Abstract
7S and 11S soy proteins were mixed with various salts to investigate the coagulating effects of cations and anions of the coagulants. When coagulants were added to mixture of 7S and 11S proteins (ratio of 7S:11S from 0:10 to 10:0) in such a way that the resultant mixture contained protein content of 4.0% (w/v) and coagulants at 0.008M, different states of curds were formed. Depending on the coagulants, the mixture would remain as liquid, form uniform curds or precipitate. Coagulants which result in the formation of precipitates in most protein ratios are defined as those with the strongest coagulating power. Based on the different states of curds, the coagulating powers of various salts were in the order of CaCl2 > MgCl2 > CaSO4 > MgSO4. Similar order was found by turbidity measurements. Coagulants with strong coagulating power were able to form uniform curds of firmer texture, having lower pH and higher L* values compared those with weaker coagulating power.
INTRODUCTION
Tofu or soybean curd is a gel-like food made by adding coagulants to soybean milk. The four basic types of coagulants that are used to make tofu are: (1) “nigari-type” or chloride-type coagulants such as magnesium chloride (MgCl2) and calcium chloride (CaCl2); (2) sulfate-type coagulants such as calcium sulfate (CaSO4) and magnesium sulfate (MgSO4); (3) Glucono delta lactone (GDL); and (4) acidic coagulants including citrus juices, vinegar, and lactic acid.[Citation1] Each of these coagulants will produce tofu of different flavor and texture.[Citation2,Citation3] In a previous study, various calcium salts, as well as some non-calcium compounds were tested at various concentrations on a fixed soybean variety for their suitability as coagulants. It was found that while calcium chloride, calcium acetate, calcium gluconate, calcium lactate, glucono-δ-lactone, and acetic acid coagulated the soy milk, calcium phosphate, calcium hydroxide, and calcium carbonate did not.[Citation4]
Protein is one of the major components in tofu and the two major proteins in soybean are 7S and 11S. These two proteins have different molecular weights, isoelectric points, thermal transition temperatures and gel-forming properties.[Citation5] Salts and proteins form coagulates when metal ions such as Ca2+or Mg2+ form bridges with the negatively charged protein. This cross-link is due to the electrostatic interactions between the cations and the proteins.[Citation3]
In our work, we investigated the gelation effects by various coagulants on soy protein mixtures made up of varying ratios of 7S:11S globulins. Different ratio of 7S and 11S proteins will form different extent of aggregations with coagulants. Different extent of aggregations will result in different types of curd formation. Based on these differences, we will be able to determine the coagulating power of the coagulants. In the past the effects of cations like Ca2+ and Mg2+ on aggregation were studied but only a few studies have been done on the effects of anions on aggregation. Lately, researchers have found that anions like chloride and citrate also play an important role in the protein aggregation process.[Citation6] Thus, further tests were carried out to investigate the coagulating effects of cations and anions of different salts.
MATERIALS AND METHODS
Isolation of 7S and 11S globulin
Defatted soybean flour was mixed with 15-fold volume of distilled water, and then pH was adjusted to 7.5 with 1 N NaOH. The water-extractable soybean protein was obtained by centrifugation (9000 g × 30 minutes) at 20°C. Sodium bisulfite (0.98 g/L) was added to the supernatant and the pH was adjusted to 6.4 with 1 N HCL, and the mixture was kept in an ice bath overnight. The following procedure was performed at 4°C. The insoluble 11S fraction was obtained by centrifugation at 6500 g for 20 minutes. The supernatant was adjusted to contain 0.25 M NaCl and pH of 5.0 with HCl. After 1 hour, the insoluble fraction was removed by centrifugation at 9000 g for 30 minutes. The supernatant was diluted 2-fold with ice water, adjusted to pH 4.8 with HCl, and then centrifuged again at 6500 g for 20 minutes. The 7S globulin was obtained as a sediment. The protein fraction obtained was rinsed with de-ionized water and centrifuged. Both 11S and 7S fractions were adjusted to pH 7.5 with NaOH, and then freeze-dried.[Citation8]
Protein Assay
The protein contents of soy isolates were determined by the Bradford dye assay.[Citation9] A calibration curve was prepared by using a series of standard solutions of bovine serum albumin. Absorbance was measured at 595 nm. The protein concentrations of 7S and 11S were determined by comparison with the calibration curve.
Preparation of Gels
Protein gels were prepared according to the method of Kohyama and Nishinari[Citation7] modified as follows. The 7S, 11S and mixtures of the two proteins at concentration of 4.35% (w/v) in aqueous solutions were heated for 10 minutes at 100°C and then were cooled in tap water (25°C) for 30 minutes to 25°C. Four coagulants were chosen and they were calcium chloride calcium sulfate, magnesium chloride and magnesium sulfate. The coagulant solution (0.1 M) was added to these protein solutions such that the resultant mixture contained a protein content of 4.0% (w/v) and a coagulant concentration of 0.008 M. These mixtures taken in a wide-mouthed glass bottle having a diameter of 5cm and a flat base were allowed to incubate at 60°C for 20 minutes. After which, they were cooled in tap water (25°C) for 60 minutes. The gels were then aged at room temperature (25°C) for 60 minutes before the analyses were carried out.
Instrumental Texture Profile Analyses (TPA)
Analysis of the force against time curve generated by compressing a gel was used to evaluate the instrumental textural properties. Samples of gel cylinders of 15 mm diameter and 10 mm height were cut using a cork borer. They were compressed twice to 30% deformation, at a test speed of 1.0 mm/s, using a 35 mm diameter probe in a Stable Micro Systems model TA.XT2i texture analyzer (Godalming, Surrey, UK). Three samples from each gel ratio were tested and the experiments were repeated twice with fresh gels (altogether 6 analyses per data point). The mean values for the Hardness (peak force of first compression) were calculated and noted.[Citation10]
Color Analysis
The color was analyzed using Minolta spectrophotometer model CM–3500d (Osaka, Japan). The measurements were replicated three times on each gel sample, and the experiment was repeated twice as before. The mean values for L* were calculated and noted.
pH of the Gel
Gel cylinders of 15 mm diameter and 10 mm height were placed on a 50 ml Maxi-Spin® centrifuge tube with a plain nylon membrane (4.5 μ m) in the middle position (Vivascience AG, Hanover, Germany). The tubes were centrifuged at 120 × g for 5 minutes. The water collected in the centrifuge tube was used to measure the pH of the gel. Three samples from each gel ratio were tested, and the experiments were repeated twice with fresh gels.
Turbidity Measurement
Turbidity due to aggregation was determined according to Molina and Wagner,[Citation6] modified as given below. Turbidity was measured as the Absorbance at 600 nm (A600) of a protein solution with added coagulant using Shimadzu UV–visible spectrophotometer model UV–1601PC (Tokyo, Japan). The absorbance of 1ml of 7S and 11S protein solutions at 0.5 mg/ml in a semi micro cuvette with successive addition of 10 μl of 1M of salt solution was measured. Readings were taken 1 minutes after each addition with agitation.
Statistical Analysis
Three replicates of duplicate samples per treatment (altogether 6 analyses per data point) were used for the analysis of texture, color, WHC, and pH. All data points represent the mean ± standard deviation. Comparison of the means of two samples was carried out by paired T-test with a 5% significance level.
RESULTS
Coagulating Power
To facilitate the discussion of the results, the following five descriptions of the protein mixtures with coagulants — “ppt”, “gel”, “soft”, “semi”, and “liq” were used and defined in . illustrates the relationship of various states of curds and their coagulating powers. A coagulant that readily form precipitates or aggregates with a protein solution was defined in this paper as one that had a good coagulating power while a coagulant that remains in solution when added to a protein solution was defines as one that had a poor coagulating power. Using this guideline, the coagulating power of coagulants for various states of curds were in the order of “ppt” > “gel” > “soft” > “semi” > “liq.”
Table 1 Descriptions of the protein mixtures with coagulants.
Different States of Curds
Different coagulants have different coagulating powers. shows that the coagulating power of the salts varied across 7S and 11S protein ratios. Salts often cause protein mixtures with higher proportions of 11S to aggregate more and form precipitates or gels, than protein ratios with higher proportion of 7S. Salts often cause the protein mixtures with high proportions of 7S to aggregate less and form mostly liquid states. Thus by varying the ratio of 7S and 11S protein, the coagulating power of different salts can be found by comparing the state of curds at various ratios of 7S and11S. By comparing the state of curdling at various ratios of 7S and11S, the coagulating power of the salts were found to be in the order CaCl2 > MgCl2 > CaSO4 > MgSO4 (). The coagulating power seems to be affected by both the cations as wells as the anions. From these results, it was deduced that at the same concentration, calcium salts influenced the coagulation more than magnesium salts and chloride salts influenced the coagulation more than the sulfate salts. shows that CaSO4can result in the formation of curds that are firm, with low pH and high L* value. Based on the earlier results, the coagulating power of CaSO4 was greater than that of MgSO4. This shows that sulfate salts having stronger coagulating power could give rise to protein gels with firmer texture, lower pH and higher L* value than those having weaker coagulating power.
Table 2 Coagulation results of protein mixtures (4%, w/v) after addition of various coagulants (0.008M).
Table 3 Physico chemical properties of protein mixture (4%, w/v) after coagulated by calcium sulphate and magnesium sulphate.
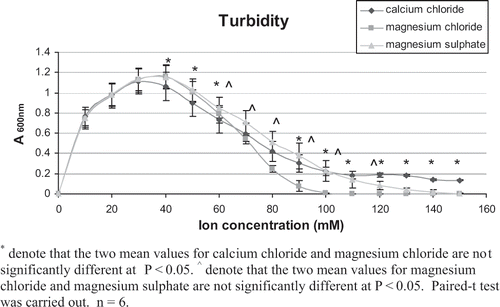
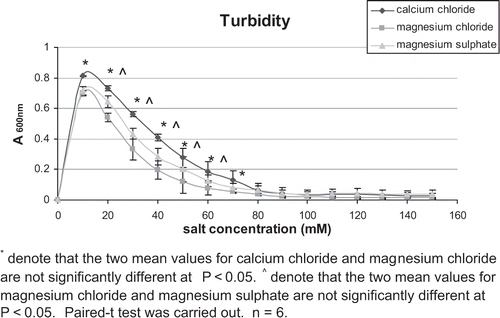
Turbidity Measurement
Further investigation was carried out to verify that for the same concentration of coagulant used, the coagulating power was affected by both the cations and anions. Researchers have found that turbidity (A600 nm) may be used to estimate the degree of protein aggregation.[Citation6] When CaCl2, MgCl2 and MgSO4 were added to 7S and 11S protein solutions, the turbidity of the mixture increased to a maximum and then decreased as the concentration of salts increased. This result was consistent with what was found earlier by other researchers.[Citation6,Citation11] When a small amount of salt was added to a proteins solution, increased in turbidity could be due to the electrostatic interactions between the cations and the proteins. These electrostatic interactions will cause the cations to form bridges with the proteins, which result in the formation of aggregates. This process was defined as the “aggregation effect”.[Citation6] The decreased in turbidity as the concentration of salts increased could be due to the decrease in electrostatic interaction between the cations and proteins caused by the anions. And this process was defined as the “dissociation effect”.[Citation6] The decrease in the electrostatic interaction will result in lower amount of cross linking and thus fewer aggregates. The turbidity measurement was carried out to investigate the effects of cations as well as anions when added to 7S and 11S proteins.
shows that the turbidity of calcium chloride from concentration of 40 mM to 150 mM was higher than that of magnesium chloride, except at 70 mM (p < 0.05). The only difference between these two salts were the cations, hence, the difference in the turbidity was due to the cations. The reason why the turbidity of calcium chloride with 11S protein solution was higher than that of magnesium chloride might be due to stronger electrostatic interaction between Ca2+ and protein than with Mg2+. According to the “aggregation effect”,[Citation6] the electrostatic interaction between cations and proteins can be used to explain turbidity of protein solution when a salt was added. In , the turbidity of magnesium chloride from concentration of 60 mM to 110 mM was lower than magnesium sulfate (p < 0.05). This showed that the Cl- ion caused the decrease of electrostatic interaction more than SO4 2- ion. Similarly, according to the “dissociation effect”,[Citation6] the decrease in the electrostatic interaction caused by anions can be used to explain turbidity of protein solutions when salt was added. A similar trend was observed for 7S protein with the various salts as shown in .
DISCUSSION
MgCl2 when added to protein solutions was found to form precipitates in fewer 7S:11S protein ratios than CaCl2. MgSO4 when added to 11S protein solutions formed curds that were softer, with higher pH and lower L* value than those formed with CaSO4. MgCl2 when added to both 11S and 7S protein solutions gave lower turbidity than when CaCl2 was added to the same solutions. Results suggest that the coagulating power of Mg2+ salts were weaker than that of Ca2+ salts. The coagulating power of the cations was proportional to their ability to form cross-links with proteins, resulting in the formation of aggregates.
Though it was not mentioned how the anions play a role in the gelation mechanism, it had been shown that anions have a strong effect on the water-holding capacity of the gels.[Citation12] It was found in this experiment that anions affect the coagulation of proteins. CaSO4 and MgSO4, when added to protein solutions, were found to form precipitates in fewer 7S:11S protein ratios than CaC12 and MgCl2. MgSO4 when added to both 7S and 11S protein solutions gave higher turbidity than MgCl2 added to the same 7S and 11S protein solutions. These results suggest that the chloride salts influenced the coagulation more than the sulfate salts. SO4 2− ion is larger in size than chloride ions.
CONCLUSION
The coagulating power of calcium and magnesium salts vary with the anions they posses, hence both cation and anion species are involved in the coagulation. From the results, it was deduced that calcium salts influenced the coagulation more than magnesium salts, and chloride salts influenced the coagulation more than the sulfate salts.
ACKNOWLEDGMENT
This research was funded by NUS Academic research grant #R–143–000–179–112.
REFERENCES
- Shurtleff , W. and Aoyagi , A. 2000 . “ Tofu and soymilk production ” . In The book of tofu , 3rd , Vol. II , 150 Lafayette, CA : Soyfood Center .
- de Man , J.M. , de Man , L. and Gupa , S. 1986 . Textural and microstructure of soybean curd (tofu) as affected by different coagulants . Food Microstructure , 5 : 83 – 89 .
- Karim , A.A. , Sulebele , G.A. , Azhar , M. E. and Ping , C.Y. 1998 . Effect of carrageenan on yield and properties of tofu . Food Chemistry , 66 : 159 – 165 . [CSA] [CROSSREF]
- Lu , J.Y. , Carter , E. and Chung , R.A. 1980 . Use of calcium salts for soybean curd preparation . Journal of Food Science , 45 : 32 – 34 . [CSA]
- Yuan , Y.J. , Velev , O.D. , Chen , K. , Campbell , B.E. , Kaller , E.W. and Lenoff , A.M. 2002 . Effect of pH and Ca2+-induced associations of soybean proteins . Journal of Agricultural Food Chemistry , 50 : 4953 – 4954 . [CSA] [CROSSREF]
- Molina , M.I. and Wagner , J.R. 1999 . The effects of diavalent cations in the presence of phosphate, cirate and chloride on the aggregation of soy protein isolate . Food Research International , 32 : 135 – 143 . [CSA] [CROSSREF]
- Kohyama , K. and Nishinari , K. 1993 . Rheological studies on the gelation process of soybean 7S and 11S proteins in the presence of glucono-δ-lactone . Journal of Agricultural Food Chemistry , 41 : 8 – 14 . [CSA] [CROSSREF]
- Nagano , T. , Hirotsuka , M. , Mori , H. , Kohyama , K. and Nishinar , K. 1992 . Dynamic viscoelastic study on the gelation of 7S globulin from soybeans . Journal of Agricultural Food Chemistry , 40 : 941 – 944 . [CSA] [CROSSREF]
- Boyer , R. 2000 . Modern Experimental Biochemistry , 3rd , 43 – 45 . San Francisco, CA : Addison Wesley Longman .
- Bourne , M.C. 2002 . Food Texture and viscosity: Concept and measurement , 2nd , 400 New York : Academic Press .
- Sorgentini , D.A. , Wagner , J.R. and Añón , M.C. 1995 . Effects of thermal treatment of soy protein isolate on the characteristics and structure-function relationship of soluble and insoluble fractions . Journal of Agricultural Food Chemistry , 43 : 2471 – 2479 . [CSA] [CROSSREF]
- Wang , H.L. and Hesseltine , C.W. 1982 . Coagulation Conditions in Tofu Processing . Process Biochemistry , 1 : 8 – 12 . [CSA]