temperature vacuum dehydration—a new approach for low-temperature and low-pressure food drying . J. Food Sci. , 54 : 1573 – 1579, 1593 .
- King , V.A.E. and Zall , R.R. 1992 . A response surface methodology approach to the optimization of controlled low-temperature vacuum dehydration . Food Res. Intl. , 25 : 1 – 8 . [CROSSREF]
- King , V.A.E. and Su , J.T. 1993 . Dehydration of Lactobacillus acidophilus . Process Biochem , 28 : 47 – 52 . [CROSSREF]
- Lombrana , J.I. and Villaran , M.C. 1993 . Kinetic modeling of sublimation and vaporization in low-temperature dehydration processes . J. Chem. Eng. Jpn. , 26 : 389 – 394 . [CROSSREF]
- Narasimham , P and John , P.J. 1995 . Controlled low-temperature vacuum dehydration as an alternative to freeze-drying of diced breadfruit . J. Food Sci. Technol. , 32 : 305 – 309 .
- King , V.A.E. and Chen , J.F. 1998 . Oxidation of controlled low-temperature vacuum dehydrated and freeze-dried beef and pork . Meat Sci , 48 : 11 – 19 . [CROSSREF]
- King , V.A.E. , Liu , C.F. and Liu , Y.J. 2001 . Chlorophyll stability in spinach dehydrated by freeze-drying and controlled low-temperature vacuum dehydration . Food Res. Intl. , 34 : 167 – 175 . [CROSSREF]
- López , A. 1987 . A Complete Course in Canning and Related Processes. Book III – Processing Procedures for Canned Food Products , 12th , 516 Baltimore, MD : The canning trade Inc .
- AOAC . 1995 . “ Assn. of Official Analytical Chemists ” . In Official Methods of Analysis , 16th Arlington, VA
- Crank , J. 1975 . The Mathematics of Diffusion , 2nd , 424 Oxford : Clarendon Press .
- Lin , M.J.Y. , Humbert , E.S. and Sosulski , F.W. 1974 . Certain functional properties of sunflower meal products . J. Food Sci. , 39 : 368 – 370 .
- Billmeyer , F.W. and Saltzman , M. 2000 . Principles of Color Technology , 3rd , 304 New York : John Wiley & Sons .
- Clydesdale , F.M. 1974 . Measuring the color of foods . Food Technol. , 28 ( 7 ) : 45 – 51 .
- Ganjyal , G.M. , Hanna , M.A. and Devadattam , D.S.K. 2003 . Processing of Sapota (Sapodilla): Drying . J. Food Sci. , 68 : 517 – 520 .
- Treybal , R.E. 1981 . Mass Transfer Operations , 3rd , New York : McGraw-Hill .
- Perry , R.H. and Green , D.W.Perry's . 1997 . Chemical Engineer's Handbook , 7th , New York : McGraw-Hill .
- Karathanos , V.T and Saravacos , G.D. 1993 . Porosity and pore size distribution of starch materials . J. Food Eng. , 18 : 259 – 280 . [CROSSREF]
- Guillard , V , Broyart , B , Bonazzi , S , Guilbert , S and Gontard , N. 2003 . Moisture diffusivity in sponge cake as related to porous structure evaluation and moisture content . J. Food Sci. , 68 : 555 – 562 .
- Kadam , S.S. , Dhumal , S.S. and Jambhale , N.D. 1991 . “ Structure, Nutritional Composition, and Quality ” . In Potato: Production, Processing, and Products , Edited by: Salunkhe , D.K. , Kadam , S.S. and Jadhav , S.J. 9 – 35 . Boca Raton, Florida : CRC Press .