Abstract
The emulsifying properties of bovine globin, extracted by the acidified acetone method, were studied at different pH values and after several times of peptic-hydrolysis. The emulsifying capacity, the emulsifying activity index, and the emulsion stability were determined. In general, the peptic hydrolysis was disadvantageous for the emulsifying properties of globin, since it only increased the emulsifying capacity (at pH = 3.0, after 30 and 60 minutes hydrolysis; at pH = 4.0, after 10 to 60 minutes hydrolysis), and the emulsifying activity index (at pH = 5.0 after 60 minutes hydrolysis; at pH = 6.0 after 15, 30, and 60 minutes hydrolysis). This treatment reduced the emulsion stability in almost all conditions studied.
INTRODUCTION
The interest in replacing artificial ingredients with natural ones has been increasing lately due in part to the higher understanding of consumers. In this way, the use of proteins as functional agents, mainly low-cost proteins, such as those from bovine blood, could be very favorable to the food industries. The modification of protein size by enzymatic hydrolysis has been used to improve functional properties. However, this result depends on the peptide size, and normally peptides containing more than 20 amino acid residues are needed in order to produce this advantageous effect.[Citation1,Citation2] The more common method involves a partial hydrolysis employing highly specific proteases in order to control the hydrolysis degree and, therefore, the size of produced peptides.[Citation1,Citation3] It is worth stating that no report was found in the literature concerning the effect of the enzymatic treatment on the functional properties of bovine globin.
Our group studied the emulsifying properties of bovine casein aiming to use it as emulsifier in food industries.[Citation4] Considering the economical advantage of the replacement of this protein by blood ones, a factor that may be taken in consideration especially in developing countries, where milk proteins are imported and, consequently, are much more expensive than blood ones, we first decided to study the functional properties of plasma.[Citation5] Blood plasma is produced from simple centrifugation of whole blood and shows no problems concerning the development of undesirable flavor or color observed with red blood cell concentrate.[Citation6,Citation7] In previous studies, we evaluated the effect of tryptic hydrolysis on the functional properties of proteins. In this article, our interest in focused on the study of the emulsifying properties of another blood ingredient, bovine globin, extracted by the acidified acetone method, evaluating the effect of pH. We also evaluated the influence of another enzyme, pepsin, on these properties.
MATERIAL AND METHODS
Separation of Red Cells from Bovine Blood
The animals were killed in a slaughter house under federal inspection, and the blood was collected directly from the carcass in vials containing the anticoagulant (2 mL of a 10% EDTA solution/100 mL of total blood), and the contact between the collecting recipient and the animal skin was avoided. The blood was immediately taken to the laboratory, where it was centrifuged (Jouan centrifuge, Br4i model) at 1000 g for 15 minutes, in order to separate the red cells (haemaceas). These cells were stored under refrigeration until the moment of the bovine globin extraction (maximum of 24 hours).
Extraction of Bovine Globin
The acidified acetone method[Citation8] was used to extract globin. The haemaceas obtained as described above were haemolysed by the addition of distilled water at the proportion of 1:1 and the pH adjusted to 4.0 with an ascorbic acid solution (2 g /100 mL). Afterwards, the air was bubbled for an hour to oxidize the hemoglobin in colemetha-hemoglobin, the haem group being removed and the globin precipitated with the addition of an acidified acetone solution (in HCl, 99:1, v/v) at a proportion of 1:4. Next, it was filtered through a paper filter and the globin retained was washed in an ether-ethanol solution (3:1) and stored in portions of approximately 100 g in glass bottles covered with paper filters containing many small holes. After drying by forced ventilation at room temperature, the globin portions were sieved (24 mesh), weighed, transferred to glass bottles and frozen at −18°C until use.
Peptic Hydrolysis of Bovine Globin
The method described by Lacroix et al.,[Citation9] with some modifications, was used. The globin was solubilized in a buffer solution (0.02 mol.L−1 KCl-HCl), pH = 1.9, to a protein concentration of 1 mg/mL. Then, the pepsin (from pork, Sigma Chemical Co., St. Louis, Mo, USA), solubilized in the same buffer, was added to obtain a 1:100 (v/v) enzyme: substrate ratio. The mixture was held in a water bath at 37°C, with stirring for 5, 10, 15, 30, and 60 minutes producing the P1, P2, P3, P4, and P5 hydrolysates, respectively. In all assays, the hydrolytic reaction was stopped by increasing the pH to 8.0, using ammonia hydroxide. The hydrolysates were then freeze-dryed (Freezone 4.5 model, Labconco, Kansas City, MI, EUA) and stored at −18°C until the moment of use.
Sample Preparation
The globin and its hydrolysates were solubilized in a buffer solution (0.02 mol/L sodium phosphate and 0.01 mol/L citric acid), at pH varying from 3.0 to 8.0, in a concentration of 1 mg of protein/mL of solution. After 30 minutes in a water bath at 35°C, the solutions were centifuged (Jouan, Br4i model, France) at 6500 g for 10 minutes and then filtered (through paper filter Quanty, JP42 model, Curitiba, PR, Brazil). The filtrates were stored at −18°C until the moment of use.
Determination of Emulsifying Capacity (EC)
For determining the emulsifying capacity, the method of Vuillemard et al.[Citation10] with modification by our group[Citation4,Citation11] was used. Fifty milliliters of protein solution were homogenized using a mixer (Fisher, mod. 14057–5) at the highest speed. Corn oil (Mazola) was added continuously during the emulsification process from a funnel into the mixture at a rate of 25 mL/min. During emulsification, the temperature was maintained at 25 ± 3°C by immersing the reaction vessel in an ice bath. The emulsifying capacity was determined by the interruption of the electric current detected by a 120 V lamp. The EC was calculated using EquationEq. (1).
where EO and BO are the amount of emulsified oil in the sample and in the blank, respectively. Blank is the buffer solution with no emulsifying agent.
Determination of Emulsifying Activity Index (EAI)
The method of Pearce and Kinsella,[Citation12] with the modifications described by our group,[Citation4,Citation11] was used for determining the EAI. For preparing the emulsions, a volume of 30 mL of the protein solution and 10 mL of corn oil were shaken together in the same mixer cited above, at the highest speed for one minute. The temperature was maintained at 20°C. Aliquots (1 mL) of the emulsion were diluted (1/100) in a solution containing 0.1% SDS (sodium docecyl sulfate) and 0.1 M NaCl, homogeneized and the absorbance was read at 550 nm (spectrophotometer CECIL, CE 2041 model, UK). The EAI values were calculated using EquationEq. (2) proposed by Cameron et al.[Citation13]
where T is turbidity, (is the volume fraction of the oil, and C is the initial protein concentration (1 mg/mL). The turbidity was calculated by multiplying the absorbance by 2.203 and by the dilution factor (100) and then dividing this result by the optical path length of the cuvette (0.01 m).
Determination of the Emulsion Stability (ES)
The method of Chobert et al.,[Citation14] as modified by our group,[Citation4,Citation11] was used for determining the emulsion stability. The stock emulsions prepared above were held at 20°C for 24 hours. After stirring, aliquots were diluted in 0.1% SDS and turbidity was measured as described above (EAI, 20°C). The 24 hour old emulsions were then heated at 80°C for 30 minutes. After the aliquots were cooled to room temperature and stirred, the turbidity was again measured as described above (EAI, 80°C). The (EAI% was calculated by the EquationEq. (3).
where EAImax is the maximum value obtained just after emulsion formation, and EAImin is the lowest value obtained for the aliquots after 24 hour-storage and 80°C heating. ES values were calculated using EquationEq. (4).
Statistical Analysis
All experiments were replicated three times. Analysis of variance was performed for the determination of optimal protein concentration, in order to investigate the presence of significant effects among treatments (P < 0.05). The Duncan test was applied to establish the differences among means.[Citation14] The effect of pH and hydrolysis time on EC, EAI, and ES was analysed using split-plot design (in which the main plots were hydrolysis times and the values of pH the subplots). Analysis of variance for each property (P < 0.05) and then the Duncan test was applied to compare means.[Citation15]
RESULTS AND DISCUSSION
Effect of pH and Peptic Hydrolysis on the Emulsifying Capacity
As shown in , the EC increased until reach a maximum at pH = 5.0. After this point, the EC decreased and at pH = 6.0 to 8.0, where globin shows the lowest solubility,[Citation16] the EC was zero. According to Mangino,[Citation17] the EC measures the capacity of proteins to migrate to the water/oil interface and for doing that proteins must be sufficiently soluble. The pH affects protein charge and hence its solubility, which is minimum in the region of pI. Thus, at pH values close to pI, proteins show low emulsifying properties.[Citation18,Citation19] Working with the same type of globin, but using a different method for measuring the EC (sudden fall of the viscosity), Crenwelge et al.[Citation20] reported a maximum and a minimum value for EC at pH = 3.0 and pH = 8.0, respectively.
Figure 1 Effect of pepsinic hydrolysis and pH on the emulsifying capacity (g of oil/mg of protein) of bovine globin. P1, P2, P3, P4, P5: globin hydrolysates with hydrolysis time of 5, 10, 15, 30, and 60 minutes, respectively. (Each value represents the mean of triple determinations. ±Standard error vertical bars).
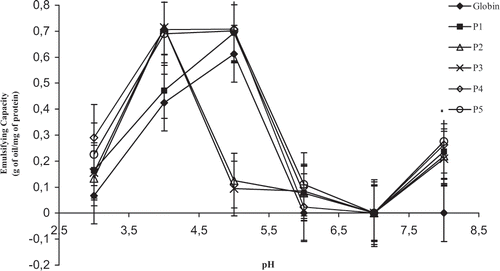
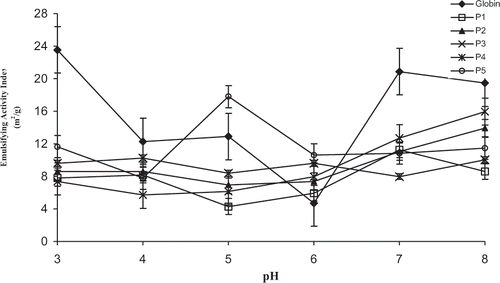
In two previous studies of our group, working with other protein sources in the same pH conditions used here, different results from those of globin were found. Thus, for the commercial bovine casein, we showed that even in the pH region of the lowest solubility (pH = 3.0 to 5.0), a moderate emulsifying capacity was observed.[Citation4] In case of bovine plasma, the pH had little effect on the EC which remained high and almost constant at all pH studied.[Citation5]
Regarding the effect of peptic hydrolysis, one can see in that it was advantageous for the EC at pH = 3.0 for 30 and 60 minutes, as well as at pH = 4.0 for almost all reaction times studied, since this favorable effect was not observed only after 5 minutes of hydrolysis. Some researchers have been considering the beneficial effect of moderate enzymatic hydrolysis on emulsifying properties of proteins and stated that besides improving protein solubility, it can also increase the number of contact points between proteins and the water/oil interface favoring the emulsion formation. The enzymes break up the native conformation of proteins increasing the exposure of hydrophobic groups which react with lipids during the emulsion formation.[Citation14,Citation21,Citation22] In fact, the results of the present work add new data to the literature showing that the beneficial effect of hydrolysis on the emulsifying properties depend not only on the extension of the reaction but also on the conditions employed (pH, duration of the reaction).
Effect of pH and Peptic Hydrolysis on the Emulsifying Activity Index
The maximum values for the EAI of globin were reached at pH = 3.0, and even at pH situated in its pI region (7.0 and 8.0), contrarily to the EC (). Commercial bovine casein behaved similarly since it showed high EAI values in its pI region (pH = 3.0 to 5.0), as shown before by our group.[Citation4] Considering that the EAI measures the protein capacity to stay at the water/oil interface after the emulsion formation,[Citation17] the low solubility could hinder the protein to pass to the aqueous phase and hence to improve its attachment to the interface water/oil, leading to an increase in the EAI. This was confirmed by our results with bovine plasma, where the solubility remained high (between 70% and 80%) and almost unchangeable at all pH values (from pH = 3.0 to 8.0) and the EAI was low in the same range of pH.[Citation5] Contrarly to our results, a globin extracted by a different method used here, employing carboxymethylcellulose, showed an EAI minimum at pH = 7.0. According to Autio et al.,[Citation6] the functional properties of proteins depend not only on their sources but also on the extraction conditions. In general, the peptic hydrolysis was disadvantageous for the EAI of globin () This enzymatic treatment only increased the EAI at pH = 5.0 after 60 minutes as well as at pH = 6.0 after 15, 30, and 60 minutes.
Effect of pH and Peptic Hydrolysis on the Emulsion Stability
No effect of pH on the emulsion stability (ES) was observed up to pH = 5.0, since the ES remained unchanged (). Next, the ES increased sharply and reached the maximum at pH = 6.0, close to the pI of the protein. Then, The ES decreased until the minimum value at pH = 7.0 and pH = 8.0, region where the solubility of this protein shows the lowest value. Also, we showed that casein had a high value for ES at pH = 4.0 near to its pI, but the maximum value was reached at pH = 7.0, where this protein is highly soluble.[Citation4] Concerning plasma, the pH had no effect on ES in the pH range studied (pH = 3.0 to 8.0).[Citation5]
Figure 3 Effect of pepsinic hydrolysis and pH on the emulsion stability (g/m2) of bovine globin. P1, P2, P3, P4, P5: globin hydrolysates with hydrolysis time of 5, 10, 15, 30, and 60 minutes, respectively. Each value represents the mean of triple determinations.
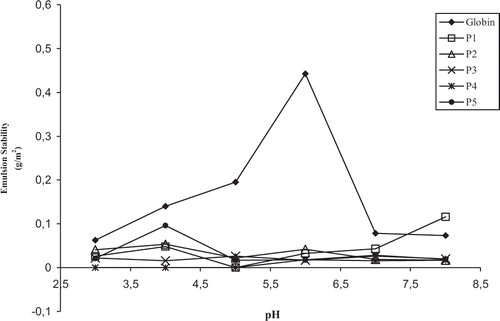
The effect of pH on the stability of emulsions shows a certain complexity. At pH values close to the pI, the proteins are able to form more firm and viscous interfacial films which are beneficial to the stability.[Citation17,Citation19] Das and Kinsella[Citation21] reported that the results coming from different laboratories concerning the effect of pH on emulsion stability are contradictory. Some groups mention that the maximum value for ES was reached at the pI of the protein while others described the opposite. The absence of standardization of the methods used as well as the use of different protein concentrations which give rise to interfacial protein films with varied properties and forces, can partly explain this contradiction. As shown in , the peptic hydrolysis had no beneficial effect on the ES of globin, in all pH region and reaction times studied.
CONCLUSIONS
The emulsifying properties of globin was affected by pH and peptic hydrolysis. The EC was improved in pH region far from the pI of this protein, while the best results for EAI and ES were reached in pH region close to its pI. The action of the pepsin produced varied effects on these properties, depending on the pH and on the hydrolysis time, but in general it was disadvantageous.
ACKNOWLEDGMENTS
The authors thank CNPq and FAPEMIG, in Brazil, for the support of this work.
References
- Brekke , C.J. and Smith , D.M. 1985 . Enzymatic modification of the structure and functional properties of mechanically deboned fowl proteins . J. Agric. Food Chem. , 33 : 631 – 637 . [CSA]
- Kinsella , J.E. 1984 . Milk proteins: physicochemical and functional properties . Crit. Rev. Food Sci. Nutr. , 21 : 197 – 262 . [INFOTRIEVE] [CSA]
- Ma , C.Y. and Wood , D.F. 1987 . Functional properties of oat proteins modified by acylation, trypsin hydrolysis or linoleate treatment . JAOCS , 64 : 1726 – 1731 . [CSA]
- Duarte , A.J. , Carreira , R.L. , Junqueira , R.G. , Coelho , J.V. and Silvestre , M.P.C. 1998 . Propriedades emulsionantes e solubilidade da caseína bovina e de seus hidrolisados trípticos: 1. Efeito do pH e do tempo de hidrólise . Ciênc. Tecnol. Aliment , 18 : 295 – 302 . [CSA]
- Silva , V.D.M. and Silvestre , M.P.C. 2003 . Functional properties of bovine globin intended for use as a functional ingredient in human food . Food Sci. Technol. , 36 : 709 – 718 . [CSA]
- Autio , K. , Lyytikäinen , H. , Mälkki , Y. and Kanko , S. 1985 . Penetration studies of blood globin gels . J. Food Sci. , 50 : 615 – 617 . [CSA]
- Ockerman , H.W. and Hansen , C.L. 1994 . Industrialización de subproductos de origem animal , 244 – 263 . Zaragoza, , Spain : Acribia .
- Tybor , P.T. , Dill , C.W. and Landmann , W.A. 1975 . Functional properties of proteins isolated from bovine blood by a continuous pilot process . J. Food Sci. , 40 : 155 – 159 . [CSA]
- Lacroix , M. and Amiot , G.J. 1983 . Brisson, Hydrolysis and Ultrafiltration Treatment to Improve the Value of Rapessed proteins . J. Food Sci. , 48 : 1644 – 1645 . [CSA]
- Vuillemard , J.C. , Gauthier , S.F. , Richard , J.P. and Paquin , P. 1990 . Development of a method for measurement of the maximum value of emulsifying capacity of milk proteins . Milchwissenschaft , 45 : 572 – 575 . [CSA]
- Duarte , A.J. , Carreira , R.L. , Junqueira , R.G. , Coelho , J.V. and Silvestre , M.P.C. 1998 . Propriedades emulsionantes e solubilidade da caseína bovina: 2. Efeito da adição de NaCl, Ciênc . Tecnol. Aliment. , 18 : 303 – 308 . [CSA]
- Pearce , K.N. and Kinsella , J.E. 1978 . Emulsifying properties of proteins: evaluation of a turbidimetric technique . J. Agric. Food Chem. , 26 : 716 – 723 . [CSA] [CROSSREF]
- Cameron , D.R. , Weber , M.E. , Idziak , E.S. , Neufeld , R.J. and Cooper , D.G. 1991 . Determination of interfacial areas in emulsions using turbidimetric and droplet size data: correction of the formula for emulsifying activity index . J. Agric. Food Chem. , 39 : 655 – 659 . [CSA] [CROSSREF]
- Chobert , J.M. , Bertrand-Harb , C. and Nicolas , M.G. 1988 . Solubility and emulsifying properties of caseins and whey proteins modified enzymatically by trypsin . J. Agric. Food Chem , 36 : 883 – 892 . [CSA] [CROSSREF]
- Pimentel-Gomes , F. 1990 . Curso de estatística experimental , 13 , 468 Piracicaba : Nobel .
- Kuppevelt , A.V. , Levin , G. and Reizenstein , P. 1976 . Small scale production and net protein utilization of globin. Globin fortification of cereals . Nutr. Rep. Inter. , 13 : 429 – 443 . [CSA]
- Mangino , M.E. 1994 . “ Protein interactions in emulsions: protein-lipid interactions ” . In Protein functionality in food system , Edited by: Hettiarachchy , N.S. and Ziegler , G.R. 147 – 180 . New York : Marcel Dekker .
- Cheftel , J.C. , Cuq , J.-L. and Lorient , D. 1989 . Proteinas alimentarias , 179 – 220 . Zaragoza : Acribia .
- McClements , D.J. 1999 . Food emulsions: principles, practice and technique , New York : CRC Press LLC .
- Crenwelge , D.D. , Dill , C.W. , Tybor , P.T. and Landmann , W.A. 1974 . A comparison of the emulsification capacities of some protein concentrates . J. Food Sci. , 39 : 175 – 177 . [CSA]
- Das , K.P. and Kinsella , J.E. 1990 . Stability of food emulsions: physicochemical role of protein and non protein emulsifiers . Adv. Food Nutr. Res. , 34 : 81 – 129 . [CSA]
- Gauthier , S.F. , Paquin , P. , Pouliot , Y. and Turgeon , S. 1993 . Surface activity and related functional properties of peptides obtained from whey proteins . J. Dairy Sci. , 76 : 321 – 328 . [CSA]