Abstract
Kinetics of lycopene and visual color degradation of tomato peel was studied at selected temperatures (50–100°C). Models based on lycopene and Hunter (a × b) values of fractional conversion were applied to determine the kinetic parameters. The degradation of lycopene and Hunter color values adequately followed first order reaction model with R2 > 0.97. The temperature dependence of the rate constants was adequately modeled by the Arrhenius equation. The activation energies of lycopene and color parameters were 18.27 and 29.07 kJ/mol, respectively, indicating greater temperature sensitivity of visual color parameters. Correlation of lycopene content and Hunter (a × b) values showed that they can be used interchangeably with good accuracy.
INTRODUCTION
Tomato (Lycopersicon esculentum), a member of solanaceae family, is one of the most important crops around the world. Commercial processing of tomato utilizes only juice or pulp while the pomace consisting of seed, peel, and fibrous material is discarded.[Citation1] Pomace creates environmental pollution during the course of decomposition. The seeds can be isolated, dried, expressed, or solvent extracted to yield orangish-red edible oil,[Citation2,Citation3] while the meal can be used in feeds, fertilizers, or for the preparation of surfactants such as wetting agents, detergents, and soon.[Citation4] The peel is another important component of the tomato processing waste that could be utilized for extracting the red pigment by using the organic solvents.[Citation5–7]
The tomato peel contains many carotenoids but lycopene is the most important responsible for red color. It has a polyenic chromophore with 11 conjugated double bonds, which absorb and reflect light.[Citation8] Lycopene exhibits the highest antioxidant activity and singlet oxygen quenching ability of all dietary carotenoids.[Citation9–11] Use of carotenoids such as β-carotene and lycopene in the diet has been positively correlated with reduced cancer incidence.[Citation12–14] Lycopene is susceptible to isomerization and oxidation when exposed to light and heat that decrease its biological activity.[Citation15] It is generally presumed that isomerization resulting in conversion of the all-trans isomers to the cis-isomers. The present study was undertaken to access the thermal stability of the lycopene and the kinetics of its degradation in tomato peel isolated from the major tomato waste pomace.
MATERIALS AND METHODS
Sample Preparation
Tomato pomace was obtained from a tomato paste-manufacturing unit located in Amritsar, India. Peel was separated from pomace by a continuous flotation-cum-sedimentation system[Citation16] and dried at 50°C in a cabinet dryer. The dried sample was kept in an airtight container stored at −20°C temperature until used.
Chemical analysis
Moisture, ash, crude protein, crude fiber and crude fat content were determined according to AOAC.[Citation17] Carbohydrates were computed by subtracting all parameters from the sample.
Heat treatment
Triplicate dried samples (25 g) were placed in covered petridishes and heated in hot air oven adjusted to 50–100°C for 0–10 hrs.
Lycopene Estimation
Sample (2 g) was extracted using solvent (hexane: acetone: alcohol 2:1:1) containing 0.05% (w/v) butylated hydroxytoluene (BHT) till it become colorless. Cold distilled water (15 mL) was added and the suspension was agitated. The solution was allowed to stand for 15 minutes for separation of polar layer and non-polar layer containing lycopene. The total lycopene was obtained by measuring the absorbance of the lycopene solution at 503 nm using a UV visible spectrophotometer (Shimadzu Co., Ltd., Japan) and expressed as mg/100 g using extinction coefficient of 17.2 × 104 mol cm−1.[Citation6]
Color Measurement
Visual color was measured using a Hunter colorimeter (Hunter associates Laboratory, U.S.A.) in terms of L (lightness), a (redness and greenness), and b (yellowness and blueness). The instrument was calibrated with a standard white tile (L = 90.55, a = −0.71, b = 0.39). A glass petri dish containing the ground peel was placed above the light source, covered with a white plate and Hunter L, a, b values were recorded.
Kinetics of Lycopene and Visual Color Degradation
The kinetics of degradation of both pigment and visual color has been reported to follow first order reaction adequately.[Citation18–22] The first order kinetic model based on pigment concentration is
where, L = amount of lycopene content at time t (mg/100 g), Lo = initial amount of lycopene (mg/100 g), k1 = reaction rate constant (h−1), and t = heating time (h). Fractional conversion is a convenient variable and often used in place of concentration[Citation23] and has been reported to increase the accuracy of the calculation.[Citation22]
First order reaction in terms of the fractional conversion may be represented as
where f1 = (LO – L)/(LO – Lα), k2 = Reaction rate constant (h−1), and Lα = lycopene content at infinite time (mg/100 g). Different combinations of hunter values (a/b, a × b and b/a) were plotted against time to find out the variables that vary in linear fashion. Hunter (a × b) values was analyzed for color degradation following model 1 and 2 after replacing lycopene concentration with Hunter (a × b) values, respectively.
where f2 = [(ao × bo) – (a × b)/(ao × bo) – (aα × bα)], k3 and k4 = Reaction rate constant for EquationEqs. (3) and Equation(4), respectively (h−1), (ao × bo) = initial value, (a × b) = value at time (t), and (aα × bα) = value at infinite time.
Effect of Temperature on Lycopene and Color Degradation
The Arrhenius model was applied to describe the temperature dependence of lycopene degradation.
where ko = frequency factor (h−1), Ea = activation energy (kJ/mol), R = universal gas constant (8.314 J/(mol. K), and T = absolute temperature (K).
Relationship Between Lycopene and Visual Color
Change in visual color is a direct manifestation of change in pigment content. The Hunter (a × b) values were therefore correlated with pigment concentration of tomato peel heated for 10 hours at selected temperatures. The relationship between visual color and lycopene content:
where ka and kb are the coefficients.
Statistical Analysis
Regression analysis and Pearson's correlation coefficient was completed using the statistical package, Statistica, 5.0 version (Stat Soft Inc, USA).
RESULTS AND DISCUSSION
Chemical Characteristics of Tomato Peel
The chemical analysis of tomato peel isolated from pomace is shown in . The dried peel contained 5.74% moisture, 14.27% protein, 3.72% crude fat, 1.28% ash, 71.25% crude fiber, and 3.46% carbohydrate. Earlier studies have reported 6.69–10% moisture, 10–10.7% crude protein, 1.7–3.96% crude fat, 1.13–5.6% ash, 46.1–55.9% crude fibre, and 26.69% carbohydrate.[Citation1,Citation24,Citation25] Therefore, moisture, ash, and crude fat values of present study were in the range of previously reported values. The high protein content of the peel might be due to the differences in agronomical practices where as higher fiber content of peel might be due to hot break treatment that resulted in leaching of soluble material.
Table 1 Chemical composition of dried tomato peel (n = 3).
The lycopene content of the waste tomato peel was 1.75 mg/100 g peel. In the common variety of tomatoes, lycopene is found in the concentration of 3.1–7.7 mg/100 g.[Citation15] Lower lycopene content of the tomato peel might be due to the hot break process (85°C/ 5 min) that reduced the pigment content.
Lycopene Degradation
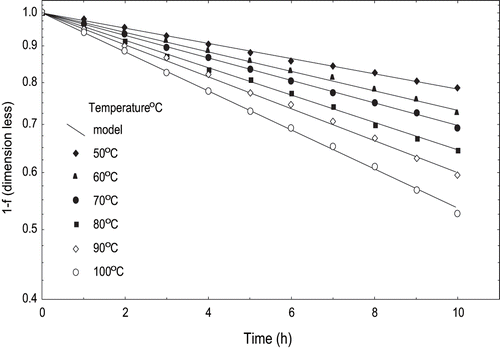
Heat treatment resulted in accelerated color degradation in tomato peel. At 50°C lycopene content decreased from 1.75 to 1.38 mg/100 g while at 100°C, it decreased from initial value to 0.92 mg/100 g after 10 hours. The lycopene content decreased in tomato pulp as the temperature was increased from 29 to 40°C.[Citation26] Decline in lycopene content was probably due to the destruction by heat and oxidation resulting in fragment products like acetone, methyl-heptenone, laevulinic aldehyde, and glyoxal.[Citation27,Citation28]
The fractional conversion factor was computed from the lycopene and was used in first order model to explain degradation.[Citation22,Citation29] The fractional conversion gave good results () and its reaction rate constants ‘k2’ increased from 0.024743 to 0.054076 h−1. Anguelova and Warthesen[Citation30] reported ‘k’ in the range of 0.02116–0.01823 h−1 for lycopene, 0.01487–0.01447 h−1 for β-carotene, and 0.00923–0.00751 h−1 for α-carotene at 37°C. The coefficients of determination (R2) were 0.9967–0.9988, and the standard error ranged from 0.004 to 0.008 (). Thus predicted values were in good agreement with experimental value. However, the values reported earlier were lower than the present values.
Table 2 The kinetics parameters of lycopene and visual color degradation (n = 3).
Color Loss
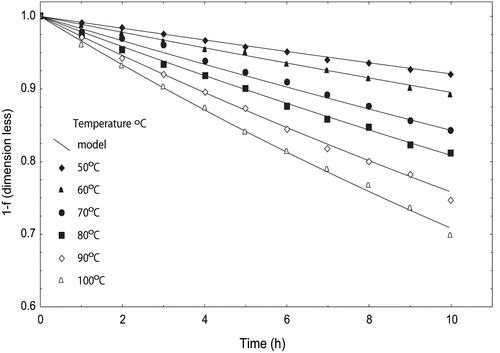
Various combinations of Hunter color values (a/b, a × b and b/a) were selected to describe the visual color change. The combination (a × b) was found to describe the first order degradation kinetics adequately. Previous studies have also stated that Hunter (a × b) values to describe the visual color degradation as it measures the color changes at the surface.[Citation22,Citation29] Hunter (a × b) decreased with increase in temperature (50°–100°C) and time (0–10 h). Fraction conversion factor was computed and plotted versus time (). The reaction rate constant ‘k4’ values varied from 0.008678 to 0.03368 h−1. Ahmed et al.,[Citation29] reported ‘k’ in the range of 0.131–0.378 h−1for visual color of papaya puree. The coefficients of determination were 0.9915–0.9962, and the standard errors were 0.001 to 0.007 ().
Temperature Dependence
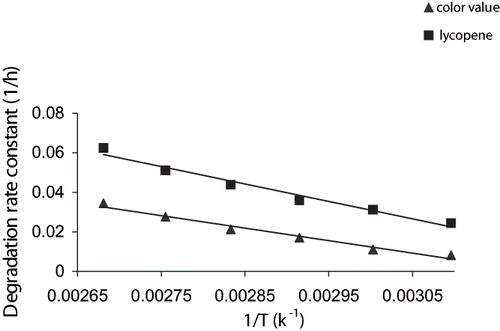
The dependence of rate constant for both lycopene (k2) and visual color (k4) on temperature followed the Arrhenius equation. An Arrhenius plot between the ln k versus 1/T followed a linear relationship with R2 more than 0.97 (). The activation energies obtained for lycopene and visual color were 18.27 and 29.07 kJ/mol, respectively. The lower activation energy of lycopene was probably because dry heating was used for lycopene extraction and decreased stability might occur.[Citation31] Higher activation energy signified greater heat sensitiveness of visual color at selected temperature. The activation energy for lycopene degradation in tomato pulp was ranged from 19.9 to 27.74 kJ/mol at −20.5 and 25°C.[Citation29] Nakagawa et al.,[Citation32] reported activation energy value from 30.80 to 37.08 kJ/mol for lycopene degradation in organic solvents heated at 100–130°C. Thus, the previous findings support the current study of lycopene degradation.
Relationship Between Visual Color and Lycopene Content
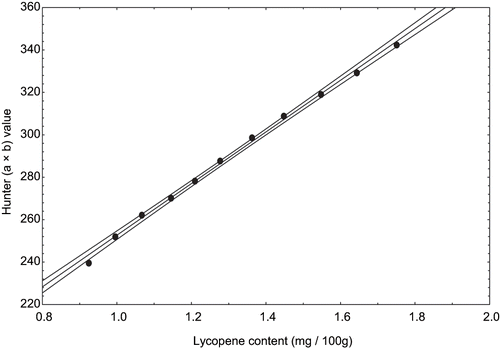
The plots of Hunter (a × b) and lycopene content of peel exhibited linear relationship with R2 0.995–0.998 and S.E was 0.55–1.85 (). Typical relationship between visual color and lycopene content of tomato peel heated at 100°C is shown in . Similarly, Ahmed et al.,[Citation29] found typical relationship between visual color and carotenoids of papaya puree at 70°C. There is a direct relationship between visual color and lycopene content in tomato peel. So visual color parameters may adequately be used in place of lycopene content in tomato peel.
Table 3 Regression parameters for the correlation of visual color and lycopene content (EquationEq. 6).
CONCLUSION
Lycopene content in tomato peel decreased during heating at selected temperatures resulting in decreased Hunter (a × b) values. The kinetics of lycopene and visual color degradation followed first order reaction model. Lycopene degradation and color loss followed Arrhenius model, while higher activation energy of visual color indicated to be more heat sensitive as compared to lycopene. Visual color and lycopene content can be used interchangeably with coefficient of determination more than 0.995.
REFERENCES
- Sogi , D.S. and Bawa , A.S. 1998 . Studies on Dehydration of Tomato Processing Waste . Indian Food Packer , 52 ( 2 ) : 26 – 29 .
- Sogi , D.S. , Kiran , J. and Bawa , A.S. 1999 . Characteristics and Utilization of Tomato Seed Oil from Tomato Processing Waste . J. Food Sci. Technol , 36 ( 3 ) : 248 – 249 .
- Sogi , D.S. , Bawa , A.S. and Garg , S.K. 2000 . Sedimentation System for Seed Separation from Tomato Processing Waste . J. Food Sci. Technol , 37 ( 5 ) : 539 – 541 .
- Lal , G. , Siddappa , G.S. and Tandon , G.L. 1986 . Preservation of Fruits and Vegetables , New Delhi : I. C. A. R .
- Chandler , L.A. and Schwartz. , S.J. 1987 . HPLC Separation of Cis-trans Carotene Isomers in Fresh and Processed Fruits and Vegetables . J. Food Sci , 52 : 669 – 672 .
- Sadler , G. , Davis , J. and Dezman , D. 1990 . Rapid Extraction of Lycopene and β-carotene from Reconstituted Tomato Paste and Pink Grape Fruit Homogenates . J. Food Sci , 55 ( 5 ) : 1460 – 1461 .
- Hakala , S.H. and Heinonen , I.M. 1994 . Chromatographic purification of natural lycopene . J. Agri. Food Chem , 42 : 1314 – 1316 .
- Davies , B.H. 1976 . “ Carotenoids ” . In Chemistry and Biochemistry of Plant Pigment , 2nd , Edited by: Goodwin , T.W. Vol. 2 , 38 – 165 . New York : Academic Press .
- DiMascio , P. , Kaiser , S. and Sies , H. 1989 . Lycopene as the Most Efficient Biological Caroteniod Singlet Oxygen Quencher . Arch. Biochem. Biophy , 274 : 532 – 538 .
- Tinkler , J.H. , Bohm , F. , Schalch , W. and Truscott , T.G. 1994 . Dietary Carotenoids Protect Human Cells from Damage . J. Photochem. Photobiol , 26 : 283 – 285 .
- Miller , N.J. , Sampson , J. , Candeias , L.P. , Bramley , P.M. and Rice-Evans , C.A. 1996 . Antioxidants Activity of Carotenes and Xaynthophylls . FEBS. Letter , 284 : 240 – 246 .
- Gerster , H. 1997 . The Potential Role of Lycopene for Human Health . J. Am. Col. Nutr , 16 : 109 – 126 .
- Giovannucci , E.L. , Ascherio , A. , Rimm , E.B. , Stampfer , M.J. , Colditz , G.A. and Willet , W.C. 1995 . Intake of Carotenoids and Retinol in Relationship to Risk of Prostate Cancer . J. National Cancer Inst , 87 : 1767 – 1776 .
- Rao , A.V. and Agarwal , S. 1998 . Effect of Diet and Smoking on Serum Lycopene and Lipid per Oxidation . Nutr. Res , 18 : 713 – 721 .
- Nguyen , M.L. and Schwartz. , S.J. 1999 . Lycopene: Chemical and Biological Properties . Food Technol , 53 ( 2 ) : 38 – 45 .
- Kaur , D. , Sogi , S.D. , Garg , S.K. and Bawa , A.S. 2005 . Flotation-cum-sedimentation System for Skin and Seed Separation from Tomato Pomace . J. Food. Eng , 71 ( 4 ) : 341 – 344 .
- Association of the Official Analytical Chemists . 1990 . Official Methods of Analysis of the Association of Official Analytical Chemists , 15th , Virgina, , USA : AOAC .
- Steet , J.A. and Tong , C.H. 1996 . Degradation Kinetics of Green Color and Chlorophyll in Peas by Colorimetery and HPLC . J. Food Sci , 61 : 924 – 927 . 931
- Weemaes , C.A. , Ooms , V. , Loey , A.M. and Hexdrix , M.E. 1999 . Kinetics of Chlorophyll Degradation and Color Loss in Heated Broccoli Juice . J. Agri. Food Chem , 47 : 2404 – 2409 .
- Ahmed , J. , Shivhare , U.S. and Raghavan , G.S.V. 2000 . Rheological Characteristics and Kinetics of Color Degradation of Green Chilli Puree . J. Food Eng , 44 : 239 – 244 .
- Ahmed , J. , Shivhare , U.S. and Singh , G. 2000 . Chlorophyll and Color of Green Chilli Puree as Affected by Mesh Size and Temperature . Int. J. Food Prop , 4 : 305 – 316 .
- Gunawan , M.I. and Barringer , S.A. 2000 . Green Color Degradation of Balanced Broccoli (Brassica oleracea) Due to Acid and Microbial Growth . J. Food Processing Preservation , 24 : 253 – 263 .
- Levenspiel , O. 1974 . Chemical Reaction Engineering; , New Delhi : Willey Eastern Publication .
- Tsatsaronis , G.C. and Boskou , D.G. 1975 . Amino Acid and Mineral Salt Content of Tomato Seed and Skin Waste . J. Sci. Food Agri , 26 : 421 – 423 .
- Lazos , E.S. and Kalathenos , P. 1988 . Composition of Tomato Processing Waste . Int. J. Food Sci. Technol , 23 : 649 – 652 .
- Akanbi , C.T. and Oludemi , F.O. 2004 . Effect of Processing and Packaging on Lycopene Content of Tomato Products . Int. J. Food Prop , 7 : 139 – 152 .
- Cole , E.R. and Kapur , N.S. 1957 . The Stability of Lycopene I. Degradation by Oxygen . J. Sci. Food Agri , 8 : 360 – 365 .
- Cole , E.R. and Kapur , N.S. 1957 . The Stability of Lycopene II. Oxidation During Heating of Tomato Pulps . J. Sci. Food Agri , 8 : 366 – 368 .
- Ahmed , J. , Shivhare , U.S. and Sandhu , K.S. 2002 . Thermal Degradation Kinetics of Carotenoids and Visual Color of Papaya Puree . J. Food Sci , 67 ( 7 ) : 2692 – 2695 .
- Anguelova , T. and Warthesen , J. 2000 . Degradation of Lycopene, α-carotene and β-carotene During Lipid Peroxidation . J. Food Sci , 65 : 71 – 75 .
- Lee , M.T. and Chen , B.H. 2002 . Stability of Lycopene During Heating and Illumination in a Model System . Food Chem , 78 : 425 – 432 .
- Nakagawa , H. , Kushida , T. , Ogura , N. and Takehana , H. 1971 . Studies on Color of Tomatoes. V. Thermal Degradation of Lycopene and β-carotene. . Nihon Shokuhin Kogyo Gakkai , 18 : 259 – 263 .