Abstract
Raw-peeled chestnuts were treated with citric acid or sodium metabisulphite, steamed, and dipped into sugar solutions containing dextrose and sucrose, or dextrose and fructose. Composition, mineral content, weight change, rheological properties, and color were measured at each step. Carbohydrate content increased during processing. Candied chestnuts were low in protein (1.31–1.35%) and lipids (0.29–0.78%) but high in carbohydrates (73.48–76.13%). Their mineral concentrations were: Ca 19.08–46.70, Cu 0.19–0.52, Fe 0.88–1.98, K 180.5–659.1, Mg 26.83–69.57, Mn 0.70–2.42, Zn 1.51–6.95 mg/100 g sample. Rheological properties were affected by processing steps. Dipping into sugar solutions did not affect rheological properties. Color changes were quantified, and average L*, a*, and b* values measured.
INTRODUCTION
Turkey was the third largest producer of chestnuts (Castanea sativa Mill and its hybrids) in the world between 1991 and 2000.[Citation1] Production was about 47,000 and 50,000 metric tons in 2001 and 2002, respectively.[Citation2] Chestnuts are grown in the Aegean, Marmara, and Black Sea regions of Turkey.[Citation3] Fresh chestnuts are chilled and consumed throughout winter, especially around the New Year. Prefered preparations are boiled or roasted chestnuts; the shell is then peeled and the nutmeat is eaten. Other preparations are candied chestnuts, marron glacés and chestnut purees.
Research has been performed to determine the compositional properties of raw chestnuts. McCarthy and Meredith [Citation4] reported the nutrient data on American, European, and Chinese chestnuts consumed in the United States. Üstün et al.[Citation5] determined selected physical and chemical properties of 55 different chestnut types grown in Sinop, Turkey. Ferreira-Cardoso et al.[Citation6] determined the composition of 23 shelled chestnut kernel samples, corresponding to 7 different cultivars of C. sativa Mill in Portugal. These studies mainly focused on raw chestnuts. The effect of roasting on chemical composition and quality of different (C. sativa Mill) varieties were studied.[Citation7] Shin et al.[Citation8] determined the effect of boiling, steaming (107°C), and roasting in an oven (200°C) on the composition of chestnuts. Morini and Maga[Citation9] reported the changes in fatty acid composition of chestnuts during roasting at 182°C. However, the composition, color, and texture of candied chestnuts, as well as the changes that occur during candied chestnut processing, have not been reported before.
Nutritionally, chestnuts were one of the staple foods in the southern valleys of the Italian, Swiss and French Alps.[Citation10] Unlike most other nuts, they are low in protein and fat, but high in carbohydrate.[Citation4,Citation11] Minerals play an important and vital role in metabolism, health, and disease. Inductively coupled plasma-atomic emission spectrometry (ICP-AES) or inductively coupled plasma-optical emission spectrometry (ICP-OES) are considered excellent techniques for the determination of major, minor, and trace elements in foods due to their low detection limits, high sensitivity, wide linear dynamic range, relatively high freedom from spectral, chemical and ionization interferences, precision, high speed, and versatility in terms of the number of elements analyzed.[Citation12,Citation13] Dos Santos and de Oliveira[Citation14] determined the mineral nutrients and toxic elements in Brazilian soluble coffee by ICP-AES. The levels of 26 elements in infant formulas from USA, UK, and Nigeria,[Citation15] and the levels of 15 elements in commonly consumed Kuwaiti dishes[Citation16] were analyzed using ICP-OES.
The color and rheological properties of candied chestnuts are important quality attributes that affect consumer preference. According to the Turkish Candied Chestnut Standard (TS9400), the color of the candied chestnuts should not be dark brown, and the texture of the product should be neither too soft nor too hard.[Citation17] The development of brown color results from the Maillard reactions because heating speeds up the reaction between sugars and amino acids. Image processing techniques can be applied to determine the color of foods, as well as their shape and size. Ramesh[Citation18] applied image processing to determine the kinetics of hydration of milled rice in hot water. Some researchers studied the instrumental textural properties of fura made from different cereal grains[Citation19] and from millet flour,[Citation20] and they determined the development of color and texture of breads during microwave and conventional baking.[Citation21] No research has been found on the determinaton of color and rheological properties of candied chestnuts. The objectives of this study were to determine the composition, mineral contents, color, and rheological properties of candied chestnuts during processing, and to investigate the effects of treatment of raw chestnuts with distilled water, predetermined amounts of citric acid and sodium metabisulphite, and using either sucrose or fructose in the dipping solution on the color of the final product.
MATERIAL AND METHODS
Chestnut Samples
Raw peeled chestnut samples (C. sativa Mill) were obtained from Kafkas Co. in Bursa, Turkey. The raw chestnuts were originally from Aydın, Turkey. The samples were kept frozen until processing.
Candied Chestnut Production
The processing steps for candied chestnut production are dipping pre-treatment, steaming, and dipping into a sugar solution. Frozen-peeled chestnuts were divided into 3 groups and each group was immersed into either distilled water (control), 0.2% citric acid (CA) (Fisher Scientific Co., Fair Lawn, NJ) or 0.1% sodium metabisulphite (MS) (Acros Organics, Geel, Belgium) solutions for 1 hour. The three groups were then placed into a retort and steamed at 103°C for 45 minutes. The retort temperature and the center temperature of the chestnuts during steaming were continuously monitored using type-T 36 gauge thermocouples (Ecklund, Fort Myers, FL). Each group was then subdivided into two sub-groups. Two different sugar solutions were prepared, one containing 65% sucrose (Walmart, Inc., Bentonville, AR) and 15% dextrose (Fisher Scientific Co., Fair Lawn, NJ), and the other containing 60% fructose (Fisher Scientific Co., Fair Lawn, NJ) and 20% dextrose. Each steamed chestnut sub-group was boiled separately in one of the sugar solutions for 30 min. Chestnuts were placed in Pyrex® containers and sugar solutions were poured into them. The samples were covered with the solution and kept in the solution at room temperature for 48 hours. Experiments were duplicated.
Chemical and Physical Analyses
The moisture, fat, and ash contents of chestnut samples at each processing step were measured by AOAC methods, Ref. 925.40, Ref. 948.22 and Ref. 950.49, respectively, and the total nitrogen content was measured using the Kjeldahl method, Ref. 950.48.[Citation22] The percentages of nitrogen were transformed into protein content by multiplying by a conversion factor of 5.30, as reported by McCarty and Meredith.[Citation4] The total carbohydrate content was determined by difference. The water activity (aw) of the samples was measured using a Rotronic Hygroskop DT (Rotronic Instrument Corp., Huntington, NY). The protein, fat and ash contents were measured in duplicate, and the moisture content and aw were done in triplicate. The weight of the samples was measured at each processing step and the change was calculated. The refractive index of the sugar solution was measured using a Reichert Abbe Mark II digital refractometer (Warner-Lambert Tech. Inc., Buffalo, NY) and % soluble solid contents of the sugar solutions initially and at the end of dipping treatment were obtained.
ICP-AES Analysis
Each chestnut sample was placed in an aluminum dish, weighed and then dried to constant weight at 100°C using a Memert Model 400 oven (Memert GmbH & Co. KG, Schwabach, Germany). The samples were cooled in the desiccator and weighed as soon as they reached room temperature. Each sample was then ground using an acid washed ceramic mortar and pestle. After grinding, the samples were again dried to constant weight at 100°C and stored in a desiccator until dry ashing. Two to five g of each sample was weighed, placed in a porcelain crucible, and covered. The crucibles were put into an electric muffle furnace (Carbolite Furnaces, CSF 1100, Sheffield, England) and burned at 500°C until white ash was obtained. After cooling the crucibles to room temperature, the ash of each sample was dissolved in 5 ml concentrated HNO3 (65% v/v). The digests were diluted to 50 ml with deionized H2O in polyethylene centrifuge tubes and were ready for analysis. The final acid content of the samples was ca. 10% HNO3.
The concentrations of minerals, Ca, Cu, Fe, K, Mg, Mn, and Zn, were determined by ICP-AES (Varian Liberty-Series II, axial configuration, Varian Australia Pty Ltd., Australia). The operating parameters of ICP-AES were: power 1.0 kW; flow of argon refrigerant 15 l/min; flow of argon auxiliary 1.5 l/min; flow of argon carrier 1.0 ml/min; sample flow rate 1.5 ml/min. Lines of emission were: Ca 317.93 nm; Cu 324.75 nm; Fe 238.20 nm; K 769.90 nm; Mg 279.81 nm; Mn 257.61 nm; Zn 213.86 nm. A stock multi-element standard solution containing 1000 mg/l each of Ca, Cu, Fe, K, Mg, Mn and Zn was supplied by Merck (Gibbstown, NJ). Calibration of the equipment was done using appropriate dilutions of the stock standard solution. The data was converted to mg/100 g sample.
Due to the absence of a certified chestnut reference material with a similar composition as the samples processed in this study, accuracy and validity of the measurements were determined by analyzing spiked samples of chestnuts. Two samples, prepared for ICP-AES analysis, were chosen randomly and diluted to a ratio of 1/40. Several aliquots of diluted samples were spiked with 0.1, 0.2, 0.5, 1.0, 2.0, and 5.0 mg/l standard solution. The calibration standards were also prepared using the same dilutions. The concentrations of minerals in these samples were determined and the % recovery in each dilution was calculated.
Rheological Analysis
The Instron Universal Testing Machine (Model 4411, Instron Corp., Canton, MA) was used to determine compression for chestnut samples at each processing step. Chestnut samples were cut into cylindrical shapes using a plunger and placed in plastic tubing that had 1.51 mm wall thickness, 1.32 cm diameter, and 1.12 cm height. This was done to prevent samples from falling apart. Samples within the plastic tubing were placed in the Instron for compression. Compression was done by the probe #10 which covered the whole surface of the sample. The maximum compression force (kg) was measured with a 50 kg load cell, and 5 mm/min load cell speed. Compression was continued until the sample was compressed for 7 mm. Ten samples from each treatment were used for each processing step. The texture measurements were done at room temperature (22–23°C).
Color Analysis
The color of the candied chestnuts was measured using a color machine vision system.[Citation23] For each treatment at each processing step, eight chestnut samples were placed in a light box. A 24-bit image of the samples was taken with a CCD digital camera located inside the light box. A 512-color discrete spectrum of the colors present in the sample and average L*, a*, b* values of all the pixels representing the chestnut sample were calculated. Colors that were present above 3% of the total surface area of the sample were selected for data analysis.
Statistical Analysis
All data were analyzed using analysis of variance of the general linear procedures (Proc GLM) of SAS© software, and the LSMEANS procedure for generating standard errors of the mean (SEM).[Citation24] Any significant differences were analyzed by the multiple comparison procedure of Duncan's Multiple Range test, using a level of significance of α = 0.05. Interaction between replications was tested for significance (p < 0.05).
RESULTS AND DISCUSSION
Chemical and Physical Analysis
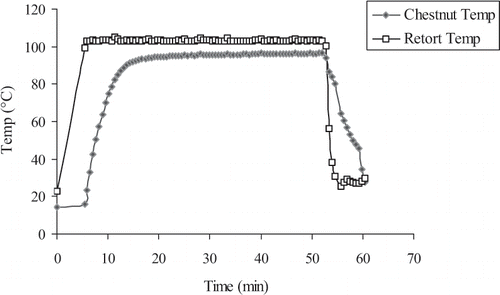
After filling the retort with chestnuts, it took approximately 6 minutes for the inside temperature to reach 103°C (). The retort temperature remained at 103 ± 2°C for 45 minutes. The data on the proximate composition of candied chestnuts, treated with CA or MS, during processing are presented in . Control or treatment with CA or MS did not significantly change the composition of chestnuts. Also, dipping into different sugar solutions did not have a significant effect on the compositions of candied chestnuts. However, the compositions changed significantly between different processing steps.
Table 1 Composition of the candied chestnuts for three different treatments at each processing step.
The average moisture content of raw chestnuts () was in agreement with the 54.88 kg/100 kg sample mean value reported by McCarthy and Meredith[Citation4] for raw European chestnuts and 54.10 kg/100 kg sample mean value reported by Künsch et al.[Citation11] for Italian commercial variety “Marrone di Cuneo.” At the end of the candied chestnut processing, the moisture content of the samples decreased () due to sugar diffusing into the chestnuts and replacing water, as well as water being osmosed out by sugar. Steaming increased the moisture content of all samples. No comparison could be done for the chestnuts during processing since data for candied chestnuts were unavailable.
The average protein content for raw samples (2.33 ± 0.37 kg/100 kg sample) was much lower than the values reported by Üstün et al.[Citation5] (3.43–8.27 kg/100 kg sample, mean value 5.683 kg/100 kg sample) for chestnuts from Sinop, Turkey. However, the value was close to that of 1.98 kg/100 kg sample reported by McCarthy and Meredith[Citation4] for raw European chestnuts. The average lipid content for raw samples (1.30 ± 0.17 kg/100 kg sample) was in agreement with the values found by Üstün et al.[Citation5] (0.66–3.08 kg/100 kg sample) for chestnuts from Sinop, Turkey. The average ash content for raw samples (2.46 ± 0.30 kg/100 kg sample) was between the ranges given by Üstün et al.[Citation5] (1.40–4.92 kg/100 kg sample) for chestnuts from Sinop, Turkey.
The protein, lipid and ash contents of the samples decreased during processing. This was due to the increase in the sugar content by diffusion. The carbohydrate content of the samples increased during processing. Treatments with CA and MS did not affect aw of the samples significantly; however, dipping into sugar solutions containing either sucrose or fructose significantly affected aw of the samples. The aw of raw, treated and steamed samples were 0.9855 ± 0.0028, 0.9872 ± 0.0031, and 0.9891 ± 0.0042, respectively. The aw of the candied chestnuts were 0.8209 ± 0.0066 (samples dipped into sugar solution having sucrose) and 0.7435 ± 0.0070 (samples dipped into sugar solution having fructose).
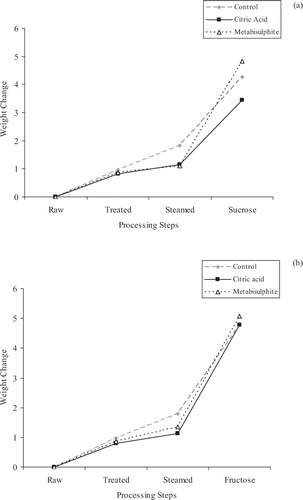
The changes in weight during processing for the candied chestnuts dipped into sucrose solution (a) or fructose solution (b) are shown in . After treatment with CA or MS, the weights of the samples increased in between 0.80 and 0.98 g, after steaming, the weights of the samples increased in between 1.09 and 1.84 g, and the weight of the candied chestnuts increased in between 3.45 and 5.06 g (compared with raw samples weighing about 15 g). Treatments with CA or MS and dipping into either sucrose or fructose solutions had significant effects on the weight change. The percent soluble solid contents of the sugar solutions were 84.17% (for sucrose) and 81.98% (for fructose), and after dipping the samples into the solution for 48 h, the % soluble solid contents of the sugar solutions decreased to 76.1% (for sucrose) and 74.65% (for fructose).
ICP-AES Analysis
The mineral contents of the candied chestnuts during processing are represented in . The processing steps had a significant effect on all of the mineral contents, except Cu for samples treated with MS, Fe for samples treated with CA and MS, Mn for samples treated with CA, and Zn for all samples. The data showed that K was found at high concentrations in all chestnut samples. Also Mg and Ca were higher than the other minerals in all chestnut samples. K, Mg, Ca, Zn, Mn, Fe and Cu were ranked from highest to lowest concentrations in all chestnut samples. Raw samples had higher concentrations of minerals than candied chestnuts. Dipping in sugar solutions caused a relative decrease in mineral contents of the chestnuts/unit weight. Sugar diffusion could be the reason for this decrease since the carbohydrate content of the samples increased.
Table 2 The concentration of elements in the chestnut samples for three different treatments during candied chestnut processing.
According to Üstün et al.,[Citation5] the mineral contents of raw chestnuts in mg/100 g dry matter were: Ca 69.71–201.70; Mg 59.71–202.89; Zn 2.63–21.87; Fe 1.84–16.99; Cu 0.33–1.29. McCarthy and Meredith[Citation4] also reported the concentrations of Ca as 23 ± 1.0, Fe as 0.71 ± 0.01, Mg as 32 ± 0.0, K as 378 ± 15.0, Zn as 0.44 ± 0.01, Cu as 0.208 ± 0.003 and Mn as 0.563 ± 0.019 mg/100 g for European raw chestnuts. The concentrations of all minerals for raw chestnuts in this study were higher than the values reported by McCarthy and Meredith.[Citation4] However, in this study Ca, Fe, Mg, and Zn concentrations were lower than the values given by Üstün et al.[Citation5] except for Cu concentrations, which were in the range reported by them. This variation could be attributed to variety and/or to soil and climate where the chestnuts were grown. The mineral concentrations of the candied chestnuts determined in this study could not be compared due to the lack of candied chestnut data.
The Turkish Standards has established maximum values for Cu (5 mg/kg), Zn (5 mg/kg) and, Fe (15 mg/kg) in candied chestnuts. None of the samples analyzed had values close to these for Cu and Fe, but Zn concentrations for candied chestnuts processed in this study were more than the maximum limit established by the Turkish Standards.[Citation17] The reason for the large variation among the samples as well as the higher concentrations of Zn in the samples during processing is not known.
Rheological Analysis
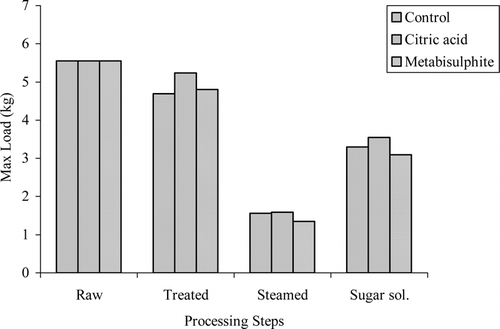
Rheological analysis was done using maximum compression force by a probe. Significant differences were detected for processing steps, treatment effects and interactions between processing steps and treatments (p < 0.05). Dipping the chestnuts into sugar solutions containing either sucrose or fructose did not have a significant effect on the rheological properties of the samples. The maximum force needed for compressing the samples treated with CA and MS and control samples at each processing step are shown in . Raw samples required more force for compression than the steamed samples. Steaming, however, reduced the force required for compression. The reason for this could primarily be linked to cell wall properties of the sample since starch swells during boiling, inducing a distension of the cell wall which facilitates cell separation.[Citation25] The maximum force for compression increased after dipping into sugar solution, presumably because of the loss of water.
Color Analysis
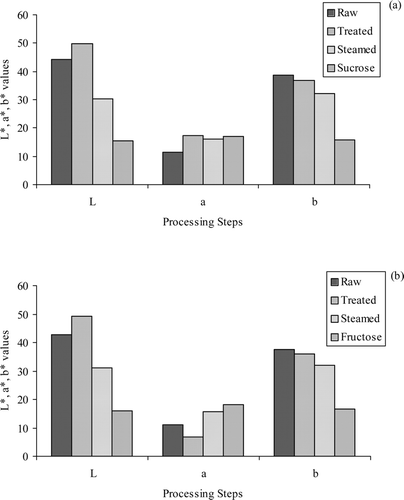
The 512-color spectra obtained from the images analyzed by the Color Analysis software[Citation26] did not show any significant differences between candied chestnuts dipped into sucrose or fructose solutions (). Dark yellow color (NBS # 88) increased during the treatment with distilled water, CA or MS, and decreased dramatically and disappeared at the last processing step. Moderate olive brown color (NBS # 95) decreased during processing. Moderate olive (NBS # 107) and moderate brown (NBS # 58) colors increased during treatment with distilled water, CA or MS, but decreased thereafter. Deep yellowish brown color (NBS # 75) increased during steaming. Dark brown (NBS # 59) and deep reddish brown (NBS # 41)—deep brown (NBS # 56) colors increased during dipping into either sucrose or fructose solutions. The changes in the average L*, a* and b* values of the candied chestnuts during processing are presented in . The treatment with distilled water, CA or MS did not affect the L*, a*, and b* values of the samples significantly. The L* values were high in raw samples and increased during the treatment with distilled water, CA or MS; however decreased during steaming and dipping into sugar solution. Dipping into sugar solutions having either sucrose or fructose did not show any significant difference between L*, a* and b* values of the samples. The a* values decreased during treatment with distilled water, CA or MS and increased during steaming and dipping into sugar solution. The b* values of the raw samples were high, but decreased during processing.
Table 3 Color spectra for candied chestnuts treated with distilled water, citric acid (CA), and sodium metabisulphite (MS) and dipped into sucrose or fructose solutions during processing. Only colors higher than 3% of the total area were considered.
CONCLUSION
The compositional and mineral contents of candied chestnuts during processing were measured. It was concluded that the % moisture, protein, lipid and ash contents were lower in candied chestnuts than in raw chestnut; however, carbohydrate content was higher. Raw and candied chestnuts contained high concentrations of K, Ca, and Mg. The rheological and colorimetric attributes of the chestnuts during processing were also investigated. It was found that steaming caused less maximum force for compression; however, dipping into sugar solutions caused more maximum force. The L* and b* values of the samples decreased during processing. Treatment with citric acid or sodium metabisulphite was not significantly different from treatment with distilled water regarding the color of the candied chestnuts. Dipping solutions having either sucrose or fructose also did not have any significant effect on the color of candied chestnuts. From a commercial point of view, candied chestnut processing leads to a good quality product due to its high carbohydrate content and high concentrations of K, Ca, and Mg.
ACKNOWLEDGMENTS
The authors would like to thank Kafkas Co., Bursa, Turkey for supplying the raw chestnuts. The assistance of Dr. Ahmet E. Eroglu and Oya Altingöz, İzmir Institute of Technology, İzmir, Turkey for the sample analyses using ICP-AES is greatly appreciated.
Notes
26. Luzuriaga, D.A. Application of computer vision and electronic nose technologies for quality assessment of color and odor of shrimp and salmon. Ph.D. thesis, University of Florida, Gainesville, FL, 1999
REFERENCES
- Bodet, L.; Ernst, M.; Allan, D.; Woods, T. The international chestnut marketing situation http://www.uky.edu/Ag/AgEcon/publications/staff411.pdf
- Food and Agricultural Organization http://www.fao.org
- Soylu , A. 1984 . Chestnut growing and specialities , Yalova, , Turkey : Atatürk Horticultural Research Institute . Publication No: 59, 39
- McCarthy , M.A. and Meredith , F.I. 1988 . Nutrient data on chestnuts consumed in the United States . Economic Botany , 42 : 29 – 36 .
- Üstün , N. , Tosun , Y. and Serdar , Ü. 1999 . Technological properties of chestnut varieties grown in Erfelek district of Sinop city . Acta Horticulture , 494 : 107 – 110 .
- Ferreira-Cardoso , J.V. , Sequeira , C.A. , Torres-Pereira , J.M.G. , Rodrigues , L. and Gomes , E.F. 1999 . Lipid composition of Castanea sativa Mill. Fruits of some native Portuguese cultivars . Acta Horticulture , 494 : 133 – 138 .
- Künsch , U. , Schärer , H. , Patrian , B. , Höhn , E. , Conedera , M. , Sassella , A. , Jermini , M. and Jelmini , G. 2001 . Effects of roasting on chemical composition and quality of different chestnut (Castanea sativa Mill) varieties . Journal of the Science of Food and Agriculture , 81 : 1106 – 1112 . [CROSSREF]
- Shin , D.H. , Oh , M.J. and Kim , S.Y. 1981 . Effect of heat treatments on the chemical composition of flesh in chestnut processing . Res. Rep. Agric. Sci. Technol , 8 : 117 – 125 . Chungnam Univ, Korea
- Morini , G. and Maga , J.A. 1995 . Volatile compounds in roasted and boiled Chinese chestnuts (Castanea molissima) . Lebensmittel Wissenschaft und Technologie , 28 : 638 – 640 . [CROSSREF]
- Conedera , M. 1996 . Die Kastanie, der Brotbaum . Bündnerwald , 49 : 28 – 46 .
- Künsch , U. , Schärer , H. , Patrian , B. , Hunter , J. , Conedera , M. , Sassella , A. , Jermini , M. and Jelmini , G. 1999 . Quality assessment of chestnut fruits . Acta Horticulture , 494 : 119 – 127 .
- Montaser , A. and Golightly , D.W. 1987 . Inductively couple plasmas in analytical atomic spectrometry , New York : VHC Publishers, Inc. .
- Munilla , M. , Gomez-Pinilla , I. , Rodenas , S. and Larrea , M.T. 1995 . Determination of metals in seaweeds used as food by inductively coupled plasma atomic emission spectrometry . Analysis , 23 : 463 – 466 .
- Dos Santos , É.J. and de Oliveira , E. 2001 . Determination of mineral nutrients and toxic elements in Brazilian soluble coffee by ICP-AES . Journal of Food Composition and Analysis , 14 : 523 – 531 . [CROSSREF]
- Ikem , A. , Nwankwoala , A. , Odueyungbo , S. , Nyavor , K. and Egiebor , N. 2002 . Levels of 26 elements in infant formula from USA, UK and Nigeria by microwave digestion and ICP-OES . Food Chemistry , 77 : 439 – 447 . [CROSSREF]
- Dashti , B. , Al-Awadi , F. , AlKandari , R. , Ali , A. and Al-Otaibi , J. 2004 . Macro- and microelements contents of 32 Kuwaiti composite dishes . Food Chemistry , 85 : 331 – 337 . [CROSSREF]
- Turkish Standards . 1991 . Candied Chestnut TS 9400 , Ankara, , Turkey : Turkish Standards Institute .
- Ramesh , M.N. 2001 . An application of image analysis for the study of kinetics of hydration of milled rice in hot water . International Journal of Food Properties , 4 ( 2 ) : 271 – 284 . [CROSSREF]
- Jideani , V.A. and Danladi , I.M. 2005 . Instrumental and sensory textural properties of fura made from different cereal grains . International Journal of Food Properties , 8 : 49 – 59 .
- Jideani , V.A. 2002 . Instrumental and sensory textural properties of fura . International Journal of Food Properties , 5 ( 7 ) : 367 – 377 . [CROSSREF]
- İçöz , D. , Sumnu , G. and Şahin , S. 2004 . Color and texture development during microwave and conventional baking of breads . International Journal of Food Properties , 7 ( 2 ) : 201 – 213 . [CROSSREF]
- Association of Official Analytical Chemists . 1999 . Official Methods of Analysis of AOAC International , 16th , Gaithersburg, MD : AOAC . 5th Revision
- Luzuriaga , D.A. , Balaban , M.O. and Yeralan , S. 1997 . Analysis of visual quality attributes of white shrimp by using machine vision . Journal of Food Science , 62 ( 1 ) : 113 – 118 . 130
- SAS . 1998 . Statistical Analysis Systems. User's Guide: Statistics, Version 6.12 , Cary, NC : SAS Institute .
- McComber , D.R. , Osman , E.M. and Lohnes , R.A. 1988 . Factors related to potato mealiness . Journal of Food Science , 53 ( 5 ) : 1423 – 1426 .
- 26. Luzuriaga, D.A. Application of computer vision and electronic nose technologies for quality assessment of color and odor of shrimp and salmon. Ph.D. thesis, University of Florida, Gainesville, FL, 1999