Abstract
Volatile compounds of wheat, corn, and potato starches were determined prior to and upon extraction of the starches with aqueous solutions of sodium hydroxide (NaOH), ethanol, and sodium dodecyl sulfate (SDS). Aqueous NaOH extraction was effective in reducing the level of total volatiles and removing certain volatiles from both wheat and corn starches without increasing the level of hexanal, an important lipid autoxidation product. However, the extraction did not considerably influence the composition and abundance of volatiles in potato starch. Aqueous ethanol extraction reduced the level of total volatiles only in wheat starch. Aqueous SDS extraction was not practical in the removal of volatiles from cereal starches. However, SDS extraction was effective in removing volatiles from potato starch, as well as reducing its hexanal level. It is evident that NaOH extraction is suitable for the removal of volatiles associated with cereal starches, whereas SDS extraction is more appropriate for tuber starches.
INTRODUCTION
Flavor is defined as the overall sensation provided by the combination of taste, odor (smell, aroma), and textural feelings upon a particular food material is consumed.[Citation1] Most food products carry a unique desirable flavor; however, a bland flavor is the main objective in the case of starch as a food ingredient. In other words, any taste or odor associated with starch is considered off-flavor. Pure starch is theoretically tasteless and odorless since starch granule is neither water-soluble nor volatile at the body temperature.[Citation2] Yet, commercial starches often have odor notes characteristics of their sources. Potato starch has an “earthy” odor, whose origin is unknown. Corn, wheat, and sorghum starches have unique “cereal” odors that may become less apparent upon cooking or may be masked by other flavors, whereas tapioca and waxy cereal starches have less pronounced odors. Cereal starch odors have been attributed to the autoxidation of lipids present on the surface of the starch granules.[Citation3,Citation4,Citation5] Starch lipids inside the granules are reportedly stable to autoxidation,[Citation6,Citation7] as they appear to form water-insoluble inclusion complexes with amylose,[Citation8] but the amylose-complexed lipids may be released and become susceptible to autoxidation upon partial digestion of the starch granules. Due to its bland flavor, native or pregelatinized tapioca (cassava) starch has been preferably used in the delicately flavored puddings, pie and pastry fillings, and baby foods.[Citation9,Citation10,Citation11] Of the commercially available starches, tapioca was reported to contribute the least off-flavor, waxy corn starch slightly more, and corn starch the most.[Citation12]
Volatiles in the sources of starches, namely, corn,[Citation13] wheat,[Citation14,Citation15,Citation16] rice,[Citation17] and potato,[Citation18] have been identified, but how those volatiles impact the odor of their starches was not reported. Volatile compounds associated with various starches were recently studied by Sayaslan and co-workers.[Citation5] Hexanal was found to be the most abundant compound in corn, potato, and wheat starches. Among the volatile organic compounds, the level of aldehydes was determined to be the highest, followed by alcohols, ketones, benzenes, esters, and terpenes. Some of the identified compounds, the majority of which are the degradation products of lipid autoxidation, were hexanal, heptanal, octanal, nonanal, decanal, benzaldehyde, 2-propanone, 2-propanol, 1-butanol, 2-ethyl-1-hexanol, methylbenzene, and tetradecane. Many volatiles detected in wheat and corn starches also were detected in the kernels of their commercial samples. Based on those findings, it was concluded that the major volatiles in cereal and root/tuber starches were common breakdown products of lipid autoxidation, especially from linoleic acid. It was speculated that bruised or damaged tissue in stored grains or roots/tubers contributed to those volatiles.[Citation5]
Volatile compounds can be bound to or retained by the starch granules either by adsorption or by complex formation.[Citation19] Binding of flavor components to starch varies by the type of starch. For instance, starches with a low amylose content, including tapioca starch, have a very weak flavor binding capacity, whereas those with a high amylose content, such as corn and wheat starches, have a greater tendency to bind flavors.[Citation20] Since starch granules have a large surface area,[Citation21] the volatiles may be adsorbed on the external surface of the granules or on the internal surface of the pores in the granules.[Citation22] Furthermore, due to the presence of amorphous regions in the granules, some volatile molecules may also diffuse into those regions and be entrapped there.
Little research regarding the removal of starch volatiles that cause off-flavor has been conducted. A patented work[Citation23] disclosed a procedure in which cereal starches were extracted with aqueous sodium hydroxide (NaOH) at a pH just below their pasting pH (pH 10.4–12.5) for 30 minutes to 3 hours at starch solids contents of 35–43% (w/w). The extracted cereal starches were claimed to be free from off-tastes and off-odors. The procedure was believed to swell and open up the amorphous regions of the granules as the pH of the extraction medium approached to that of gelatinization, so that materials associated with off-taste and off-odor could be removed by washing with water. The benefits to the flavor were measured by a sensory panel and no objective chemical data were provided as to what compounds were removed. Matsunaga[Citation24] also argued that aqueous NaOH-extracted wheat starch was free of characteristic cereal odor. In addition to removal of off-flavors, NaOH extraction of cereal starches has been reported to remove glutinous material, prevent microbial growth, increase the solubility of laundry starch,[Citation23] and improve the clarity and shortness of cooked puddings.[Citation25] Aqueous NaOH also has been suggested to remove lipids from rice starch.[Citation26] Azudin and Morrison[Citation27] extracted rice starch with 0.1% aqueous NaOH to remove lipids. They reported that the treatment reduced the amount of lysophospholipids (LPL), but increased the level of free fatty acids (FFA) due to saponification. Matsunaga and Seib[Citation28] also found similar results when wheat starch was extracted with NaOH at different pH and temperatures with and without sodium sulfate. All the fatty acids were reported to remain in the starch, but phosphate was removed.
Another patent[Citation19] disclosed a process that allegedly removed off-flavors from cereal starches. The process involved washing starch with 28% aqueous ammonia in methanol at 60–65°C for 2 to 6 hours followed by washing with water. The phosphatides and lipids were proposed to be the possible sources of off-odors. The removal of starch surface lipids was implied as they are suspected of leading to off-flavor development.[Citation3,Citation4,Citation5] Starch surface lipids can be easily removed from starches with varying polarity of solvent mixtures at room temperature, including chloroform-methanol-water, ethanol-diethylether-water, benzene-ethanol-water, water-saturated butanol, methanol, or ethanol.[Citation29,Citation30] Ethanol is more suitable for food use because of its ease of availability and low toxicity.[Citation31] Also, washing starches with aqueous sodium dodecyl sulfate (SDS) removed some starch surface lipids and LPL along with surface proteins when 2–5 mL of 1–2% aqueous SDS per gram of starch was employed at 20°C for 30 minutes.[Citation32,Citation33] It was reported that extraction of wheat starch with 1% aqueous SDS solution containing 1% of 2-mercaptoethanol (15–25°C, 24 h) removed surface proteins without gelatinizing the starch granules, and that the extraction dissolved and removed various trace materials from the surface of the granules.[Citation34] It was also reported that the extraction removed surface lipids and LPL, which in turn gradually loosened the framework of the granules that allowed the SDS solution to penetrate the granules and began the dissolution process.[Citation35] Extraction of starch granules with 2% aqueous SDS at a starch-SDS solution ratio of 1/20 (w/v) at 20°C for 2 hours was reported to remove surface proteins without disturbing the granules, and that SDS extraction at 40–60°C caused swelling of wheat starch and extracted integral proteins and some LPL. The extraction released 50–75% of palmitic acid from amylose-palmitic acid complex, indicating that fatty acids were displaced by the detergent.[Citation7] The objective of this study was to determine the effects of extractions of different starches with various aqueous solutions on the removal or reduction of starch-associated volatile compounds.
MATERIALS AND METHODS
Materials
Wheat starch was isolated by wet-milling of wheat flour (70% extraction), freshly roller-milled from a blend of hard wheats, according to the method of Shogren et al.[Citation36] Corn starch was isolated from yellow dent corn by Eckhoff et al.[Citation37] Potato starch was isolated according to the method of Willigen.[Citation38] Unless otherwise stated, all starches were stored in screw-capped glass containers at 5°C.
General Methods
Moisture contents of starches were determined by the AACC Method 44–15A[Citation39], and protein contents (N × 5.7) by combustion in a nitrogen determinator (Leco FP-2000, Leco Corp., St. Joseph, MI). All starches contained < 0.3% protein.
Aqueous NaOH Extractions of Starches
Wheat, corn, and potato starches were separately extracted with aqueous NaOH solution at 25°C as previously described.[Citation24] Starch (60 g, wb) was mixed with 80 mL of distilled water (∼38% starch solids) at 25°C, and 1 N NaOH solution was added to the starch slurry drop-wise with stirring over a period of 15 minutes to pH 12.3. After 1-h stirring, the pH of the slurry was readjusted to 12.3 by adding 1 N NaOH solution. The slurry was stirred at 25°C for a total of 4 hours and the starch was collected by centrifugation at 2,000 × g for 20 minutes. The off-colored material and gelled starch atop the granular starch was carefully scraped off and discarded. The granular starch was then dispersed in distilled water (60 mL) and the mixture was stirred for 1 hour and centrifuged again. After one more wash, the starch was resuspended in distilled water (60 mL) and 1 N hydrochloric acid solution was added to pH 6.0. The slurry was stirred for 30 minutes, centrifuged at 3,500 × g for 5 minutes, and then collected and washed once more with water. All extracted starches were air-dried at room temperature for two days, ground with a mortar and pestle, and stored in screw-capped glass containers at 5°C.
Aqueous Ethanol Extractions of Starches
Wheat, corn, and potato starches were separately extracted with 75% (v/v) aqueous ethanol at a starch-aqueous ethanol ratio of 1/10 (w/v) at 25°C for 2 hours with gentle stirring. After the first extraction, starch was collected by centrifugation at 2,000 × g for 20 minutes, and extracted again with 75% aqueous ethanol under the same conditions for 1 hour. After centrifugation, the starch was resuspended in distilled water and centrifuged, repeating the process three times. The extracted starches were dried and stored as described above.
Aqueous SDS Extractions of Starches
Wheat, corn, and potato starches were separately extracted with 1.5% (w/v) aqueous SDS solution at a starch-SDS solution ratio of 1/5 (w/v) at 25°C for 30 minutes with gentle stirring. After the extraction, the starch was collected by centrifugation at 2,000 × g for 20 minutes and then resuspended in distilled water and centrifuged. After repeating this process three times, the extracted starches were dried and stored as described above.
Collection, Separation, and Identification of Volatiles Associated with Starches
Volatiles from the control and extracted starches were separately collected through dynamic headspace sampling using a purge and trap instrument (LSC 2000, Tekmar Co., Cincinnati, OH), separated on a 5890 Series II gas chromatograph (GC) coupled with a 5965A Fourier-transform infrared detector (FTIRD), and a 5970 mass selective detector (MSD), all from Hewlett Packard Co. (Palo Alto, CA), and identified by computer matching of the observed mass spectral data with the standard mass spectra in the HP Wiley 138 spectral database (Hewlett Packard Co., Palo Alto, CA). Volatiles of the starches were analyzed in duplicate and the mean values were reported. The details of the instrumental setup and experimental conditions were similar to those published elsewhere.[Citation5]
RESULTS AND DISCUSSION
Effects of Aqueous NaOH Extractions on Starch Volatiles
As previously reported by Sayaslan et al.,[Citation5] hexanal, which is an important autoxidation product of linoleic acid,[Citation40] was found to be the most abundant compound in corn, potato, and wheat starches. Among the volatile organic compounds, the level of aldehydes was determined to be the highest, followed by alcohols, ketones, benzenes, esters, and terpenes. Some of the identified compounds, the majority of which are the degradation products of lipid autoxidation, were hexanal, heptanal, octanal, nonanal, decanal, benzaldehyde, 2-propanone, 2-propanol, 1-butanol, 2-ethyl-1-hexanol, 2-pentylfuran, methylbenzene, nitromethane, 1-nitrohexane, tetradecane, pentadecane, and hexadecane ().
Table 1 Abundance of selected volatile compounds associated with wheat starch prior to and upon extractions with aqueous NaOH, ethanol, and SDS solutions.
Table 2 Abundance of selected volatile compounds associated with corn starch prior to and upon extractions with aqueous NaOH, ethanol, and SDS solutions.
Table 3 Abundance of selected volatile compounds associated with potato starch prior to and upon extractions with aqueous NaOH, ethanol, and SDS solutions.
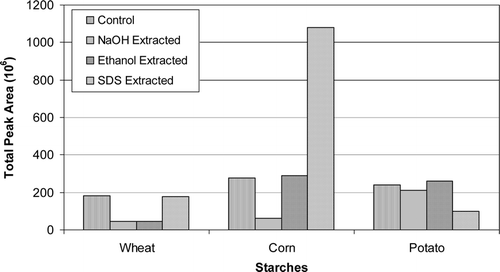
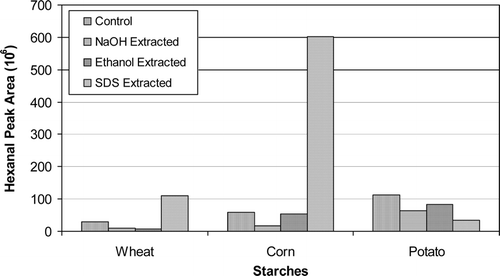
The extractions with aqueous NaOH at pH 12.3 reduced the level of total volatiles noticeably in wheat and corn starches, but did not affect total volatiles in potato starch (). As listed in and , NaOH extractions removed certain volatiles associated with cereal starches or reduced their levels. This result can be explained as previously hypothesized by Seidel et al.[Citation23] that as the pH of the medium approached to the pasting pH of cereal starches, the amorphous regions of the granules swelled and opened up leading to readily removal of volatiles entrapped there. The instrumentally obtained results in this study were in line with the sensory results of Seidel et al.[Citation23] that off-tastes and off-odors associated with starch granules were removed upon aqueous NaOH extraction. In the case of potato starch, however, NaOH extraction was not so effective on reducing the level of total volatiles (). This was possibly caused by the variation in the pasting pH and other characteristics of potato starch. However, hexanal levels were reduced in all starches upon NaOH extractions ().
Effects of Aqueous Ethanol Extractions on Starch Volatiles
The level of total volatiles in wheat starch upon ethanol extraction decreased significantly, but did not change much in corn and potato starches (). The quantity of some volatiles decreased, or they were removed totally ( ). Some new volatiles also were introduced to the starches after ethanol extraction. In the case of corn starch; 2-hexenal, benzene, styrene, chloroform, 1-bromo-2-flourobenzene, and 4-nonenal were introduced, and the level of benzaldehyde, nonanal, 2-nonenal, and decanal, which are the products of lipid oxidation,[Citation40] increased. This indicates that ethanol extraction probably solubilized the lipids; however, the lipids precipitated on the surface of the granules and resulted in oxidation. Ethanol extraction of potato starch did not change the abundance and composition of volatiles (). Hexanal levels were somewhat reduced in all starches upon their extractions with ethanol (). It appears that the granular structure of starch is required to be loosened without gelatinization in order to remove the volatiles entrapped inside the pores and amorphous regions of the granules.
Effects of Aqueous SDS Extractions on Starch Volatiles
The extraction of the starches with SDS did not affect the abundance of total volatiles in wheat starch, but it caused a substantial increase in the level of total volatiles in corn starch (). However, SDS extraction reduced the level of total volatiles in potato starch (). In wheat and corn starches, the levels of hexanal () and of other oxidation products, which include pentanal, 2-hexenal, heptanal, 2-pentylfuran, 2-heptenal, 3-octen-2-one, 2-octenal, 2-nonenal, 4-nonenal, nonanal, decanal, and t,t-2,4-nonadienal, markedly elevated (). The reason for the elevated levels of volatiles in cereal starches upon their extractions with aqueous SDS solution might stem from the fact that SDS loosens the framework of starch granules and allows penetration of SDS as a result of removal of some trace materials from the granules along with surface proteins,[Citation35] which in turn help displace the fatty acids that are complexed with amylose.[Citation7] It was reported by Seguchi and Yamada[Citation34] that extraction of wheat starch with 1% aqueous SDS solution at 15–25°C for 24 hours removed surface proteins without gelatinizing the starch granules, and that the extraction dissolved and removed various trace materials from the surface of the granules. Extraction of starch granules with 2% aqueous SDS at a starch-SDS solution ratio of 1/20 (w/v) at 20°C for 2 hours was reported to remove surface proteins without disturbing the granules, and that SDS extraction at 40–60°C caused swelling of wheat starch and extracted integral proteins and some LPL. The extraction released 50–75% of palmitic acid from amylose-palmitic acid complex, indicating that fatty acids were displaced by the detergent.[Citation7] As opposed to the absence of internal lipids in root/tuber starches, cereal starches do contain internal lipids, mainly LPL and FFA. It is possible that those lipids might have been displaced by SDS, and that they might have been deposited on the surface of the granules causing their rapid autoxidation. However, extraction of potato starch with SDS appeared to be effective in reducing its total volatiles () without increasing its hexanal level (). This observation is consistent with the above approach because potato starch does not contain internal lipids. However, it is not clear whether the extraction with SDS under the conditions in this study (1.5%, 25°C, 30 min, 1/5 (w/v) starch-SDS ratio) can cause swelling of the starch granules and displacement of the fatty acids. Further research is required to better understand the differences in the obtained results.
CONCLUSIONS
Aqueous NaOH extraction was effective in reducing the level of total volatiles or removing certain volatiles from both wheat and corn starches without increasing the abundance of hexanal, an important oxidation product often used to monitor the course of lipid autoxidation in food products. However, NaOH extraction did not influence the composition and abundance of volatiles in potato starch. Aqueous ethanol extraction was effective in removal of volatiles only from wheat starch. The extraction had almost no effect on the total volatiles of corn and potato starches. Aqueous SDS extraction did not change the level of total volatiles in wheat starch but elevated the total volatiles considerably in corn starch. The extraction also introduced new oxidation products and increased the level of hexanal noticeably in wheat and corn starches, ruling out its application to cereal starches. However, SDS extraction was interestingly effective in removal of volatiles from potato starch as well as reducing its hexanal level. It seems that an aqueous solution that swells and opens up the starch granules without causing their gelatinization is a prerequisite for the removal or reduction of starch-associated volatile compounds.
ACKNOWLEDGMENTS
This research was conducted as part of the author's graduate work. The author sincerely acknowledges the invaluable contributions of Dr. Paul A. Seib, Department of Grain Science and Industry, Kansas State University, and Dr. Okkyung K. Chung and Dr. Larry M. Seitz, USDA/ARS Grain Marketing and Production Research Center, Manhattan, KS, USA.
REFERENCES
- Belitz , H.D. and Grosch , W. 1987 . Food Chemistry , New York : Springer Verlag .
- Ramirez , I. 1991 . Chemoreception for an insoluble nonvolatile substance: starch taste . Amer. J. Physiol. , 260 : R192 – R199 . [INFOTRIEVE]
- Radley , J.A. 1976 . Industrial Uses of Starch and Its Derivatives , London : Applied Science Publishers .
- Swinkels , J.J.M. 1985 . “ Sources of Starch, Its Chemistry and Physics ” . In Starch Conversion Technology , Edited by: Beynum , G.M.V. and Roels , J.A. 15 – 46 . New York : Marcel Dekker .
- Sayaslan , A. , Chung , O.K. , Seib , P.A. and Seitz , L.M. 2000 . Volatile compounds identified in five starches . Cereal Chem. , 77 : 248 – 253 .
- Morrison , W.R. 1995 . Starch lipids and how they relate to starch granule structure and functionality . Cereal Foods World , 40 : 437 – 446 .
- Morrison , W.R. and Coventry , A.M. 1989 . Solvent extraction of fatty acids from amylose inclusion complexes . Starch/Stärke , 41 : 24 – 27 .
- Morgan , K.R. , Furneaux , R.H. and Larsen , N.G. 1993 . Solid-state NMR studies on the structure of starch granules . Cereal Chem. , 70 : 385 – 389 .
- Balagopalan , C. , Padmaja , G. , Nanda , S.K. and Moorthy , S.N. 1988 . Cassava in Food, Feed, and Industry , Boca Raton, FL : CRC Press .
- Rutenberg , M.W. 1980 . “ Starch and Its Modifications ” . In Handbook of Water-Soluble Gums and Resins , Edited by: Davidson , R.L. 22.1 – 22.83 . New York : Mc-Graw Hill Book Company .
- Moore , C.O. , Tuschhoff , J.V. , Hastings , C.W. and Schanefelt , R.V. 1984 . “ Application of Starches in Foods ” . In Starch: Chemistry and Technology , 2nd , Edited by: Whistler , R.L. , BeMiller , J.N. and Paschall , E.F. 575 – 592 . New York : Academic Press .
- Langan , R.E. 1987 . “ Food Industry ” . In Modified Starches: Properties and Uses , Edited by: Wurzburg , D.B. 199 – 212 . Boca Raton, FL : CRC Press .
- Haugen , F.W. , Quilliam , M.A. and Curran , W.A. 1971 . Headspace vapors from cereal grains . J. Agr. Food Chem. , 19 : 182 – 183 . [CROSSREF]
- Maga , J.A. 1978 . Cereal volatiles, a review . J. Agr. Food Chem. , 26 : 175 – 178 . [CROSSREF]
- McWilliams , M. and Mackey , A.C. 1969 . Wheat flavor compounds . J. Food Sci. , 34 : 493 – 496 .
- Seitz , L.M. 1995 . “ Volatile Compounds in Wheat Cultivars from Several Locations in Kansas ” . In Food Flavors: Generation, Analysis, and Process Influence , Edited by: Charalambous , G. 2183 – 2203 . Amsterdam : Elsevier .
- Wilkie , K. , Wootton , M. and Paton , J.E. 2004 . Sensory testing of Australian fragrant, imported fragrant, and non-fragrant rice aroma . Int. J. Food Prop. , 7 : 27 – 36 . [CROSSREF]
- Solms , J. and Wyler , R. 1979 . “ Taste Components of Potatoes ” . In Food Taste Chemistry , Edited by: Boudreau , J.C. 175 – 184 . Washington, D.C. : American Chemical Society .
- Harris , G.C. Cereal Starches and Process . U.S. Patent No. 3,102,054 . Issued August 27 1963 .
- McGorrin , R.J. 1996 . “ Introduction ” . In Flavor-Food Interactions , Edited by: McGorrin , R.J. and Leland , J.V. IX – XII . Washington, D.C. : American Chemical Society .
- Soulaka , A.B and Morrison , W.R. 1985 . The amylose and lipid contents dimensions and gelatinization characteristics of some wheat starches and their A- and B-granule fractions . J. Sci. Food Agr. , 36 : 709 – 718 .
- Fannon , J.E. , Hauber , R.J. and BeMiller , J.N. 1992 . Surface pores of starch granules . Cereal Chem. , 69 : 284 – 288 .
- Seidel , W.C. , Orozovich , G.E. and Medcalf , D.G. Method of Preparing a Clean Flavored Cereal Starch . U.S. Patent No. 4,477,480 . Filed July 6, 1982 issued October 16, 1984 .
- Matsunaga , N. 1996 . Alkali-Extraction of Wheat Starch Granules . M.S. Thesis, Kansas State University . 1996 , Manhattan, KS.
- Daly , R.E. , Frankenfield , F.A. and Schopmeyer , H.H. Method of Making Modified Corn Starch . U.S. Patent No. 2,373,016 . Issued April 3, 1945 .
- Sowbhagya , C.M. and Bnattacharya , K.R. 1979 . Simplified determination of amylose in milled rice . Starch/Stärke , 31 : 159 – 165 .
- Azudin , M.N. and Morrison , W.R. 1986 . Non-starch lipids and starch lipids in milled rice . J. Cereal Sci. , 4 : 23 – 31 .
- Matsunaga , N. and Seib , P.A. 1997 . Extraction of wheat starch with aqueous sodium hydroxide . Cereal Chem. , 74 : 851 – 857 .
- Morrison , W.R. , Mann , D.L. , Soon , W. and Coventry , A.M. 1975 . Selective extraction and qualitative analysis of non-starch and starch lipids from wheat flour . J. Sci. Food Agr. , 26 : 507 – 521 .
- Morrison , W.R. , Tan , S.L. and Hargin , K.L. 1980 . Methods for the quantitative analysis of lipids in cereal grains and similar tissues . J. Sci. Food Agr. , 31 : 329 – 340 .
- Morrison , W.R. and Coventry , A.M. 1985 . Extraction of lipids from cereal starches with hot aqueous alcohols . Starch/Stärke , 37 : 83 – 87 .
- Morrison , W.R. and Karkalas , J. 1990 . “ Starch ” . In Methods in Plant Biochemistry , Edited by: Dey , P.M. Vol. II , 323 – 352 . London : Academic Press .
- South , J.B. and Morrison , W.R. 1990 . Isolation and analysis of starch from single kernels of wheat and barley . J. Cereal Sci. , 12 : 43 – 51 .
- Seguchi , M. and Yamada , Y. 1989 . Study of proteins extracted from the surface of starch granules with sodium dodecyl sulfate . Cereal Chem. , 66 : 193 – 196 .
- Seguchi , M. 1995 . Surface staining of wheat starch granules with Remazolbrilliant Blue R Dye and their extraction with aqueous sodium dodecyl sulfate and mercaptoethanol . Cereal Chem. , 72 : 602 – 607 .
- Shogren , M.D. , Finney , K.F. and Hoseney , R.C. 1969 . Functional (breadmaking) and biochemical properties of wheat flour components. I. Solubilizing gluten and flour protein . Cereal Chem. , 46 : 93 – 102 .
- Eckhoff , S.R. , Singh , S.K. , Zehr , B.E. , Rausch , K.D. , Fox , E.J. , Mistry , A.K. , Haken , A.E. , Niu , Y.X. , Zou , S.H. , Buriak , P. , Tumbleson , M.E. and Keeling , P.L. 1996 . A 100-g laboratory corn wet-milling procedure . Cereal Chem. , 73 : 54 – 57 .
- Willigen , A.H.A. 1964 . “ Potato Starch Isolation ” . In Methods in Carbohydrate Chemistry , Edited by: Whistler , R.L. Vol. IV , 9 – 13 . New York : Academic Press .
- American Association of Cereal Chemists . 1995 . Approved Methods of the AACC , 9th , St. Paul, MN : AACC .
- Frankel , E.N. 1982 . Volatile lipid oxidation products . Lipid Res. , 22 : 1 – 33 . [CROSSREF]