Abstract
Turkish White Cheese (TWC), manufactured from pasteurized cows' milk, was ripened over 90 days at 4°C, and proteolytical, chemical, textural, and sensorial changes were investigated. The main compositional characteristics of TWC are low content of total solids (TS) (39.80–41.75 g/100 g of cheese), high content of fat (49.12–49.91 g/100 g of TS) and salt (8.69–9.35 g/100 g of TS). The lactose level, pH values, and titratable acidity of samples were found between 0.74–2.05 g/100 g of TS, 4.39–4.68, and 0.73–1.12 g lactic acid/100 g of cheese, respectively. Changes in nitrogenous fractions, Urea-PAGE electrophoregram and RP-HPLC chromatograms showed that TWC undergoes a slight proteolysis. Textural attributes of TWC were significantly (p < 0.05) affected by the ripening period. The highest overall acceptance scores were found at the 60 days of ripening. Bitterness was identified at the end of the 60 and 90 days of ageing period.
INTRODUCTION
Turkish white cheese (TWC) is one of the most popular cheese varieties manufactured and consumed in Turkey. It constitutes 60–80% of Turkey cheese production.[Citation1] An important part of TWC is traditionally manufactured in small dairy plants without any standard production methods. Therefore, it is difficult to find a standard product with respect to composition and other qualities. In the last decade, the cheese has been produced on a large scale in well-organized dairy plants, where standardized production methods are used.[Citation2]
TWC is a semi-soft, brined cheese variety with a slightly acid and salty taste. The typical color of TWC is white both inside, and on the surface. It has a soft texture with no gas holes. Generally, it is cubical in shape (7 × 7 × 7 cm) and weighs about 350 g. This type of cheese is manufactured originally from raw or pasteurized sheeps', goats', cows' milk, or a combination of these milks and preripened by mesophilic lactic culture (pasteurized type) before renneting.[Citation2,Citation3] It can be consumed fresh, but it is mostly utilized after ripening in brine solution. It is matured at 4–8°C for a period of 1 to 3 months in cold storage.
Physical, chemical, biochemical, microbiological, and sensorial changes occur during the ripening period. Proteolysis is the principal and most complex biochemical reaction that occurred during the ripening process of most cheese varieties.[Citation4] Proteolysis, like in most cheese varieties, has a significant role in the development of cheese characteristics in TWC. The rate, extent, and the pattern of proteolysis, the amount and nature of the degradation products vary according to the enzymes involved, the type of cheese, and the environmental conditions during cheese ripening.[Citation5] The extent of proteolysis in cheese can vary from very limited, e.g., in Mozzarella, to very extensive, e.g., in mould-ripened cheeses (blue-moulded cheese, camembert) and contributes to cheese ripening through a direct formation of flavor via the decomposition of proteins into peptides and amino acids, and by changing the texture of cheese owing to breakdown of the protein network.[Citation6,Citation7]
During cheese ripening, it has been reported that α-casein is firstly hydrolyzed by the action of rennet and afterwards by the action of microbial proteinases.[Citation8] McGoldrick and Fox,[Citation6] who compared proteolysis in several cheese varieties, found that in most cases, αs1-casein is degraded more extensively than β-casein. They reported that during cheese ripening, the concentration of β-casein decreases while γ1- and γ2-caseins remain almost constant. Fox[Citation9] also reported distinct differences between the RP-HPLC profiles of a range of cheese varieties (Cheddar, Emmental, Gouda, Parmesan, Brie, Appenzeller). The peptide profile of cheeses of the same variety can show considerable differences, such as those observed between profiles of Cheddar cheese made from raw and pasteurized milk.[Citation10]
Some chemical, sensory, and ripening characteristics of TWC have been reported in previous studies,[Citation11,Citation12,Citation13] but these investigations do not reflect the detailed characteristics and their effects on the quality of the end product. Also, the proteolytical characteristics have not been studied in detail by Urea-PAGE and RP-HPLC techniques. The objective of this article is to obtain better and detailed knowledge on proteolytical, chemical, sensorial, and textural properties of TWC during the ripening period at 4°C. This data could be valuable in establishing the scientific basis for the large-scale production of Turkish White Cheese made from pasteurized cows' milk
MATERIALS AND METHODS
Cheesemaking and Sampling Procedure
Cheese production was carried out in industrial scale in Bahçivan Food Inc. Whole cows' milk was filtrated, clarified, and then pasteurized at 72°C for 15 seconds in the plate heat exchanger. After pasteurization, the milk was cooled to 32°C. It was transferred to cheese vats and 30 ml of 50% (wt/vol) CaCl2 solution/100L was added to milk. Milk with mixed freeze-dried culture (Dri-Vac®, R-704, Chr. Hansen's Laboratory, Copenhagen, Denmark) of Lactococcus lactis subsp. lactis and Lactococcus lactis subsp. cremoris was preripened for 15 minutes. Liquid calf rennet (Naturen®; Peyma Hansen, Istanbul, Turkey) was added at a level sufficient to coagulate the milk in 75 minutes at 30–32°C. Formation of gel was completed in 70–75 minutes, then curd was cut into 1 cm3 with a knife and allowed to rest in the whey for 20 minutes. The curd was transferred into the cheese cloths and whey was drained. After 2 hours of draining at room temperature (∼ 21–23°C), pressure was applied until whey drainage had stopped or decreased to a low level and until the acidity of curd had reached to pH 5.30. The pressure was adjusted to 20–30 kg weights for approximately 100 L of cheese milk. The raw cheese mass was divided into blocks of about 7 × 7 × 7 cm after the removal of the whey. It was salted in brine (14%) for 5–10 hours, or until the cheese reaches to pH 4.7 at 20–22°C (pH of the brine was adjusted to 4.5 by using lactic acid). Salted cheese blocks were placed in a tin-plated can filled with 6% brine solution to cover the surface of the cheese blocks. The cans containing about 1 kg of cheese were closed hermetically and ripened at 4°C for 90 days. Two batches of TWC were manufactured in the same day and the same bulk milk was used at TWC production. Samples were selected randomly for analysis during the 1, 30, 60, and 90 days of ripening and the analysis were carried out in duplicate. A 1-kg was taken at each sampling time and cheese was homogenized before taking the cheese portions requested for the analyses.
Chemical Analysis
Cheese milk and whey were analysed for fat, protein, lactose, and total solids content by a Milko-Scan (Milko-Scan F50, Foss Electric, Denmark).[Citation14] The pH and titratable acidity of the milk and whey samples were determined according to AOAC Methods.[Citation15] Total solids (TS), titratable acidity, and salt contents of the cheeses were determined according to AOAC Methods.[Citation15] Fat content of cheese samples was determined by Van-Gulik method.[Citation16] The pH of the samples was measured by placing an electrode within a cylinder filled with grated cheese; the pH value was recorded within 30 to 45 seconds. The total nitrogen content of cheese (TN) was determined using 1-g samples by the Kjeldahl method.[Citation15]
Lactose content was determined by HPLC method with slight modification of the method of Mullin and Emmons.[Citation17] Thermo Finnigan SpectraSystem (U.S.A.) integrated with by an autosampler including temperature control for the column (SpectraSystem AS3000), a degasser system (SpectraSystem SCM1000), a quaternary gradient pump (SpectraSystem P4000), a refractive index detector (SpectraSystem RI-150), and a personal computer with a software package for system control and data acquisition (ChromQuest 4.0) were used for analyses. For the extraction of lactose from cheese, 5 g samples weighed into a 100 ml beaker. Deionized water (50 ml) at 50°C was added to the cheese and dispersed at high speed homogenizer (Heidolph, Diax 900, Germany) for 5 minutes then centrifuged at 10,000 rpm and 4°C for 10 minutes. A portion of aqueous solution filtered through nylon 0.45 μm filter. The analysis was performed isocratically at 1 ml/min flow rate and at 40°C with Phenomenex Luna NH2 analytical column (5 μm, 250 × 4.6 mm). Mobile phase was prepared as a mixture of Acetonitrile:Deionized water (75:25).
Proteolysis Analyses
Water soluble extract (WSE) of the cheese sample was prepared according to the procedure of Kuchroo and Fox,[Citation18] but the ratio of cheese to deionized water was modified to 1:5 (wt/wt) instead of 1:2 (wt/wt).[Citation14] Samples of WSE were freeze-dried and kept −25°C before further RP-HPLC analysis. The trichloroacetic acid soluble nitrogen (TCA-SN) fraction was prepared by adding 10 ml of 24% TCA to 10 ml of WSE. The mixture was allowed to stand for 30 minutes at 20°C and filtered through Whatman No. 42 filter paper. The nitrogen content was determined on an aliquot of the filtrate. Phosphotungstic acid soluble nitrogen (PTA-SN) fraction was prepared by adding 14.0 ml of 3.95 M sulfuric acid and 6 ml of PTA (33.3%, w/v) to 20 ml of WSE. The mixture was allowed to stand overnight at 4°C and subsequently filtered through Whatman No. 42. The water-soluble nitrogen content (WSN), TCA-SN, and PTA-SN were determined by the micro-Kjeldahl procedure. The ripening extension index, represented by WSN (%TN), the ripening depth index, represented by TCA-SN (%TN), and the free amino acid index, represented by PTA-SN (%TN) were then calculated.[Citation19]
The degradation of caseins in cheese during ripening was monitored by Urea-polyacrylamide gel electrophoresis method (urea-PAGE) (12.5% C, 4% T, pH 8.9), essentially as described by Andrews[Citation20] with modifications.[Citation21]; the gels were stained with Coomassie Blue G-250 (Bio-Rad) using the method of Blakesley and Boezi.[Citation22] The analysis was performed on cheese samples by using a Scie-Plas V20-CDC vertical electrophoresis units (Warwickshire, U.K.) equipped with Apelex PS1006P power supply (Cedex, France). Quantitation of γ-caseins, β-casein, αs1-caseins and αs1-I-casein was evaluated by densitometry using a Bio-Rad molecular analysist software (Version 1.5, Bio-Rad Lab., USA).
Peptide profiles of the WSE were detected by RP-HPLC system equipped with a Varian 9050 Variable wavelength UV–VIS Detector. The HPLC equipment used was the same as above. Separation was achieved a phenomenex Jupiter C18 wide pore analytical column (5 μm, 300 A°, 250 × 4.6 mm) equipped with phenomenex cartridge holder contains widepore C18 guard cartridge (4 × 3 mm). Separations were conducted at 30°C using a mobile phase of two solvents at eluant flow rate of 0.8 ml/min. Eluant A was 0.1% (vol/vol) trifluoroacetic acid (TFA, HPLC grade, J.T.Baker,USA) in deionized water, and eluant B was 0.1% (vol/vol) TFA in acetonitrile (ultragradient grade; Merck, Germany), starting with 100% eluant A for 10 minutes, and continuing with a linear gradient to 50% B over 80 minutes, a linear gradient to 60% B over 5 minutes, and 60% B for 5 minutes. The column was finally eluted with 100% eluant B for 2 min. Samples (10 mg/ml) of freeze-dried WSE were dissolved in eluant A and filtered through 0.45 μm cellulose acetate filter, and an aliquot (100 μl) of the filtrate was injected to the HPLC system. The absorbance of the eluate was monitored at 214 nm.
Instrumental Texture Profile Analysis
TA plus Texture Analyzer (Ametek Lloyd Inst. Ltd., UK) equipped with a cylinder probe (10 mm diameter) was used for instrumental texture profile analysis (TPA). Hardness, cohesiveness, springiness, chewiness, gumminess, and adhesiveness were evaluated during the ripening period. Cheese samples were taken from at least 2 cm deep in the cheese blocks and their dimensions were 2 × 2 × 2 cm. After cutting, the samples were immediately covered airtightly and allowed to equilibrate to room temperature (20 ± 2°C) for 30 minutes prior to testing. The samples were compressed by 33% from the initial sample's height, using two consecutive compression cycles at a speed of 0.5 mm/s which was modified from the method used by Romeih et al.[Citation14] The data obtained from curve were used for the calculation of the textural parameters as follows; the compressive force (N) recorded at maximum compression during the first cycle, as a measure of cheese hardness; strength of the internal bonds making up the body of the product, as a measure of cheese cohesiveness (A2/A1); the distance recovered by the sample during the time between end of first cycle and start of second cycle, as a measure of springiness (d2, mm); energy needed to disintegrate the cheese sample until it is ready for swallowing (hardness × cohesiveness), as a measure of gumminess (N); energy needed to chew cheese sample until it is ready for swallowing (gumminess × springiness), as a measure of chewiness (N mm); work necessary to overcome the attractive forces between the surface of the cheese and surface of other materials with which the cheese comes in contact, as a measure of adhesiveness (the negative force area, A3, Nmm).[Citation23,Citation24] All the measurements were replicated twice.
Sensory Analysis
Sensorial properties of the cheese samples were carried out with scoring test by five panelists of Food Engineering Department's staff (3 females and 2 males, between 27 and 55 years of age) on the basis of interest and experience in sensory evaluation of TWC during the ripening period at 1, 30, 60, and 90 days. The samples were presented to panelists as cheese blocks (7 × 7 × 3 cm) in standard panel room under white fluorescent light at room temperature (20 ± 2°C).[Citation25] Samples were served with water and bread. Cheese samples were evaluated for flavor, odor, body, and texture and appearance according to the Turkish Standard for white cheese.[Citation26] For the scoring system, the product was graded on a 100 point scale as follows: 20 points maximum for appearance, 35 points maximum for body and texture, 10 points maximum for odor and 35 points maximum for flavor.[Citation26] The intensity of bitterness was scored on a scale from 0 (not bitter) to 4 (very strongly bitter).[Citation27]
Statistical Analysis
The effects of ripening period on the chemical, sensorial, textural properties, and the level of proteolysis was assessed using analysis of variance (ANOVA) by the general linear model procedure of the SPSS 10.0 statistical package programme. Significance level was considered as p < 0.05.
RESULTS AND DISCUSSION
Milk Composition
Generally, chemical composition of the milk affects the quality of the final dairy products. The average composition of cheese milk, whey, and cheese yield are given in . The average yield of fresh TWC from cows' milk is 14–16 kg/100 kg.[Citation28] The cheese yield was found to be between these ranges in present study. Casein:fat ratio was calculated to be 0.67 in cheese milk. Tekinsen[Citation29] reported that casein to fat ratio of 0.7 was optimum for TWC quality and cheese yield.
Table 1 Chemical composition of cows' milk used in the manufacture of Turkish White Cheese and whey composition after cheese productionFootnote a
Cheese Composition
The mean values of cheese composition at 1, 30, 60 and 90 days of ripening are given in . The chemical compositions of the cheese samples showed changes over the ripening period. The analysis of variance (ANOVA) of chemical analysis (except fat in total solid, salt in total solid) showed that the ripening period had an important effect on cheese composition (p < 0.05).
Table 2 Changes of chemical composition in Turkish White Cheese during the ripening periodFootnote a
Total solids, fat in total solid, salt in total solid, titratable acidity, and pH values of cheeses fluctuated between 39.80–41.75 g/100g of cheese, 49.12–49.91 g/100g of TS, 8.69–9.35 g/100g of TS, 0.73–1.12 g/100g of cheese, and 4.39–4.68, respectively during the ripening period (). Similar observations have been reported by other researchers.[Citation3,Citation11] Fat in total solid values of TWC were almost stable during the ripening period. The salt content of the cheeses fluctuated throughout the ripening days, probably due to diffusion of NaCl between brine and TWC. The salt concentration of samples has a great importance in cheese ripening due to its influence on the proteolytic activity of enzymes, and on the growth and activity of lactic acid bacteria. Salt content also affects the sensitivity of rennet to casein fractions.[Citation30]
Lactose content decreased continuously throughout the maturation but did not disappear totally. The high salt concentration may cause slow lactose reduction in TWC (it occurred over a time scale of months) when compared with other cheeses such as Cheddar, Gouda, Emmental, Picón, and Bejes-Tresviso cheese (the lactose concentration in these cheeses essentially decreased to zero).[Citation30]
High quantity of whey (moisture) was retained in the curd during the manufacturing of TWC, so the appropriate quantity of lactose was left in the product for fermentation by the starter culture. Titratable acidity content increased slightly at the 30 days of aging and remained almost constant until end of the ripening. Similar values in titratable acidity were reported for TWC.[Citation3,Citation12,Citation31]
Changes in Nitrogenous Fractions
Average values and standard deviations in total nitrogen (TN), TN (%TS) and different soluble nitrogen fractions such as WSN, TCA-SN, and PTA-SN in %TN during the ripening of cheese are shown in . The values of TN (%TS) fluctuated during the ripening period. The TN (%TS) content increased at the 30 days of ripening however slightly decreased at 60 and 90 days. This result may reflect the transportation of soluble nitrogenous compounds into the brine.[Citation2] The changes both TN and TN (%TS) were found to be statistically insignificant.
Table 3 Changes of nitrogen fractions in Turkish White Cheese during the ripening periodFootnote a
The values of WSN (%TN) in the samples continuously increased throughout ripening. An increase of 33.7% was found at the 90 days of ripening compared with the 1 day of ageing. It is well known that the water soluble nitrogen compounds in cheese are produced mainly by the action of rennet but may be produced by starter bacteria used in cheese production and plasmin in raw material.[Citation32] The effect of ripening time on WSN (%TN) fractions were found important in present study (p < 0.05). These results were lower then Dagdemir, Celik and Özdemir,[Citation31] and Güven and Karaca,[Citation13] but higher then the Saldamli and Kaytanli[Citation11] findings. Results are in agreement with those obtained by Uraz and Şimşek.[Citation33]
TCA-SN also increased in ripening period (p < 0.05). An increase of 76.0% at the 90 days of ripening has been found when compared with 1 day of aging. O'Keeffe et al.[Citation34] observed from experiments made with model cheeses that the proteolytic activity of starter organisms is the source of most of the 12% TCA-SN fraction, leading to the formation of smaller peptides and amino acids. Some authors also use the ratio 12% TCA-SN to evaluate the action of lactic acid bacteria in the formation of soluble nitrogen compounds in cheese.[Citation35] According to Ardö[Citation32] peptides that are soluble in 12%TCA vary in size from 2 to 22 amino acid residues (600 Da < Molecular Mass < 15,000 Da). These authors also suggested that the increase in 12% TCA-SN throughout maturation was derived from the degradation of αs1-casein. The values of TCA-SN of TWC in present study are in conformity with Güven and Karaca.[Citation13]
PTA-SN fractions contain nitrogen compounds with molecular masses < 600 Da, i.e., mainly amino acids and very small peptides produced primarily by the action of microbial peptidases.[Citation32,Citation36] In TWC, the concentration of PTA-SN fluctuated during maturation (p < 0.05). This result probably reflects microbial consumption of free amino acids and/or diffusion into brine.[Citation19]
Protein Degradation by Urea-PAGE and RP-HPLC
shows the Urea-PAGE electrophoregram of the casein subgroups and their degradation products in TWC. It is well known that αs1-casein is the principal substrate for rennet during cheese ripening. It is converted to αs1-I-casein initially and later to the other degradation products.[Citation36] The αs1-casein degradation in TWC can be easily observed at the . The high relative level of αs1-I-casein was found at 1d of ripening, probably due to the high retention of rennet in TWC. Quantitation of β and αs1-caseins were evaluated by densitometry and values were given as total peak area for each fraction in . The electrophoretic results revealed that, in TWC, the β-casein did not undergo as much proteolysis as αs1-casein, which was in agreement with other studies already reported in the literature.[Citation4,Citation5,Citation36] High salt content may have an effect on casein hydrolysis (). The hydrolysis of αs1-casein by rennet is greatly influenced by the concentration of NaCl. The optimum salt concentration for αs1-casein hydrolysis is about 6% (salt in moisture). But the proteolysis of β- casein by rennet is strongly inhibited at this salt concentration.[Citation30]
Figure 1 Urea-PAGE electrophoretograms of Turkish White Cheese during ripening for 1, 30, 60, and 90 days. (C: Sodium caseinate).
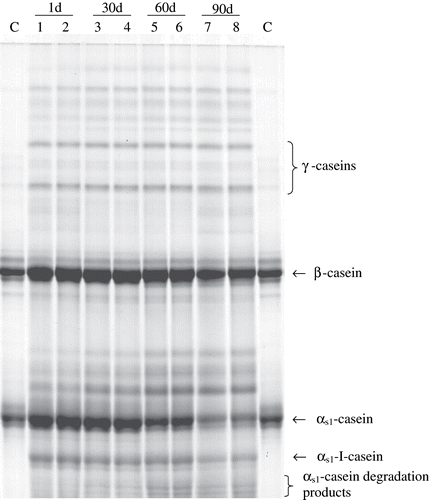
Table 4 Changes in casein fractions in Turkish White Cheese during the ripening periodFootnote a, Footnote b
Proteolysis increased gradually throughout the ripening period and the quantity of β- casein, αs1- casein and αs1-I-casein were decreased at a rate of 12.02%, 43.89%, and 50.40% respectively at the 90 days of the ripening. The decrease in the αs-casein fraction was accompanied by an increase in the fractions which corresponded to its degradation products. The αs1-casein and β- casein degradation were found to be significantly correlated (p < 0.05) with ripening period. The changes in αs1-I-casein and αs1-casein degradation products (αs1- CNDP) of TWC were also found statistically significant during the ripening (p < 0.05). Level of residual αs1-casein, αs1-I-casein and β-casein showed a linear decrease with high correlation values of r2 = 0.9915, r2 = 0.9259, and r2 = 0.8739, respectively. The αs1/ β ratio decreased during the ripening, and the changes were found to be significant (p < 0.05) and show high linearity (r2 = 0.9914) during the ripening period.
Total γ-casein level fluctuated during the ripening period (). It has been evaluated an increase of 13.26% at the end of the ripening (p < 0.05). Many authors have emphasized that plasmin hydrolyses all caseins, especially β- casein, resulting in the formation of γ-caseins.[Citation8,Citation36] The fluctuation of total peak area of γ-casein may indicate a small enhancement of plasmin activity in TWC. However, the degradation may be generated from bacterial proteases and peptidases action due to the fact that plasmin activity decreases at low pH values (~4.3–4.4) in the product.[Citation37]
The results of the quantification of the caseins and their degradation products show the limited proteolysis undergone at TWC during ripening, and the rennet used had a great importance in the protein degradation. The action of enzyme mainly focused on αs1-casein, where it was much lesser on β-casein. The dominant role of the rennet in the proteolysis has also been shown in other cows' milk cheeses such as cheddar[Citation10], feta,[Citation38] and ahumoda de aliva cheese.[Citation39] In addition to rennet, the lactic acid bacteria in cheese have slight activity on intact caseins which could also contribute to the low level of proteolysis in TWC during ripening.[Citation36,Citation40]
The RP-HPLC profiles of the WSE of the cheeses are given at . It has been found an increase total peak area during the ripening period. HPLC chromatograms are in agreement with electrophoretic and chemical results. The retention times of three aromatic amino acids (tyrosine, phenylalanine, and tryptophan) were used to define hydrophilic and hydrophobic zones, at a detection wavelength of 214 nm. These amino acids were identified by comparison of their retention time with that of standard solutions that were injected separately under the same conditions. The hydrophilic peptide portion consisted of the peptides that eluted between Tyr and Trp (from 22 to 41 minutes). The group of hydrophobic peptides consisted of peptides with retention times from 41 to 80 minutes.[Citation41]
Figure 2 Reverse-phase HPLC profiles of the water soluble fraction of Turkish White Cheese at 1, 30, 60, and 90 days of ripening (Tyr: Tyrosine; Phe: Phenylalanine; Trp: Tryptophan, see text).
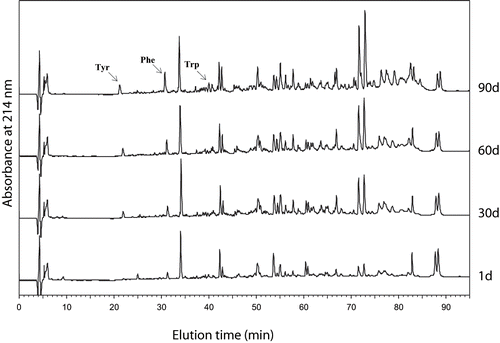
As shown in , the peptide profiles of WSE of the cheeses were quite different both qualitatively and quantitatively during the ripening period. The number and corresponding concentration of peptides were very low during early stages of ripening (data not shown), but increased quickly after 30, 60, and 90 days. The most of the peptides were eluted in the hydrophobic region in TWC. The ratio of the total peak area of hydrophobic to hydrophilic peptides are 3.35 ± 0.13, 4.54 ± 0.14, 4.92 ± 0.12, and 4.94 ± 0.05 (means of duplicated determination of two trials) at the 1, 30, 60, and 90 days of ripening respectively (p < 0.05). A major peak (at a retention time 34 minutes) in the hydrophilic region, and the following two major peaks (with retention times between 71–73 minutes) in the hydrophobic region considerably increased during the ripening period. As shown in , tyrosine, phenylalanine and tryptophan peaks (with retention times 22, 31, and 41 minutes) increased during the ripening period.
In many cheese varieties it has been found that tyrosine, tryptophan and also hydrophobic peptide level is highly related to β-casein hydrolysis.[Citation42,Citation43] There is a positive correlation between β-casein hydrolysis and the intensity of bitterness.[Citation42] Undesirable bitter flavors in cheese have been associated with late-eluted, hydrophobic peptides.[Citation44] In present study, bitterness was evaluated by sensorial evaluation in cheese samples at the 60 and 90 days of ripening () and hydrolysis of β-casein was confirmed by electrophoretic analysis.
Table 6 Changes of sensorial properties in cheese samples during the ripening periodFootnote a
Instrumental Texture and Sensory
The typical textural profile (force-time) curve obtained with one complete run is presented in , and the results of the texture profile analysis (TPA) of TWC at the 1, 30, 60, and 90 days of ageing are shown in . Most of the data of instrumental textural properties showed irregular fluctuations. The analysis of variance of TPA values showed that the ripening period had an important effect on cheese texture (p < 0.05).
Figure 3 A typical TPA curve of Turkish White Cheese at 1 day of ripening. Hardness (N); cohesiveness (A2/A1); springiness (d2); gumminess (hardness ¥ cohesiveness); chewiness (gumminess ¥ springiness); adhesiveness (A3).
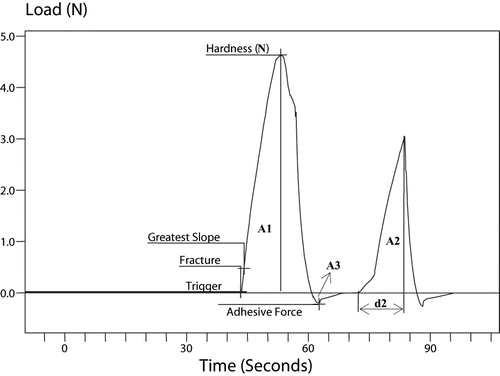
Table 5 Texture Profile Analysis in cheese samples during the ripening periodFootnote a
An increase of 70% hardness values has been found at 90 days of ripening when compared with 1 day old cheese. It may be generated from salt diffusion into cheese. It can be concluded that high quantity of NaCl and low pH values may affect the hardness, cohesiveness, springiness, gumminess, chewiness and adhesiveness of TWC. Cohesiveness and springiness values decreased at a level of 24% and 15% at the 90 days of ripening, respectively. Degradation of protein and reduction of free water may tent to reduce springiness. Gumminess, chewiness and adhesiveness values increased during the ageing. These findings are in agreement with the results of Özer et al.[Citation45]
The means of the sensory scores of all sensory characteristics are given at . The appearance, flavor, body and texture, and overall acceptance of TWC were significantly affected by the ripening period (p < 0.05) excluding odor. The sensory data indicated that TWC flavor and odor developed at an earlier stage of aging and the scores decreased during the ripening period. Body and texture scores were weak for cheeses and increased until 60 days of ripening and then decreased at the end of 90 days. In general, cheese samples showed the highest overall acceptance score at the 60 days of ripening. Bitterness was also identified at 60 days of ageing and increased at the 90 days. But the level of bitterness does not affect the overall acceptance of cheese adversely at the 60 days of ripening.
CONCLUSIONS
High content of moisture, salt and low pH value are the major chemical characteristic features of Turkish White Cheese (TWC) produced from pasteurized cows' milk. Textural, sensorial, and proteolytical properties are affected by these components. It was also shown that proteolysis was realized at low levels during the storage when the values of nitrogen fractions were taken into consideration. The results of the urea-PAGE and RP-HPLC analysis showed a slight increase in proteolysis of TWC during the ripening period. It has been found that proteolysis of αs1-casein was higher than the other subgroups of casein. The hydrolysis of β-casein and γ-casein was observed at limited levels. It was also estimated that insufficient proteolysis adversely affected the formation of aromatic compounds of TWC during the ripening. As a result, the quantities of the proteolysis products were inadequate for the cheese aroma. So, high salty taste and acidic flavor became dominant at the end of the maturation.
ACKOWLEDGMENTS
This work was supported by Turkish Scientific Research Council (TUBITAK), (Project Number: TOGTAG–2596) and Bahçivan Food Inc. The authors wish to thank Bahçivan Food Inc. staff for their help.
Notes
1. Anonymous. Sekizinci Bes Yillik Kalkinma Plani Gida Sanayi Ozel Ihtisas Komisyonu Raporu Sut ve Sut Urunleri Sanayi Alt Komisyon Raporu. [The Progress Planning of 8th five years.] No: DPT: 2636-OIK: 644; Ankara, Turkey, 2001
16. Anonymous. Cheese-Determination of Fat Content-Van Gulik Method, TS3046; Turk Standartlari Enstitusu, [The Institute of Turkish Standards] Ankara, 1978
26. Anonymous. White cheese, TS591; Turk Standartlari Enstitusu, [The Institute of Turkish Standards]: Ankara, 1995
28. Ucuncu, M. Süt teknolojisi. İzmir: Ege Universitesi Mühendislik Fakültesi Yayınları, Turkey, 2002
29. Tekinşen, O.C. Sütürünleri teknolojisi. Konya: Selçuk Universitesi Veteriner Fakültesi Yayınları, Turkey, 1997
REFERENCES
- 1. Anonymous. Sekizinci Bes Yillik Kalkinma Plani Gida Sanayi Ozel Ihtisas Komisyonu Raporu Sut ve Sut Urunleri Sanayi Alt Komisyon Raporu. [The Progress Planning of 8th five years.] No: DPT: 2636-OIK: 644; Ankara, Turkey, 2001
- Hayaloglu , A.A. , Guven , M. and Fox , P.F. 2002 . Microbiological, biochemical and technological properties of Turkish White cheese “Beyaz Peynir.” . International Dairy Journal , 12 : 635 – 648 .
- Akın , N. , Aydemir , S. , Koçak , C. and Yıldız , M.A. 2003 . Changes of free fatty acid contents and sensory properties of white pickled cheese during ripening . Food Chemistry , 80 : 77 – 83 .
- Fox , P.F. 1989 . Proteolysis during cheese manufacture and ripening . Journal of Dairy Science , 72 : 1379 – 1408 .
- Grappin , R. , Rank , T.C. and Olson , N.F. 1985 . Primary proteolysis of cheese proteins during ripening . Journal of Dairy Science , 68 : 531 – 540 .
- McGoldrick , M. and Fox , P.F. 1999 . Intervarial comparison of proteolysis in commercial cheese . Z Lebensm Unters Forsch A , 208 : 90 – 99 .
- Guizani , N. , Kasapis , S. , Al-Attabi , Z.H. and Al-Ruzeiki , M.H. 2002 . Microbiological, physicochemical, and biochemical changes during ripening of camembert cheese made of pasteurized cow's milk . International Journal of Food Properties , 5 : 483 – 494 .
- Farkye , N.Y. and Fox , P.F. 1992 . Contribution of plasmin to cheddar cheese ripening: effect of added plasmin . Journal of Dairy Research , 59 : 209 – 216 .
- Fox , P.F. 1999 . “ Cheese: An Overview ” . In Cheese: Chemistry, Physics and Microbiology , 2nd , Edited by: Fox , P.F. Vol. 1 , 1 – 32 . Gaithersburg, MD : Aspen Publishers .
- McSweeney , P.L.H. , Fox , P.F. , Lucey , J.A. , Jordan , K.N. and Cogan , T.M. 1993 . Contribution of the indigenous microflora to the maturation of cheddar cheese . International Dairy Journal , 3 : 613 – 634 .
- Atasever , M. , Ceylan , Z.G. and Alisarli , M. 2002 . Changes in the sensory, microbiological and compositional properties of Turkish white cheese during ripening . Acta-Alimentaria , 31 : 319 – 326 .
- Saldamli , I. and Kaytanli , M. 1998 . Utilisation of Fromase, Maxiren and Rennilase as alternative coagulating enzymes to rennet in Turkish White cheese . Milchwissenschaft , 53 ( 1 ) : 22 – 25 .
- Güven , M. and Karaca , O.B. 2001 . Proteolysis levels of white cheeses salted and ripened in brines prepared from various salts . International Journal of Dairy Technology , 54 : 29 – 33 .
- Romeih , E.A. , Michaelidou , A. , Biliaderis , C.G. and Zerfiridis , G.K. 2002 . Low-fat white-brined cheesemade from bovine milk and two commercial fat mimetics:chemical, physical and sensory attributes . International Dairy Journal , 12 : 525 – 540 .
- Association of Official Analysis Chemists . 1990 . Official Methods of Analysis , 15th , Washington, DC : AOAC .
- 16. Anonymous. Cheese-Determination of Fat Content-Van Gulik Method, TS3046; Turk Standartlari Enstitusu, [The Institute of Turkish Standards] Ankara, 1978
- Mullin , W.J. and Emmons , D.B. 1997 . Determination of organic acids and sugars in cheese, milk and whey by high performance liquid chromatography . Food Research International , 30 : 147 – 151 .
- Kuchroo , C.N. and Fox , P.F. 1982 . Soluble nitrogen in cheddar cheese: comparison of extraction procedures . Milchwissenschaft , 37 : 331 – 335 .
- Sousa , M.J. and Malcata , F.X. 1997 . Comparison of plant and animal rennets in terms of microbiological, chemical and proteolysis characteristics of ovine cheese . Journal of Agricultural and Food Chemistry , 45 : 74 – 81 .
- Andrews , A.T.J. 1983 . Proteinases in normal bovine milk and their action on caseins . Journal of Dairy Research , 50 : 45 – 55 .
- Shalabi , S.I. and Fox , P.F. 1987 . Electrophoretic analysis of cheese: comparison of methods . Irish Journal of Food Science and Technology , 11 : 135 – 151 .
- Blakesley , R.W. and Boezi , J.A. 1977 . A new staining technique for proteins in polyacrylamide gels using Coomassie Brillant Blue G-250 . Analytical Biochemistry , 82 : 580 – 581 .
- Gunasekaran , S. and Ak , M.M. 2003 . Cheese Rheology and Texture , 437 Boca Raton, FL : CRC Press .
- Uprit , S. and Mishra , H. N. 2004 . Instrumental textural profile analysis of soy fortified pressed chilled acid coagulated curd (paneer) . International Journal of Food Properties , 7 : 367 – 378 .
- Awad , R.A. , Abdel-Hamid , L.B. , El-Shabrawy , S.A. and Singh , R.K. 2004 . Physical and sensory properties of block processed cheese with formulated emulsifying salt mixtures . International Journal of Food Properties , 7 : 429 – 448 .
- 26. Anonymous. White cheese, TS591; Turk Standartlari Enstitusu, [The Institute of Turkish Standards]: Ankara, 1995
- Stadhouders , J. , Hup , G. , Exterkate , F.A. and Visser , S. 1983 . Bitter flavour in cheese.1. Mechanism of the formation of the bitter flavour defect in cheese . Neth. Milk Dairy J , 37 : 157 – 167 .
- 28. Ucuncu, M. Süt teknolojisi. İzmir: Ege Universitesi Mühendislik Fakültesi Yayınları, Turkey, 2002
- 29. Tekinşen, O.C. Sütürünleri teknolojisi. Konya: Selçuk Universitesi Veteriner Fakültesi Yayınları, Turkey, 1997
- Guinee , T.P. and Fox , P.F. 1999 . “ Salt in Cheese: Physical, Chemical and Biological Aspects ” . In Cheese Chemistry Physics and Microbiology , 2nd , Edited by: Fox , P.F. Vol. 1 , 257 – 302 . Gaithersburg, MD : Aspen Publishing .
- Dagdemir , E. , Celik , S. and Özdemir , S. 2003 . The effects of some starter culture on the properties of Turkish white cheese . International Journal of Dairy Technology , 56 : 215 – 218 .
- Ardö , Y. 1999 . “ Evaluation of proteolysis by analyzing the N content of cheese fractions ” . In Bulletin of the international dairy federation , 4 – 9 . Brussels : International Dairy Federation . No 337
- Uraz , T. and Şimşek , B. 1998 . Ankara piyasasinda satılan Beyaz peynirlerin proteoliz düzeylerinin belirlenmesi . Gida dergisi , 23 : 371 – 375 .
- O'Keeffe , A.M. , Fox , P.F. and Daly , C. 1978 . Proteolysis in cheddar cheese: role of coagulant and starter bacteria . Journal of Dairy Research , 45 : 465 – 477 .
- Furtado , M.M. and Partridge , J.A. 1988 . Characterization of nitrogen fractions during ripening of a soft cheese made from ultrafiltration retentates . Journal of Dairy Science , 71 : 2877 – 2885 .
- Fox , P.F. and McSweeney , P.L.H. 1996 . Proteolysis in cheese during ripening . Food Review International , 12 : 457 – 509 .
- Gruerty , M.B. and Fox , P.F. 1988 . Milk alkaline proteinase . Journal of Dairy Research , 55 : 609 – 630 .
- Katsiari , M.C. and Voutsinas , L.P. 1994 . Manufacture of low-fat feta cheese . Food Chemistry , 49 : 53 – 60 .
- Franco , I. , Prieto , B. , Urdiales , R. , Fresno , J.M. and Carballo , J. 2001 . Study of the biochemical changes during ripening of Ahumado de Aliva cheese: a Spanish traditional variety . Food Chemistry , 74 : 463 – 469 .
- Sousa , M.J. , Ardö , Y. and McSweeney , P.L.H. 2001 . Advances in the study of proteolysis during cheese ripening . International Dairy Journal , 11 : 327 – 345 .
- Gomez , M.J. , Garde , S. , Gaya , P. , Medina , M. and Nunez , M. 1997 . Relationship between level of hydrophobic peptides and bitterness in cheese made from pasteurized and raw milk . Journal of Dairy Research , 64 : 289 – 297 .
- Lemieux , L. and Simard , R.E. 1991 . Bitter flavour in dairy products, I.A review of the factors likely to influence its development, aminly in cheese manufacture . Lait , 71 : 599 – 636 .
- Marcos , A. and Esteban , M.A. 1999 . “ Iberian Cheeses ” . In Cheese: Chemistry, physics and microbiology , 2nd , Edited by: Fox , P.F. Vol. 2 , 173 – 219 . Gaithersburg, MD : Aspen Publishing .
- Najafi , M.B.H and Lee , B.H. 1996 . Bitterness in cheese: a review . Critical Reviews in Food Science and Nutrition , 36 ( 5 ) : 397 – 411 .
- Özer , B.H. , Robinson , R.K. and Grandison , A.S. 2003 . Textural and microstructural properties of urfa cheese (a white-brined Turkish cheese) . International Journal of Dairy Technology , 56 : 171 – 176 .