Abstract
Our objective was to investigate the glass transition and crystallization of trehalose-sucrose mixtures at various moisture contents. Samples were freeze-dried, rehumidified, and scanned with Differential scanning calorimetry (DSC) to obtain Tg values for all mixtures and pure sugars. Amorphous cotton candy samples for crystallization studies were prepared, humidified, and monitored for crystallinity as a function of time using powder X-ray diffraction (XRD). The Tg of pure dry trehalose was found to be 106 °C, while sucrose had a Tg of 60 °C. Glass transition, as expected, occurred at an intermediate temperature for sucrose-trehalose mixtures. Of the dry samples, only those containing less than 16% trehalose showed sucrose crystallization during scanning. In cotton candy made from a 25% trehalose-75% sucrose mixture, humidified to 33%, sucrose did not crystallize after 30 days, whereas pure sucrose cotton candy at that humidity crystallized completely after 11 days. These data show that trehalose may be a useful crystallization inhibitor in foods with high sucrose content, although small amounts of trehalose did not significantly raise the Tg.
Introduction
Knowledge of and the ability to manipulate sucrose-water state data are important in the effort to increase shelf-life of high-sucrose containing foods, such as cotton candy and soft cookies. Detrimental texture changes, such as stickiness and collapse, in high-sucrose food systems often accompanies the crystallization of the glassy sugar.[Citation1,Citation2] Furthermore, it is known that the crystallization rates of glasses depend on the temperature in relation to the glass transition temperature, Tg. Amorphous systems subjected to temperatures and humidities that fall on a point above the glass transition line can crystallize. Those systems with a large (T-Tg) will crystallize faster than systems close to or below their Tg.[Citation3] Therefore knowledge of the Tg line of pure or mixed sugar systems is critical to stability prediction. Trehalose has been touted as a special molecule used in nature to protect biological systems in dry or cold environments, perhaps by slowing crystallization.[Citation4] Thus, due to the relatively high glass transition temperature of trehalose, amorphous sucrose-trehalose mixtures deserve attention from the high-sucrose foods industry. If the presence of trehalose can sufficiently slow crystallization of sucrose under ordinary storage conditions, which can exceed Tg, shelf life can be extended.
is a composite state diagram of the trehalose-water system based on data reported in available sources,[Citation5–25] including those compiled previously by Chen et al., 2000.[Citation26] The equilibrium lines shown include the liquidus line (freezing point depression line), and the solidus line (solubility or solute crystal melting line). The glass transition curve shows a large variation among different studies, up to ±20 °C at any given solids content. In these studies the dry Tg of trehalose falls between 75 and 120 °C (see ). As seen in , at low moistures the Tg rises dramatically. Therefore, the range of values in could be due to unmeasured, and thus unreported, residual moisture. The same phenomenon can be seen in the case of sucrose-water systems, as shown in , where Tg values taken from publications also do not agree well with each other,[Citation3,Citation12,Citation20,Citation22,Citation24,Citation27–41] particularly in the driest region where the glass transition curve has a steep slope.
Figure 1 Literature values for the state diagram of the trehalose-water system, including the glass transition line.
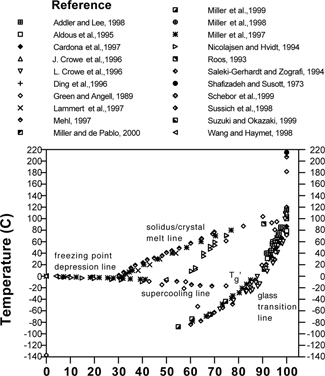
Figure 2 Literature values for the state diagram of the sucrose-water system, including the glass transition line.
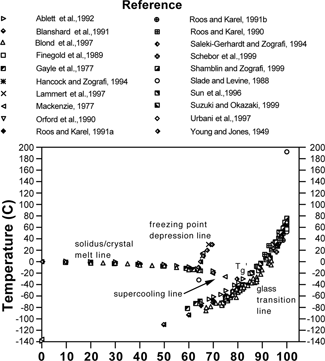
Table 1 Literature values for the glass transition temperature of dry amorphous trehalose and sucrose
shows that in general the reported Tg curve for trehalose is higher than that of sucrose using only those references that reported either onset or midpoint or both. In no case was the onset of glass transition for trehalose reported as less than the onset for sucrose. The Tg values for dry, amorphous sucrose reported in the literature is also listed in . As noted above, a factor contributing to inaccuracy in Tg versus moisture reporting may arise from failure to remove all water from the dry samples. In this case presumed dry samples would actually have moisture contents greater than that reported, and thus the reported Tgs would be lower than their true values. Using the Gordon-Taylor model for the trehalose-water system with values derived in Chen et al. (2000), (k = 5.2), the Tg falls more than 12 °C as the moisture content increases from 0% to 1%.
Figure 3 Trehalose-water and sucrose-water Tg data points from literature sources, as measured by DSC, showing onset and midpoint values as a function of solids percent.
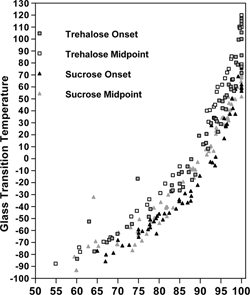
A variety of methods have been reported for the drying of trehalose and sucrose samples, most of which involve freeze-drying over several days with an increasing temperature program. For trehalose, reports of a dry Tg between 105 and 120 °C—which constitute the upper range of the reported values—come from samples that were dried at high temperature (∼60 °C) during the final day of drying.[Citation10,Citation14,Citation16,Citation17,Citation20,Citation37] Typically, reports of Tg for trehalose below 105 °C were from studies where the samples were dried only at temperatures up to and including room temperature[Citation3,Citation7,Citation22,Citation24] or even just equilibrated over P2O5 at room temperature.[Citation34] Because inadequate drying is the most prominent source of error in reporting a Tg versus moisture relationship, it follows logically that of the values reported in the literature the lower ones are less likely to be reliable, and the higher ones are perhaps more realistic.
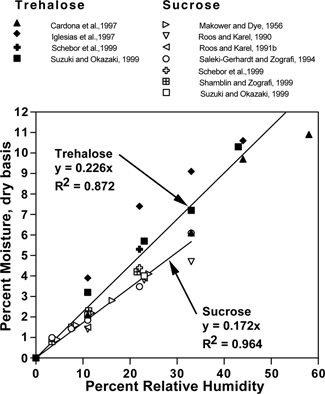
Several papers[Citation3,Citation7,Citation20,Citation22,Citation37,Citation42,Citation43] have reported data for the moisture content of amorphous sucrose and trehalose as a function of water activity. shows these data at 25 °C along with a linear regression. The purpose of this study was to evaluate the overall Tg for combined trehalose-sucrose mixtures, as well as the influence of relative humidity and added trehalose on the recrystallization of sucrose in these mixtures.
Method
Freeze-drying
Samples were prepared by first dissolving 10 grams of a pure crystalline sugar mixture in roughly 30 mL of distilled water, and poured into a plastic 40 mm diameter dish. The mixtures consisted of 0, 4, 8, 12, 16, 20, 40, 60, 80 and 100% trehalose mixtures with the balance being sucrose. Each dissolved mixture was rapidly frozen in liquid nitrogen and placed in a Dura-Top bulk tray dryer with a Dura-Dry condenser module (FTS Systems, Inc., Stone Ridge, NY) running at 13.3 Pa vacuum, with a temperature program of −40 °C for 24 hours, −20 °C for 12 hours, 0 °C for 12 hours, 25 °C for 24 hours, followed by 50 °C for 36 hours. The high-temperature step was not done in the initial trials, resulting in samples with some residual moisture.
After drying, the samples were placed into four desiccators for re-humidification at 25 °C. One set of samples were kept dry over P2O5 powder, while the other three sets were stored over the following saturated salt solutions: LiCl (aw = 0.11), CH3COOK (aw = 0.23), and MgCl (aw = 0.33). Samples were equilibrated for 3 weeks.
Cotton Candy Preparation
Amorphous cotton candy mixtures were created using an Econo Floss Model 3017 cotton candy machine (Gold Medal Products, Co., Cincinnati, OH). About 100 g of pure, crystalline sugar, or trehalose-sucrose mixtures, was heated in the machine's sugar reservoir. When necessary, in order to melt the anhydrous trehalose (Tm ∼215 °C.) additional heat was supplied by a space heater positioned above the sugar reservoir. All cotton candy samples were placed in cups and stored in desiccators humidified to 23%, 33%, and 54% relative humidity.
Differential Scanning Calorimetry
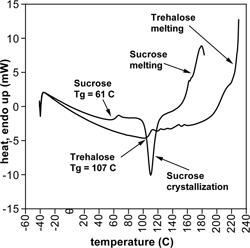
Glass transition temperatures was determined on a Perkin Elmer differential scanning calorimeter, model DSC 7 (Norwalk, CT). Roughly 10 to 20 mg of each sample from the various desiccators was transferred into aluminum pans, sealed, and scanned from −40 °C to 230 °C at a rate of 10 °C/min. All samples were prepared in a dry nitrogen glove box to minimize moisture absorption from the air. Samples that had undergone crystallization during storage exhibited a visible change in volume and texture, and were not scanned. The onset of Tg was recorded. In all cases two replicates were made. shows sample DSC plots for dry sucrose and trehalose. In some cases the Tg zone was accompanied by an enthalpic relaxation (ΔHrelax).
This phenomenon occurs when the amorphous molecules reorient themselves to a lower energy amorphous state. Since this relaxation is made possible by the onset of glass transition, it does not affect the onset of glass transition. This assumption was confirmed with DSC by heating a sample to a temperature just above the Tg, and thereby allowing it to anneal, and then recooling it below the Tg, followed by again heating it above Tg. In this case the onset of Tg did not appear to change, but the peak associated with relaxation enthalpy disappeared. Because enthalpic relaxation was neither influential nor vital to the focus of this study, no further attempts were made to suppress it. It should be clarified, however, that enthalpic relaxation can affect the midpoint of Tg (depending on how midpoint is determined.)
Moisture and Water Activity Determinations
The water activity of each sample was done on an AquaLab model 3 TE (Decagon Devices, Inc., Pullman, WA). The moisture content was measured by the Karl Fischer titration method.[Citation44]
X-ray Diffraction
X-ray diffraction (XRD) patterns were then taken periodically to monitor crystallization using a Seimens D5005 Powder X-ray diffractor. Scans went from 10 to 27 2Θ degrees with a 4-second dwell every 0.04 degrees. To verify the identity of the crystalline sugar, the diffraction angle of measured peaks were compared to database values using Jade 5.0 analysis software. One problematic feature of powder X-ray diffraction is the appearance of one or two peaks in an otherwise amorphous sample. This is most likely due to the presence of a single, small crystal in the sample that never completely melted in the cotton candy-making process. When the sample as a whole begins to crystallize all of the characteristic peaks appear at once, not just one or two. In addition, upon crystallization of the bulk sample, the intensity of the amorphous halo, typically above 500 counts, begins to diminish.
Results and Discussion
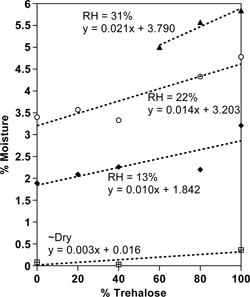
shows the moisture sorption data for the freeze-dried trehalose-sucrose mixtures as a function of water activity. If both sugars had the same sorption properties, the lines would be flat, but as seen adding trehalose, which binds more water (see ), a slight increase in water content results. The slope of the sucrose data was roughly 15.0, and for trehalose the slope was 17.9. In comparison, data collected for moisture sorption of sucrose at 25 °C by Makower and Dye,[Citation43] had a slope of about 18.5 (same units). The Saleki-Gerhardt,[Citation45] sorption at 30 °C data showed a slope of 17.8 for sucrose and 22.0 for trehalose. Although moisture sorption is slightly temperature dependent, the difference between 25 and 30 °C may actually be less than the experimental error, and therefore data at these two temperatures are worth comparing. In general, given the problems in moisture measurement, our data agree well.
Figure 6 Moisture sorption data for amorphous, freeze-dried trehalose-sucrose mixtures at different relative humidities, based on Karl Fischer titration.
As indicated previously in the onset of Tg for dry sucrose and trehalose occurred at 61 and 107 °C, respectively. Based on previously reported data, these values are about 8 or 9 °C less than what was expected for those sugars (∼115 °C for trehalose and 70 °C for sucrose.) This could be due to the fact that even under the relatively rigorous drying conditions used, a small amount of moisture remained. Karl Fischer experiments confirmed a small but not negligible amount of moisture remained in the dry samples, particularly the pure trehalose sample, which contained 0.36% water. Using data plotted in as a guide, this moisture content could explain the slightly lower Tg value determined from the DSC measurements. In this plot, a moisture content of roughly 0.5% corresponds to a 10 °C drop in Tg.
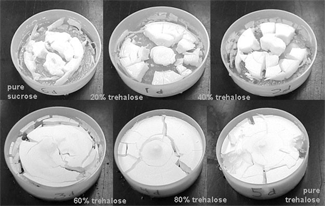
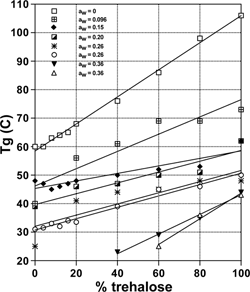
shows the results of Tg determination of sample mixtures as a function of trehalose-sucrose ratio for all runs done at each water activity. As expected the glass transition of the mixtures occurred at a temperature in between the Tgs of the pure components, similar to what is found for carbohydrate-sugar mixtures.[Citation46] In addition, the data appear to follow a roughly linear trend as a function of mixture composition at each water activity. At the highest humidity (aw = 0.36) samples with only 40% trehalose or less had Tgs lower than room temperature, and structural collapse was observed. The formerly amorphous cake became a sticky residue with a reduced volume, as seen in .
Figure 7 Glass transition temperatures of amorphous, freeze-dried sucrose-trehalose mixtures at different water activities at 25°C.
For the dry freeze-dried systems, shows the influence of trehalose addition on sucrose crystallization during the DSC scan. The interesting feature of this data is that while the Tg of the mixture shifts only slightly with small amounts of trehalose added to the sucrose, the crystallization peaks are dramatically shifted to higher temperatures. Pure sucrose crystallizes with a peak at roughly 110 °C. With the addition of just 4% trehalose the peak shifts to about 135 °C and becomes less pronounced, while the 8% trehalose mixture had a peak at 150 °C. At 12% trehalose, the crystallization peak has already disappeared, indicating some inhibitory effect of trehalose.
Figure 9 DSC thermograms of the dry amorphous sucrose-trehalose mixtures, showing the influence of added trehalose on sucrose crystallization.
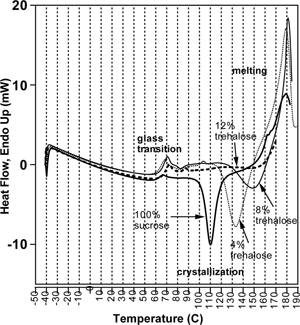
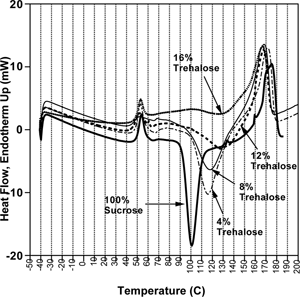
A similar behavior was observed at a water activity of 0.15 (), but in this case some crystallization still occurs with 12% trehalose, and only at 16% is crystallization inhibited prior to melting. Given that sugar crystallization is accelerated at higher moisture, it is not surprising that more trehalose is required to inhibit it than for the dry samples. It should be pointed out, however, that a sharp exothermic peak occurs at this water activity for pure trehalose, indicating that it recrystallized, while samples between 20% and 80% trehalose did not. This detail may be insightful, for it implies that perhaps crystallization is inhibited not only by the elevation of the glass transition temperature, but also by the mere fact that it is a mixture. Mazzobre et al.,[Citation47] reported analogous data using mixtures of trehalose and lactose. The addition of trehalose, which has a dry Tg slightly higher than lactose (∼115 °C compared to ∼100 °C for lactose), caused little change in Tg at 33% RH, but greatly inhibited crystallization, as indicated by the shift in the exothermic crystallization peaks. These results further support the conclusion made above that a sugar added to another sugar may inhibit crystallization, regardless of the degree to which the glass transition is affected.
Figure 10 DSC thermograms of the amorphous sucrose-trehalose mixtures, stored at 15% relative humidity, showing the influence of added trehalose on sucrose crystallization.
As noted above, cotton candy samples were prepared with 0%, 25%, 50%, and 100% trehalose, the balance sucrose, and stored in desiccators at 23%, 33%, and 54% relative humidity. One concern in the preparation of the trehalose-sucrose cotton candy mixtures was the possibility of a heterogeneous distribution of the two sugars. But based on the relative intensities of sucrose and trehalose peaks within individual cotton candy samples, this did not occur. In the 50% mixture, the peaks corresponding to the pure component scans are present in equal proportion, and in the 25% trehalose mixture they are found in the corresponding proportion. This suggests that the sugar mixture is coming out of the cotton candy machine in the same proportion as the feed.
summarizes the time to crystallize for all samples. Representative X-ray diffraction patterns are shown in Figs. . No crystallinity was observed in any sample at 23% RH, even after 30 days of storage, nor did the fluffy structure collapse. At 33% RH pure sucrose samples collapsed and crystallized within 11 days. XRD crystalline peaks for sucrose did not appear in the 25% trehalose samples at this humidity after 26 days, although some collapse of the structure occurred. At 54% RH, crystallization occurred in all samples, but notably faster in the pure sucrose sample.
Figure 11 X-ray diffraction pattern of pure amorphous sucrose cotton candy after storage at 33% relative humidity and 25°C for 11 days, showing crystalline peaks.
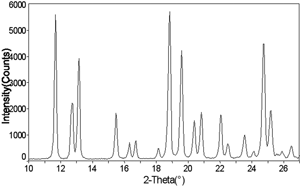
Figure 12 X-ray diffraction pattern of pure amorphous trehalose cotton candy after storage at 54% relative humidity and 25°C for 120 hours, showing crystalline peaks.
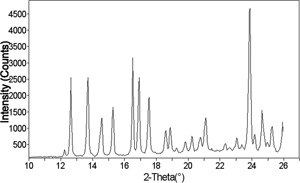
Figure 13 X-ray diffraction pattern of an amorphous 50% sucrose-50% trehalose cotton candy mixture, after storage at 54% relative humidity and 25°C for 120 hours, showing crystalline peaks.
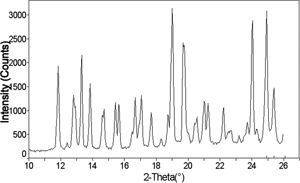
Figure 14 X-ray diffraction pattern of an amorphous 75% sucrose-25% trehalose cotton candy mixture, after storage at 54% relative humidity and 25°C for 120 hours, showing crystalline peaks.
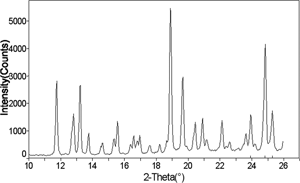
Figure 15 X-ray diffraction pattern of an amorphous 75% sucrose-25% trehalose cotton candy mixture, after storage at 23% relative humidity and 25°C for 30 days, showing retention of amorphous halo.
Table 2 Time to crystallize for sucrose-trehalose cotton candy samples stored at different relative humdities
In all samples that underwent crystallization a collapse in the cotton candy structure occurred beforehand. This phenomenon was most clearly observed at 54% RH. In this case, after 18 hours, no samples exhibited crystallinity, but the 100% sucrose cotton candy had almost fully collapsed while the 100% trehalose cotton candy remained intact. “Collapse” entails an increased density of the sample and a corresponding decrease in volume. The collapsed structure is sticky until crystallization occurs, when it becomes brittle and dry. The implication of this observation is that crystallization cannot be the cause of collapse, but more likely the result since the pickup of water reduces local viscosity and induces faster diffusion.[Citation3] These results show that the addition of trehalose to sucrose in cotton candy will both slow the collapse of the fluffy structure and delay crystallization. This is true even with only 25% trehalose.
Conclusions
The differential scanning calorimetry studies indicated that more than 20% dry weight fraction of trehalose is necessary to significantly inhibit the crystallization of pure amorphous sucrose. The results indicate that the effect is not just an increase in Tg, but may be due to diffusion resistance in the matrix. Due to the high price of trehalose further economic analysis is required to determine if its use as an additive would be a practical shelf-life enhancer. Cotton candy samples with 25% or more of added trehalose stored at various humidities showed significantly slower crystallization than pure sucrose samples, as indicated by X-ray diffraction as well as visual observation of collapse. This fact is promising since, ultimately, it is texture that determines cotton candy shelf-life, not Tg. The next step will be to determine if this same phenomenon occurs in more complex, high-sucrose food systems, such as soft cookies, with the addition of trehalose.
References
- Adhikari , B. , Howes , T. , Bhandari , B.R. and Truong , V. 2001 . Stickiness in foods: a review of mechanisms and test methods . International Journal of Food Properties , 4 ( 1 ) : 1 – 33 .
- Panda , F. and Labuza , T.P. 2004 . X-ray diffraction evaluation of sucrose crystallization in soft cookies . Journal of Food Science , in press
- Roos , Y. and Karel , M. 1991 . Plasticizing effect of water on thermal behavior and crystallization of amorphous food models . Journal of Food Science , 34 : 324 – 329 .
- Roser , B. Trehalose . 1991 . a new approach to premium dried foods . Trends in Food Science and Technology , 2 : 166 – 169 . [CROSSREF]
- Addler , M. and Lee , G. 1998 . Stability and surface activity of lactate dehydrogenase in spray-dried trehalose . Journal of Pharmaceutical Sciences , 88 : 199 – 208 . [CROSSREF]
- Aldous , B.J. , Auffret , A.D. and Franks , F. 1995 . The crystallization of hydrates from amorphous carbohydrates . Cryo-Lett , 16 : 181 – 186 .
- Cardona , S. , Schebor , C. , Buera , M.P. , Karel , M. and Chirife , J. 1997 . Thermal stability of invertase in reduced-moisture amorphous matrices in relation to glassy state and trehalose crystallization . Journal of Food Science , 62 : 105 – 112 .
- Crowe , J.H. , Hoekstra , F.A. , Nguyen , K.H.N. and Crowe , L.M. 1996 . Is vitrification involved depression of the phase transition temperature in dry phospholipids? Biochim . Biophys. Acta. , 1280 : 187 – 196 .
- Crowe , L.M. , Reid , D.S. and Crowe , J.H. 1996 . Biophys. J. , 71 : 2087 – 2093 . [PUBMED]
- Ding , S.-P. , Fan , J. , Green , J.L. , Lu , Q. , Sanchez , E. and Angell , C.A. 1996 . Vitrification of trehalose by water loss from its crystalline hydrate . J. Therm. Anal. , 47 : 1391 – 1405 . [CROSSREF]
- Green , J.L. and Angell , C.A. 1989 . Phase relations and vitrification in saccharide-water solutions and the trehalose anomaly . J. Phys. Chem. , 93 : 2880 – 2882 . [CROSSREF]
- Lammert , A.M. , Schmidt , S.J. and Day , G.A. 1997 . Water activity and solubility of trehalose . Food Chemistry , 61 : 139 – 144 . [CROSSREF]
- Mehl , P.M. 1997 . Solubility and glass transition in the system α-d-trehalose/water . J. Therm. Anal. , 49 : 817 – 822 . [CROSSREF]
- Miller , D.P. and de Pablo , J.J. 2000 . Calorimetric solution properties of simple saccharides and their significance for the stabilization of biological structure and function . J. Phys. Chem. B. , 104 : 8876 – 8883 . [CROSSREF]
- Miller , D.P. , de Pablo , J.J. and Corte , H. 1999 . Viscosity and glass transition temperature of aqueous mixtures of trehalose with borax and sodium chloride . J. Phys. Chem. B. , 103 : 10243 – 10249 . [CROSSREF]
- Miller , D.P. , Anderson , R.E. and de Pablo , J.J. 1998 . Stabilization of lactate dehydrogenase following freeze-thawing and vacuum-drying in the presence of trehalose and borate . Pharm. Res. , 15 : 1215 – 1221 . [PUBMED] [CROSSREF]
- Miller , D.P. , de Pablo , J.J. and Corte , H. 1997 . Thermophysical properties of trehalose and its concentrated aqueous solutions . Pharm. Res. , 14 : 578 – 590 . [PUBMED] [CROSSREF]
- Nicolajsen , H and Hvidt , A. 1994 . Phase behavior of the system trehalose-NaCl-water . Cryobiology , 31 : 199 – 205 . [CROSSREF]
- Roos , Y. 1993 . Melting and glass transition of low molecular weight carbohydrates . Carbohydr. Res. , 238 : 39 – 48 . [CROSSREF]
- Saleki-Gerhardt , A. and Zografi , G. 1994 . Non-isothermal and isothermal crystallization of sucrose from the amorphous state . Pharmaceutical Research , 11 : 1166 – 1173 . [PUBMED] [CROSSREF]
- Shafizadeh , F and Susott , R.A. 1973 . Crystalline transitions of carbohydrates . J. Org. Chem. , 38 : 3710 – 3715 . [CROSSREF]
- Schebor , C. , Burin , L. , del , Pilar Buera M. and Chirife , J. 1999 . Stability to hydrolysis and browning of trehalose, sucrose and raffinose in low-moisture systems in relation to their use as protectants of dry biomaterials . Lebensm.-Wiss. u.-Technol , 32 : 1 – 5 . [CROSSREF]
- Sussich , F. , Urbani , R. , Princivalle , F. and Cesàro , A. 1998 . Polymorphic amorphous and crystalline forms of trehalose . J. Am. Chem. Soc. , 120 : 7893 – 7899 . [CROSSREF]
- Suzuki , T. and Okazaki , M. 1999 . Thermal stabilizing effect of amorphous matrices of sugars on freeze-dried proteins . Bulletin of the Polish Academy of Sciences Technical Sciences , 48 : 415 – 427 .
- Wang , G.M. and Haymet , A.D.J. 1998 . Trehalose and other sugar solutions at low temperatures: modulated differential scanning calorimetry (MDSC) . Journal of Physical Chemistry B , 102 ( 27 ) : 5341 – 5347 . [CROSSREF]
- Chen , T. , Fowler , A. and Toner , M. 2000 . Literature review: supplemented phase diagram of the trehalose-water binary mixture . Cryobiology , 40 : 277 – 282 . [PUBMED] [CROSSREF]
- Ablett , S. , Izzard , M.J. and Lillford , P.J. 1992 . Differential scanning calorimetry study of frozen sucrose and glycerol solutions . J. Chem. Soc. Faraday Trans. , 88 : 789 – 794 . [CROSSREF]
- Blanshard , J.M.V. , Muhr , A.H. and Gough , A. 1991 . Crystallization from concentrated sucrose solutions . Adv. Exp. Med. Biol. , 302 : 639 – 655 . [PUBMED]
- Blond , G. , Simatos , D. , Catte , M. , Dussap , C.G. and Gros , J.B. 1997 . Modeling of the water-sucrose state diagram below 0 °C . Carbohydrate Research , 298 : 139 – 145 . [CROSSREF]
- Finegold , L. , Franks , F. and Hatley , R.H.M. 1989 . Glass/rubber transitions and heat capacities of binary sugar blends . J. Chem. Soc. Faraday Trans. , 85 : 2945 – 2951 . [CROSSREF]
- Gayle , F.W. , Cocks , F.H. and Shepard , M.L. 1977 . The H2O-NaCl-sucrose phase diagram and applications in cryobiology . J. Appl. Chem. Biotechnol. , 27 : 599 – 607 .
- Hancock , B.C. and Zografi , G. 1994 . The relationship between glass transition temperature and the water content of amorphous pharmaceutical solids . Pharmaceutical Research , 11 : 471 – 477 . [PUBMED] [CROSSREF]
- Mackenzie , A.P. 1977 . Non-equilibrium freezing behavior of aqueous systems . Phil. Trans. R. Soc. Lond. B. , 278 : 167 – 189 .
- Orford , P.D. , Parker , R. and Ring , S.G. 1990 . Aspects of the glass transition behavior of mixtures of carbohydrates of low molecular weight . Carbohydr. Res. , 196 : 11 – 18 . [PUBMED] [CROSSREF]
- Roos , Y. and Karel , M. 1991 . Amorphous state and delayed ice formation in sucrose solutions . International Journal of Food Science and Technology , 26 : 553 – 566 .
- Roos , Y. and Karel , M. 1990 . Differential scanning calorimetry study of phase transitions affecting the quality of dehydrated materials . Biotechnol. Progress. , 6 : 159 – 163 . [CROSSREF]
- Shamblin , S.L. and Zografi , G. 1999 . The effect of absorbed water on the properties of amorphous mixtures containing sucrose . Pharmaceutical Research , 16 : 1119 – 1124 . [PUBMED] [CROSSREF]
- Slade , L. and Levine , H. 1988 . Non-equilibrium behavior of small carbohydrate-water systems . Pure & Appl. Chem , 60 : 1841 – 1864 . [INFOTRIEVE]
- Sun , W.Q. , Leopold , A.C. , Crowe , L.M. and Crowe , J.H. 1996 . Stability of dry liposomesin sugar glasses . Biophys. J. , 70 : 1769 – 1776 . [PUBMED]
- Urbani , R. , Sussich , F. , Prejac , S. and Cesàro , A. 1997 . Enthalpy relaxation and glass transition behaviour of sucrose by static and dynamic DSC . Thermochimica Acta , 304/305 : 359 – 367 . [CROSSREF]
- Young , F.E. and Jones , F.T. 1949 . Sucrose hydrates: the sucrose-water phase diagram . J. Phys. Colloid Chem. , 53 : 1334 – 1350 . [CROSSREF]
- Iglesias , H.A. , Chirife , J. and Buera , M.P. 1997 . Adsorption isotherm of amorphous trehalose . J. Sci. Food Agric. , 75 : 183 – 186 . [CROSSREF]
- Makower , B. and Dye , W.B. 1956 . Equilibrium moisture content and crystallization of amorphous sucrose and glucose . Agricultural and Food Chemistry , 4 : 72 – 77 . [CROSSREF]
- Karmas , E. 1981 . Measurement of moisture content . Cereal Foods World , 26 : 332
- Saleki-Gerhardt , A. Role of Water in the Solid-State Properties of Crystalline and Amorphous Sugars . 1993 . Ph.D. Thesis, University of Wisconsin-Madison
- Biliaderis , C.G. , Lazaridou , A. , Mavropoulos , A. and Barbayiannis , N. 2002 . Water plasticization effects on crystallization behavior of lactose in a co-lyophilized amorphous polysaccharide matrix and its relevance to the glass transition . International Journal of Food Properties , 5 ( 2 ) : 463 – 483 . [CROSSREF]
- Mazzobre , M.F. , Soto , G. , Aguilera , J.M. and Buera , M.P. 2001 . Crystallization kinetics of lactose in systems co-lyofilized with trehalose. Analysis by differential scanning calorimetry . Food Research International , 34 : 903 – 911 . [CROSSREF]