Abstract
Ohmic heating is an alternative heating method for Maras-type ice cream mix, which is a traditional ice cream in Turkey. Fast and uniform heating is necessary for the pre-heating of ice cream mixes. Maras-type ice cream, produced in continuous system, was investigated in this study. The results obtained were compared with those of standard ice cream. The ice cream mix used for this study was supplied before the pasteurization step. A voltage gradient of 10–60 V/cm was used to heat the ice cream mixes ohmically from 4°C to 80°C. Temperature dependent electrical conductivity relations were obtained for different ohmic heating rates. Electrical conductivity of the standard type ice cream mix was lower than that of the Maras type ice cream mix. Fat content and temperature had an effect on the electrical conductivity values. The rheological properties of the ice cream mixes were also measured at 4, 25, 40, 60, 70, and 80°C by a Brookfield viscometer. Experimental data were evaluated according to power the law model—rheological constants (K, n) of Maras type ice cream mix were found to be greater than the standard type ice cream mix and increased with temperature.
INTRODUCTION
Maras-type ice cream is a traditional ice cream with high consistency. It is produced mainly in the South East region of Turkey. Ice cream producing companies are trying to commercialize this product worldwide. Very high consistency of the mix results in many problems, especially in heating processes.
The traditional essential ingredient of Maras-type ice cream is Salep, which is a flour of dried tubers of wild terrestrial orchids (Orchis anatolica). It is a natural stabilizer and ingredient giving typical consistency and flavour to the Maras-type ice cream. The characteristics of salep and it's use in ice cream mixes have been explained elsewhere.[Citation1] Dickinson and Stainsby[Citation2] explained the dependency of the texture of the ice cream—such as the state of the aggregation of the fat globules, the amount of air, the viscosity of the aqueous phase, and soon. The viscosity of a mix is affected by composition, type and quality of ingredients, processing and handling of the mix, and concentration. Stabilizers are used to increase ice cream mix viscosity and thereby affect the quality.[Citation1]
In recent years, studies have shown that ice creams are Non-Newtonian type fluids, and stabilizers added to the mixes influence their consistencies. Uzomah and Ahiligwo[Citation3] studied the effects of the concentration of gums, temperature, and rheological properties of ice creams and found that the pseudo-plastic nature was not changed by temperature. Bolliger et al.[Citation4] investigated the relationship between ice cream mix viscoelasticity and ice cream crystal growth in ice cream as a function of stabilizer addition. They obtained the minimum ice crystallization rate when the concentration of guar gum was 0.14% in the mix. Kaya and Tekin[Citation1] studied the rheological properties of milk-salep-sugar and water-salep-sugar mixes prepared at different salep concentrations at different temperatures. They mentioned that the gradual increase in salep concentration changed the rheological properties of water-salep-sugar mix from Newtonian to non-Newtonian and the salep concentration was found to be more effective than the temperature on the variation of the consistency of mixes. Cogne et al.[Citation5] determined the changes of thermal properties of ice cream mixes during processing.
The efficiency of thermal processes applied to the non-Newtonian fluid food mixtures and purees depends on composition and rheological properties.[Citation5,Citation6] It is difficult to obtain uniform heating in ice cream mixes having high consistency. It requires the special design of heat transfer equipment and affects the operational costs. The high consistency causes heat transfer problems during pasteurization. Uniform heating could not be achieved as a result of fouling and coagulation in the surfaces of heat transfer equipment. The design of heat transfer equipment having proper process control equipment, in which uniform heating could be obtained, is important to ensure the sufficient pasteurization of non-Newtonian fluids.
Ohmic heating is a technique based on the electrical current passing through the food material. In recent years, ohmic heating has been applied as an alternative technique in heating of pumpable foods. The uniform heat generation inside the food material depends on the electrical current and electrical conductivity of food material.[Citation7] The fouling in ohmic heating units is much less than in the conventional heat transfer equipment. So, it has advantages of reduced labour and cleaning costs. In recent years, the studies on the applicability of ohmic heating to pumpable foods have been increased due to its various advantages.[Citation7–13]
The aim of this article was (i) to obtain ohmic heating rate changes, and the electrical conductivity-temperature relationships of ice cream mixes during ohmic heating by applying 6 different voltage gradients in the range of 10–60 V/cm, and (ii) to determine the rheological properties of Maras-type and standard type ice cream mixes at different temperatures in the range of 4–80°C.
MATERIALS AND METHODS
Maras-type and standard type ice cream mixes were supplied from a modern ice cream plant. The mixes were taken from the process line before the pasteurisation process. Maras-type ice cream mix ingredients were sugar, glucose syrup, whey powder, skim-milk powder, stabilisers (guar gum, carboxy-methyl-cellulose), emulsifiers (mono and diglycerides), salep, natural identical flavour (vanillin), water, and the ingredients of the standard type ice cream mix were milk cream, sugar, skim-milk powder, glucose syrup, whey powder, emulsifiers (mono and diglycerides), stabilisers (locust bean gum, guar gum, carrageenan), natural identical flavour (vanillin), and water. The chemical compositions of the mixes were determined using methods given in AOAC,[Citation14] and are shown in .
Table 1 The compositions of ice cream mixes used (Basis: 100 g)
The ice cream mixes were heated ohmically from 4°C to 80°C. The ohmic heating rates and electrical conductivity-temperature relationships were obtained. Ohmic heating experiments were conducted in laboratory scale ohmic heating system consisting of a power supply, an isolating transformer, a variable transformer and a microprocessor board. The microprocessor board included a microprocessor (21 X, Campbell Scientific Inc., Utah) measuring the temperatures at different points of test cell, voltage and current sensors, RS 232 port. It monitored the temperatures, current and voltage applied and passed this information to the PC (Pentium IV) via an RS 232 port at constant time intervals (1 s). The detailed technical information about the system used was given in.[Citation12] Six different voltage gradients (10, 20, 30, 40, 50, and 60 V/cm) were applied to determine the effect of voltage gradient on electrical conductivities of ice cream mixes. Teflon coated electronic temperature sensors (AD590, Omega Eng. Inc., Stamford, CT) with a compression fitting were used to measure temperature at the different sections of the mix in the Pyrex test cell. Response characteristics of the Teflon coated electronic temperature sensors for high-voltage gradient heating cases were determined. The microcomputer was set-up by considering temperature lags during heating. The mix was poured through a temperature measurement port; after that the electronic temperature sensors were inserted and fitted to the system. The distance between two electrodes was 0.03 m and the diameter of the electrodes was 0.025 m. After the system was sealed, the sample was ohmically heated up to a temperature of 80°C at 50 Hz frequency. The temperature of each sample was assumed to be uniform in the cell, since the maximum difference among the measured temperatures at different locations (at two points; 0.5 cm near the each electrodes and at the center of the test cell) was 0.5 ± 0.5°C during heating from 0°C to 80°C. The experiments were replicated three times. The time constants of Teflon coated temperature sensors were determined by calibrating them in melting point standards (52°C, 79°C, and 107°C, Omega, UK).
Electrical conductivity of the samples (σ) was calculated from voltage and current data using the following equation:
L, distance between the electrodes (m); A, cross sectional area of the electrodes (m2); I, electrical current (A) and V, voltage gradient applied.[Citation7] The time-temperature data were plotted to obtain the ohmic heating curves for ice cream mixes. Electrical conductivity was plotted against the corresponding temperature to obtain the electrical conductivity curves.
Brookfield RVT model viscometer (Brookfield Engineering Laboratories, Middleboro, MA, USA) was used to determine the rheological properties of ice cream mixes. The apparent viscosities, μ app , (mPas) of ice cream mixes were measured at 7 different rotational speed, N, (0.5–50 rpm) by using a rotational spindle no 4. For Brookfield rotational viscometer, shear rate value, γ (s−1), at a given rotational speed, N′ (rps), was calculated from the EquationEq. (2);[Citation15]
Experimental data was fitted to power law model (EquationEq.3).
The consistency coefficients, K, and flow behaviour indexes, n, of the ice cream mixes at 6 different temperatures (4, 25, 40, 60, 70, and 80°C) were determined by non-linear regression analysis. The apparent viscosity and shear rate values were given as Pa.s and s−1, respectively. The temperature dependency of K was obtained by using Arrhenius type relationship, EquationEq. 4;
where temperature was taken in Kelvin (K).
Ea is activation energy (J/kgmol) and R is gas constant, in EquationEq. 5. Statistical analysis (One way Anova Post-Hoc test, paired comparison test, linear and non-linear regression) were conducted by using SPSS 11.0.1 Statistical Package.[Citation16] Confidence interval was 0.99.
RESULTS AND DISCUSSION
Ohmic heating curves of ice cream mixes at 6 different voltage gradients are given in . For both of the ice cream mixes, with the increase of voltage gradient, ohmic heating times required to heat up from 4°C to 80°C were decreased. Icier[Citation17] mentioned that the increase of voltage gradient in ohmic heating of milk samples resulted in the decrease in ohmic heating times required for heating them to the predetermined temperature. Decrease of the voltage gradient from 60 V/cm to the 10 V/cm caused an increase of ohmic heating times to heat up from 4°C to 80°C approximately 50 and 31 times for both Maras-type and standard type ice cream mixes, respectively. Maras-type ice cream mix was relatively more sensitive to voltage gradient changes than the standard ice cream mix during ohmic heating. The effect of voltage gradient on ohmic heating times were significant (Mean standard error: 0.9, p < 0.01).
Figure 1 Ohmic heating curves of ice cream mixes at different voltage gradients i) Maras-type, ii) standard type.
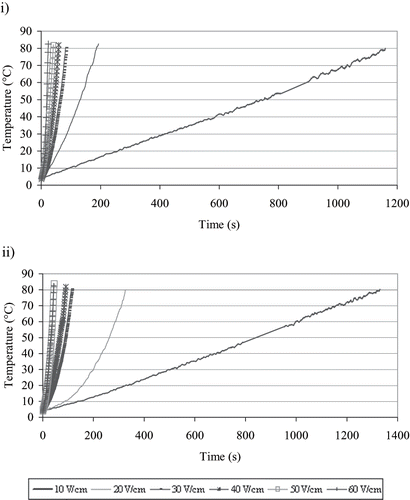
As shown in Table1, the fat content of standard type ice cream mix was higher than that of the Maras-type ice cream mix. At all voltage gradients, Maras-type ice cream mix heated up faster than standard type ice cream mix (). Fat is an ingredient having lower electrical conductivity value.[Citation18] It is thought that the fat content difference between the samples affected the electrical conductivity changes during ohmic heating and resulted in the difference in ohmic heating rates of the mixes.
The electrical conductivity changes of ice cream mixes during ohmic heating at different voltage gradients are given in . As temperature increased, electrical conductivity values increased. The linear band shape electrical conductivity change was observed at both of the ice cream mixes. The linear electrical conductivity-temperature relationship constants for different voltage gradients applied are given in . Similar results for ohmic heating of fruit juice concentrates have been obtained by Icier and Ilicali.[Citation13] Electrical conductivity of liquid foods depends on composition and the temperature. The volumetric heat generation inside the food sample increases as the electrical conductivity increases.[Citation7,Citation10,Citation19,Citation20] At the voltage gradient range of 10–40 V/cm, the electrical conductivity of Maras-type ice cream mix was higher than that of the standard type ice cream mix having lower fat content, at the same temperature. Thus, Maras-type ice cream mixes were heated up to prescribed temperature faster than the standard-type ice cream mix. At the higher voltage gradients, the bubbling was observed in Maras-type ice cream mixes at the temperatures above 50°C and resulted in decreases in the electrical conductivity change (). Therefore, its electrical conductivity was lower than that of the standard mix. Due to bubbling which occurred at the high temperatures and corresponding high electrical conductivity values, the difference in the ohmic heating rates of ice creams at high voltage gradients was not significant (p < 0.01).
Figure 2 Electrical conductivity changes of ice cream mixes during ohmic heating at different voltage gradients i) Maras-type, ii) standard type.
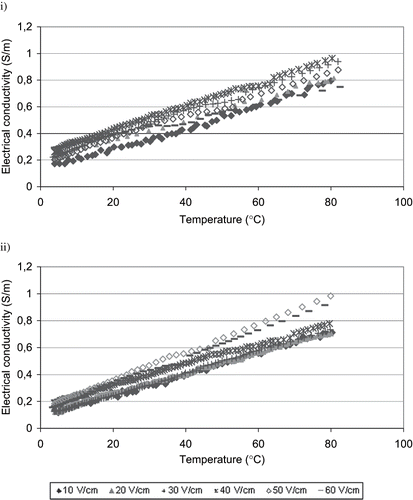
Table 2 Electrical conductivity-temperature relationships of ice cream mixes at different voltage gradients
The rheological characteristics of solutions could also affect their ohmic heating behaviour.[Citation8,Citation21,Citation22,Citation23] The differences between the rheological characteristics of ice cream mixes at the temperatures in the range of 4–80°C were obtained (). For both ice cream mixes, the power law relationship was obtained between their apparent viscosity values and shear rates applied. This means that ice cream mixes studied showed non-Newtonian behaviour as obtained similarly in Cogne et al.,[Citation5] Hegedusic et al.,[Citation24] Goff et al.,[Citation25] and Adapa et al.[Citation26] The non-linear regression coefficients of the non-Newtonian model (EquationEq. 3) for both ice cream mixes are given in . shows that the apparent viscosity of ice cream mixes decreases as the temperature increases. Since the consistency is a critical parameter affecting the flow region in piping systems, the data on the consistency changes of ice cream mixes during ohmic heating may be useful in the design of continuous cylindrical ohmic heaters, which could be used for ice cream processing.
Figure 3 The changes of apparent viscosity values of ice cream mixes by shear rate at different temperatures; i) Maras-type, ii) standard type.
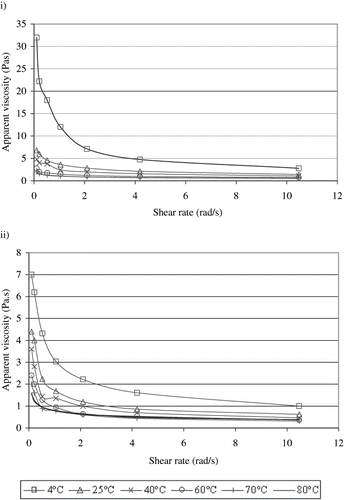
Table 3 Rheological properties of ice cream mixes at different temperatures
Consistency coefficients and flow behaviour indices of standard-type ice cream mix at different temperatures were significantly different (P < 0.01). The flow behaviour indices increased as the temperature increased in the range of 25°C to 80°C, while the consistency coefficient at 4°C was approximately 3.5 times of that at 80°C. This behaviour is also shown in .
As the temperature increased, the consistency coefficient of Maras-type ice cream mixes decreased, whereas the flow behaviour indexes increased. Thus, Maras-type ice cream mixes were more viscous at lower temperatures. The effect of temperature on rheological properties of Maras-type ice cream mixes was also statistically significant (P < 0.01). Ahmed and Ramaswamy[Citation27] similarly reported the considerable effect of temperature on rheological properties of fruit puree. Kaya and Tekin[Citation1] found that the flow behaviour indices of model ice cream mixes having different concentrations of salep were between 0.77–0.95 for the range of 10–50°C and increased with the increasing temperature. Goff et al.[Citation25] reported the flow behaviour indexes of ice cream mixes as 0.7, except those containing xanthan gum.
Rheological constants of Maras-type ice cream mixes were higher than those of the standard-type ice cream mixes for all measurement temperatures (P < 0.01). It was thought that the use of salep in Maras-type ice cream mix resulted in the differences in consistencies with standard-types in this study. Kaya and Tekin[Citation1] similarly reported that the flow behaviour indices of ice cream mixes consisting of salep were higher than other mixes. Salep plays an important role in the flavour and consistency of Maras-type ice cream and results in special ice cream.
The temperature dependency of consistency coefficients of ice cream mixes of EquationEq. (4) are given in . The corresponding activation energies for Maras-type and standard-type ice cream mixes were calculated as 25.1 kJ/mol and 14.2 kJ/mol, respectively, by using EquationEq. (5). Roberts and Tong[Citation28] used similar method in the calculation of activation energies of food material. They reported the activation energy of porous bread samples as 48.7 kJ/mol.
Table 4 The temperature dependency of consistency coefficient, K, of the ice cream mixes
Experimental results showed that the Maras-type ice cream mix was more sensitive to temperature changes than the standard-type ice cream mix. Furthermore, the ohmic heating rates of Maras-type ice cream mixes were higher than those of standard-type. Thus, the temperature control was more critical for Maras-type ice cream mixes during ohmic heating.
CONCLUSIONS
Maras-type ice cream mixes were found to have higher electrical conductivity values at all voltage gradients. The electrical conductivities of ice cream mixes increased linearly with temperature at all voltage gradients applied in the range of 10–60 V/cm. Maras-type ice cream mixes heated ohmically faster than standard-type ice cream mixes. The consistency of Maras-type ice cream mixes was higher than that of standard-type ice cream at all temperatures. Since Maras-type ice cream mixes were more sensitive to temperature changes and bubbling occurred above 50°C at high voltage gradients, its ohmic heating behaviour in continuous ohmic heaters must be studied further in future. The data on temperature dependencies of electrical conductivities and rheological properties of ice cream mixes are important factors in the design of ohmic heating systems being used in ice cream processing. From this study it is confirmed that ohmic heating may be a good alternative for heating of ice cream mixes.
ACKNOWLEDGMENTS
The authors would like to thank Unilever-Turk S.A for support of raw materials to this project.
Notes
7. Icier, F. The experimental investigation and mathematical modelling of ohmic heating of foods. PhD diss., Ege University, The Institute of Natural and Applied Sciences, Izmir, Turkey. 2003.
REFERENCES
- Kaya , S. and Tekin , A.R. 2001 . The effect of salep content on the rheological characteristics of a typical ice-cream mix . Journal of Food Engineering , 47 : 59 – 62 .
- Dickinson , E. and Stainsby , G. 1982 . Colloids in Foods , 382 – 383 . London : Applied Science Publishers .
- Uzomah , A. and Ahiligwo , R.N. 1999 . Studies on the rheological properties and functional potentials of achi (Brachystegea eurycoma) and ogbono (Irvingia gabonesis) seed gums . Food Chemistry , 67 : 217 – 222 .
- Bolliger , S. , Wildmoser , H. , Goff , H.D. and Tharp , B.W. 2000 . Relationships between ice cream mix viscoelasticity and ice crystal growth in ice cream . International Dairy Journal , 10 : 791 – 797 .
- Cogné , C. , Andrieu , J. , Laurent , P. , Besson , A. and Nocquet , J. 2003 . Experimental data and modelling of thermal properties of ice creams . Journal of Food Engineering , 58 : 331 – 341 .
- Ditchfield , C. , Tadini , C.C. , Singh , R. and Toledo , R.T. 2004 . Rheological properties of banana puree at high temperatures . International Journal of Food Properties , 7 ( 3 ) : 571 – 584 .
- 7. Icier, F. The experimental investigation and mathematical modelling of ohmic heating of foods. PhD diss., Ege University, The Institute of Natural and Applied Sciences, Izmir, Turkey. 2003.
- Khalaf , W.G. and Sastry , S.K. 1996 . Effect of viscosity on the Ohmic heating rate of solid-liquid mixtures . Journal of Food Engineering , 27 : 145 – 158 .
- Hong , H.D. , Kim , S. S. , Kim , K. T. and Choi , H.D. 1998 . Changes in heating profiles of apple juice by ohmic heating . Hanguk Nongwhahak Hoechi (from English summary) , 41 ( 6 ) : 431 – 436 .
- Marcotte , M. , Piette , J.P.G. and Ramaswamy , H.S. 1998 . Electrical conductivities of hydrocolloid solutions . Journal of Food Process Engineering , 21 : 503 – 520 .
- Reznick, D. Electroheating. 2000 www.raztek.com/electroheating.html
- Castro , A. , Teixeire , J.A. , Salengke , S. , Sastry , S.K. and Vicente , A.A. 2004 . Ohmic heating of strawberry products: electrical conductivity measurements and ascorbic acid degradation kinetics . Innovative Food Science and Emerging Technologies , 5 : 27 – 36 .
- Icier , F. and Ilicali , C. 2004 . Electrical conductivities of apple and sourcherry juice concentrates during ohmic heating . Journal of Food Process Engineering , 27 ( 3 ) : 159 – 180 .
- Association of Official Analytical Chemists . 1981 . Official Methods of Analysis , 13th , Washington, DC : AOAC .
- Saravacos , G.D. and Moyer , J.C. 1967 . Heating rates of fruit products in an agitated kettle . Food Technology , 21 : 372 – 376 .
- SPSS Statistical Package . 2001 . SPSS for Windows , Chicago : SPSS Inc . Ver. 11.0.1.;
- Icier , F. . The use of ohmic heating as a preheating method in milk processing . CD Proceedings of International Dairy Symposium . May 24–28 2004 . Turkey : Isparta . Dairy 2004,
- Icier , F. and Ilicali , C. 2005 . The use of tylose as a food analog in ohmic heating studies . Journal of Food Engineering , 69 ( 1 ) : 67 – 77 .
- Palaniappan , S. and Sastry , S.K. 1991 . Electrical conductivity of selected juices: influences of temperature, solids content, applied voltage, and particle size . Journal of Food Process Engineering , 14 : 247 – 260 .
- Fryer , P. 1995 . “ Electrical Resistance Heating of Foods ” . In New Methods of Food Preservation, , 1st , Edited by: Gould , G.V. 205 – 235 . London : Blackie Academic and Professional .
- Kim , S.H. , Kim , G.T. , Park , J.Y. , Cho , M.G. and Han , B.H. 1996 . A study on the Ohmic heating of viscous food . Foods and Biotechnology , 5 ( 4 ) : 274 – 279 .
- Marcotte , M. , Taherian , A.R. , Trigui , M. and Ramaswamy , H.S. 2001 . Evaluation of rheological properties of selected salt enriched food hydrocolloids . Journal of Food Engineering , 48 : 157 – 167 .
- Marcotte , M. , Trigui , M. and Ramaswamy , H.S. 2000 . Effect of salt and citric acid on electrical conductivities and ohmic heating of viscous liquids . Journal of Food Processing and Preservation , 24 ( 5 ) : 389 – 406 .
- Hegedusic , V. , Pilizota , V. and Subaric , D. 1994 . Rheological and thermophysical properties of model ice cream mixtures . Prehrambeno Tehnoloska-i- Biotehnoloska Revija , 32 ( 2/3 ) : 67 – 70 .
- Goff , H.D. , Davidson , V.J. and Cappi , E. 1994 . Viscosity of ice cream mix at pasteurization temperatures . Journal of Dairy Science , 77 ( 8 ) : 2207 – 2213 .
- Adapa , S. , Dingeldein , H. , Schmidt , K.A. and Herald , T.J. 2000 . Rheological properties of ice cream mixes and frozen ice creams containing fat and fat replacers . Journal of Dairy Science , 83 ( 10 ) : 2224 – 2229 .
- Ahmed , J. and Ramaswamy , H.S. 2004 . Response surface methodology in rheological characterization of papaya puree . International Journal of Food Properties , 7 ( 1 ) : 45 – 58 .
- Roberts , J.S. and Tong , C.H. 2003 . Drying kinetics of hygroscopic porous materials under isothermal conditions and the use of a first-order reaction kinetic model for predicting drying . International Journal of Food Properties , 6 ( 3 ) : 355 – 367 .