Abstract
Hygroscopy of hybrid rice affects storage, handling, and processing. The thermodynamic relationship upon which the estimate of isosteric heat is based is the Clausius-Clayperon equation. The net isosteric heat of sorption was determined from the slope of the ln(rh) versus 1/T lines at a constant moisture content. Net isosteric heat of desorption was found higher than that for the adsorption within a moisture content range of 12–20% for all of the rice kernels. The net isosteric heats of desorption and adsorption were higher for hybrid rough rice kernels followed by brown rice and milled rice kernels, respectively. An empirical equation was fitted to describe the net isosteric heats of hybrid rice kernels as a function of moisture content and was found adequate to predict the net isosteric heats. The intercept K was also found to be a function of moisture content and the model predicted the K value well (r2 = 0.85 for adsorption and r2 = 0.92 for desorption). This set of two equations would be useful in the simulation of hybrid rice during storage.
INTRODUCTION
Hygroscopy of cereal grains affects storage, handling and processing. An isostere equation gives the variation of relative humidity with temperature for a given moisture content. A knowledge of the relative humidity of air in equilibrium with the moisture content in stored grain is essential in the computation of humidity during the simulation of stored grain, and this can be estimated from the isosteric heat equation together with the parameters.[Citation1,Citation2] A knowledge of the equations of isosteric heat of adsorption/desorption together with the parameters for cereal grains is also essential in the modeling and simulation of drying of cereal grains.[Citation3]
The net isosteric heat of sorption is defined as the difference between total heat of sorption of water for the material, and the heat of vaporization of pure water. Total isosteric heat of sorption is the total heat supply for drying of a material. As a differential molar quantity, isosteric heat of sorption determines the temperature dependence of water activity of a biological material. The heat of vaporization of sorbed water may increase to values well above the vaporization of pure water as food is dehydrated to low moisture levels.[Citation4] The extent of material moisture content at which the net isosteric heat of sorption approaches the latent heat of vaporization of water is often considered as an indication of the amount of “bound water” existing in the food.[Citation5,Citation6] Two methods are used to estimate the sorption isosteric heat:[Citation7–10] (1) application of Clausius-Clayperon equation to isotherms at different temperatures — the most widely used method; and (2) calorimetric techniques and Riedel equation – based on thermal analysis, thermo-gravimetry, and DSC. Note that there is a good agreement between the two methods.[Citation9,Citation10] Theoretical prediction of the heat of sorption is not possible, due to the complexity of the physical and chemical structure of the foodstuff.[Citation11]
Many studies[Citation11–18] have been conducted on the heat of sorption of fruits, vegetables, medicinal plants, protein foods, and cereals. Most of the studies used either adsorption or desorption data to determine the heat of sorption. Oztekin et al.[Citation18] reported that total isosteric heats of grains determined from the mean value of adsorption and desorption isotherms were dramatically lower than those of special grain cultivars. This suggests that adsorption and desorption net isosteric heat of hybrid rice should be determined separately to adequately describe the real sorption characteristics. Furthermore, experimental determination of these isotherms is necessary to obtain the most accurate data on isosteric heat of sorption.[Citation18]
Tsami et al.[Citation19] proposed an empirical exponential relationship between the net isosteric heat of sorption and material moisture content for some fruits. Hossain et al.[Citation17] found isosteric heat to be a power function of equilibrium moisture content for pineapple. Although an equation for isosteric heat of wheat grain and some common seeds together with the parameters has been reported,[Citation20,Citation21] negligible information is available on the isosteric heat of hybrid rice kernels. Therefore, this study was carried out to estimate this important thermodynamic property together with the parameters for different forms of hybrid rice kernels.
MATERIALS AND METHODS
Rice kernels of hybrid variety (long grain) of brand Sonar Bangla-1 (CNSGC-6) originated from China (Hi-tech Seed Co. Ltd., Hejia Group, Sichuan) were used in this study. Samples were collected from a seed lot harvested in 2003 from a commercial seed shop in Bangladesh and stored at 5°C for five months in a refrigerated warehouse. Initial moisture content of the rough rice was about 14% (d.b.). Rough rice, brown rice, and milled rice were used for the determination of heat of sorption.
Brown rice was obtained by dehusking the raw and parboiled rough rice in an impeller-type husker (FCS type, Otake Co., Oharu, Japan) in a single pass. Brown rice was milled in a vertical friction-type laboratory milling machine (VP31T, Yamamoto Co., Tendu, Japan) to get milled rice. Degree of milling was kept at about 9%. Samples with higher moisture contents were prepared by wetting the samples. This was done by adding a calculated amount of distilled water. All the samples were then sealed in plastic packets and kept in a refrigerated warehouse at 5°C for 2 weeks so that the moisture content inside the kernel becomes uniform. Before starting the experiment, samples were kept overnight in room temperature in the sealed packets. Sample size was about 3 g.
Moisture content of the rice kernels was determined using ISO R712 standard method.[Citation22] About 5 g of ground sample was dried at 130°C for 2 hours in an air oven. Weight loss or gain of the samples was measured by an electronic balance with an accuracy of 0.0001 g (model: LIBROR AEG-220G, Shimadzu Corp., Japan). Saturated solution of different salts was used to create relative humidity in a range of 30% to 97%. The salt solutions used in the study and their percent relative humidities at various temperatures are shown in .[Citation23] The salt was of research grade (Wako Pure Chemical Industries Ltd., Japan).
Table 1 Percent relative humidity of the saturated salt solutions used in this study at various temperatures
Data on equilibrium moisture contents were obtained from the isotherms determined experimentally at three temperatures: 30°C, 40°C, and 50°C. The details of the experiment were reported by Haque et al.[Citation24] For this study an accelerated method was used,[Citation25] and a glass desiccator equipped with a small fan inside it to agitate the air inside it served as a closed chamber. The desiccators were placed inside the incubator (Eyela, SLI-600N, Tokyo Rikakikai Co. Ltd., Japan) at controlled temperatures. Temperature inside the incubator was checked by a thermometer.
Theory
The most commonly used procedure for calculation of isosteric sorption heat — the Clausius-Clayperon equation[Citation12,Citation26–29] explains the heat of sorption phenomena as follows:
The value of net isosteric sorption heat (Qst ) is calculated from the slope of EquationEq. (2).
Several researchers reported the isosteric heat of sorption as an empirical function of moisture content.[Citation17,Citation18,Citation30] The net isosteric heat of sorption of hybrid rice kernels as a function of equilibrium moisture content in the following forms were considered:
and
Where, Me is the equilibrium moisture content, %(d.b.), m and n are the two parameters. A statistical package SPSS version 10 was used to fit the EquationEqs. (4,Equation5) to the observed data by direct least square technique. To evaluate the goodness-of-fit of the equation to the observed data, three quantitative measures were used: Coefficient of determination;[Citation31,Citation32] the mean relative percentage deviation modulus;[Citation31,Citation33,Citation34] and the standard error of estimate.[Citation31,Citation32,Citation35] Mean relative percentage deviation gives an idea of the mean departure of the observed data from the predicted data. Therefore, the smaller the value of mean relative deviation, the better the goodness-of-fit. It is generally assumed that a good fit is obtained when P < 10%.[Citation11] The standard error of estimate is a measure of the amount of error in the prediction.
RESULTS AND DISCUSSION
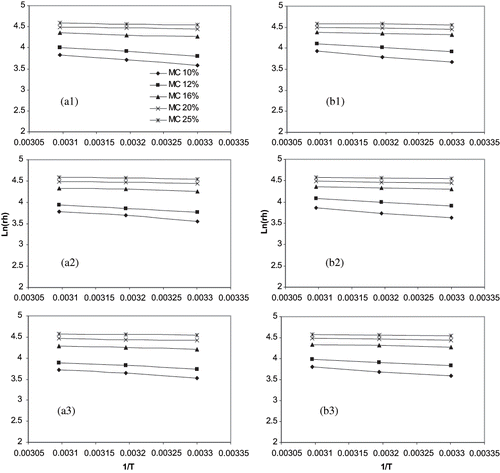
The data used in this study to estimate the isosteric heats of adsorption and desorption together with the parameters are taken from Haque et al.[Citation24] These are shown in and the isotherms follow type II classification.[Citation36] The slopes of the EquationEq. (2) were calculated by the least squares fit for the lines of ln(rh) versus 1/T . The plots of ln(rh) versus reciprocal of temperature (in Kelvin) for a constant moisture content for hybrid rough, brown, and milled rice kernels at desorption and adsorption are shown in . These isosteres have linear negative slopes and reflect the temperature effect on the sorption isotherms.
Table 2 Equilibrium moisture contents (%, d.b.) of hybrid rice kernels under various experimental conditions
Figure 1 ln(rh) vs. 1/T for sorption for hybrid rice kernel. (a) desorption and (b) adsorption; (1) rough rice, (2) brown rice, (3) milled rice.
The net isosteric heats of adsorption and desorption of hybrid rice kernels: (a) rough, (b) brown, and (c) milled rice, are presented in . It is evident that there exists a marked increase in isosteric heats at the lower moisture contents. Trends of the observed net isosteric heat for hybrid rice kernels for different moisture contents are depicted in . Above 19% (d.b.), the change is not very important and almost constant indicating the presence of free water. But below this value, the heat of sorption increased quickly indicating the presence of strongly bound water to the material by the existence of polar groups on the surface of the material. Foodstuffs are complex in structure and the main water sorbing polymers in food (e.g. starch, cellulose, etc.) exhibit water binding sites of varying degrees of activity. Initially, desorption occurs at the least active sites requiring low interactive energies in addition to the latent heat of vaporization of pure water. As desorption continues active sites require a higher interactive energies.
Table 3 Net isosteric heats of sorption of different forms of hybrid rice kernel
Figure 2 Comparison of adsorption and desorption net isosteric heat values for hybrid rice kernels. N.B. m- milled rice, b- brown rice, r- rough rice, and ads- adsorption, des- desorption.
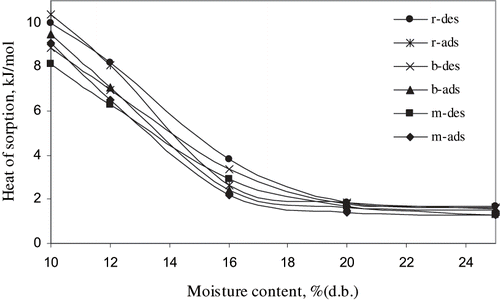
The net isosteric heats of desorption was found higher than those of adsorption within the moisture content range of 20–12% (d.b.) for all types of kernels. This indicates the requirement of higher energy in the desorption process as stated by Lahsani et al.[Citation15] Below this range, net isosteric heats of adsorption increased rapidly. At very low moisture content, the condensation of water molecule might have occurred which has contributed to increase in the heat of adsorption rapidly. Net isosteric heat of adsorption below 12% M.C. reported by Oztekin et al.[Citation18] was also higher than that of this study. But McLaughlin et al.[Citation11] reported a lower isosteric heat of adsorption than that of desorption for potato. The difference between the heats of adsorption and desorption converges as moisture content increases. These changes are probably due to changes in molecular structures during sorption which affects the degree of activation of sorption sites. Hysteresis effect on sorption isotherms also might have influenced these differences since the grain used was rewetted.
Rough rice had the highest net isosteric heat during sorption followed by brown rice and milled rice, respectively (). At a moisture content of 12% (d.b.) rough rice and brown rice had 30% and 10.5% higher net isosteric heat of desorption, respectively, over that of milled rice while net isosteric heat of adsorption for those were 24% and 8.3% higher, respectively. Statistical analysis was conducted to find the significant differences of net heats of sorption among milled rice, brown rice, and rough rice for adsorption and desorption using MSTAT-C version 2.10 and it was found that there is a significant difference among the net heats of sorption of milled rice, brown rice, and rough rice for adsorption and desorption (P < 0.01). Complexity of the sorption sites for additional layers of hull and/or bran in rough and brown rice kernels is presumed to be responsible for this.
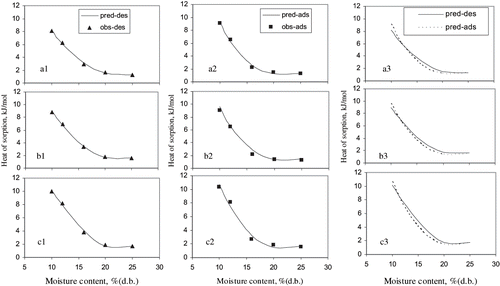
Both exponential and polynomial equations were fitted to describe the relationship between net isosteric heat of sorption of hybrid rice kernels and equilibrium moisture content. But the exponential EquationEq. (3) did not fit well to the data on isosteric heat of sorption of hybrid rice. The parameters and the standard error of estimate of the parameters for fitting the EquationEq. (4) are shown in for adsorption and desorption process for hybrid milled, brown, and rough rice kernels. High values of coefficient of determination (R 2 = 0.98 – 0.99), low values of mean relative percentage deviation (P = 2.7 – 11.50), and standard error of estimate of prediction (SE = 0.11 – 0.45) in show that the EquationEq. (4) can predict the heat of sorption well. depicts the predicted curves of heat of sorption as compared with the observed curves for hybrid milled, brown, and rough rice kernels, respectively, at both adsorption and desorption. The agreement between the observed and predicted heat values is also good.
Table 4 Parameters and standard error of estimate of the parameters of the empirical equation for the isosteric heat of sorption (EquationEq. 4) for hybrid rice kernels
Table 5 The mean relative percentage deviation (P), standard error of the prediction (SE) and coefficient of determination (R 2 ) of the isosteric heat (Q) of sorption for hybrid rice kernels (EquationEq. 4)
Figure 3 Observed and predicted net isosteric heat of sorption for (a) milled (b) brown and (c) rough rice for desorption and adsorption. Obs — observed; pred — predicted; ads — adsorption; des — desorption.
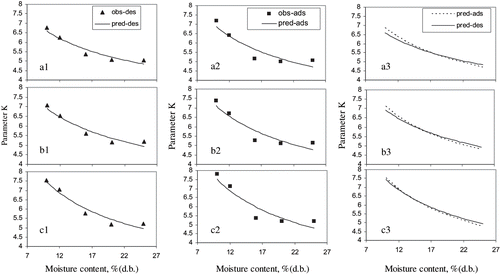
The parameters and standard error of estimate of the parameters of Equationequation (5) are shown in . Coefficient of determination (r 2 ), mean relative percentage deviation, (P) and standard error of estimate (SE) of the prediction for Equationequation (5) are presented in . Fitting and error criteria in and suggest that the Equationequation (5) can be used to predict the K values. The prediction is good for the parameter K as shown in .
Table 6 Parameters and standard error of estimate of the parameters of the equation for the K values (EquationEq. 5) of hybrid rice kernels
Table 7 The mean relative percentage deviation (P), standard error (SE) of the prediction and coefficient of determination (r 2 ) of the K values for hybrid rice kernels (EquationEq. 5)
Figure 4 Parameter K for (a) milled (b) brown and (c) rough rice at desorption and adsorption. Obs — observed; pred — predicted; ads — adsorption; des — desorption.
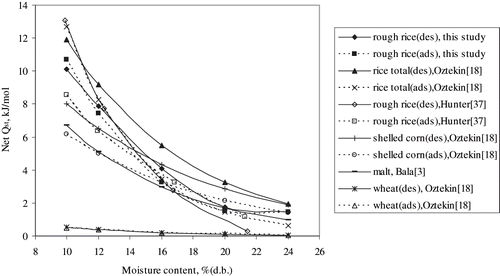
The net isosteric heat values for hybrid rice were found to be in the similar range as has been used in the literature[Citation3,Citation18,Citation37] for rice and other cereals, except wheat for which values were reported much lower than those for rice, shelled corn, and malt. The net heats of sorption of rough rice and some other cereal grains are shown in and comparisons of net isosteric heats of sorption are shown in . At higher moisture contents the net isosteric heat of desorption for rice reported by Oztekin and Soysal[Citation18] was found higher than those obtained by Hunter[Citation37] and this study. The reason might be the variations in the sorption isotherms of various data sets collected from the literatures for different cultivars, which were combined together in order to obtain the values for rice total (including rough and brown rice). At a moisture content of 14%, the net isosteric heat of desorption for hybrid rough rice was found to be 17% lower than that reported by Oztekin and Soysal[Citation18] and about 1% higher than that reported by Hunter.[Citation37] This variation justifies the individual determination of isosteric heat of sorption for different products in order to get the accurate prediction. The variation in net isosteric heat of sorption might be attributed to the physicochemical properties of the different cultivars, which are responsible for variations in isotherms.
Table 8 Net isosteric heats of sorption of rice and some other cereal grains
Figure 5 Comparison of net isosteric heat of sorption of hybrid rice with those of other varieties and crops. des — desorption; ads — adsorption.
The values of isosteric heat and the parameter K are needed to compute humidity of the air inside the stored grain to simulate the heat and moisture movement during storage of grains. Thus EquationEqs. (4) and Equation(5) would be useful in the simulation of stored grain.
CONCLUSION
Net isosteric heat of sorption increased with decreasing moisture content for hybrid rice kernels. For all 3 forms of hybrid rice kernels, net isosteric heats of desorption were higher than net isosteric heats of adsorption within 12 – 20% M.C. of the kernels, while below this moisture content, isosteric adsorption heat increased rapidly. The net isosteric heat of sorption for either adsorption or desorption for rough rice kernels was the highest, followed by brown rice and milled rice kernels. Net isosteric heats of adsorption and desorption were found to be a polynomial function of equilibrium moisture content and the intercept K of the ln(rh) versus reciprocal of absolute temperature was also found to be a function of equilibrium moisture content. This set of two equations will be useful in the simulation of hybrid rice during storage.
REFERENCES
- Thorpe , G.R. Modeling Moisture Migration in Stored Grains . Proceedings of an International Conference . 1996 . Grain Drying in Asia , Edited by: Champ , B.R. , Highley , E. and Johnson , G.I. Oct 17–20 . pp. 99 – 122 . Bangkok, , Thailand : ACIAR: Caberra .
- Prachayawarakorn , S. , Choteboon , C. and Soponronnarit , S. 2005 . Simultaneous Momentum, Heat and Mass Transfer with Color Change During Paddy Storage in Silo . Drying Technology , 23 : 205 – 223 .
- Bala , B.K. 1997 . Drying and Storage of Cereal Grains , New Delhi, , India : Oxford & IBH .
- Rizvi , S.S.H. 1986 . “ Thermodynamics of Foods in Dehydration ” . In Engineering Properties of Food , Edited by: Rao , M.A. and Rizvi , S.S.H. New York : Marcel Dekker .
- Duckworth , R.B. 1972 . The Properties of Water Around the Surfaces of Food Colloids . Proc Inst Food Sci. Technol. , 5 : 50 – 62 .
- Kiranoudis , C.T. , Maroulis , Z.B. , Tsami , E. and Marinos-Kouris , D. 1993 . Equilibrium Moisture Content and Heat of Desorption of Some Vegetables . J. Food Eng. , 20 ( 1 ) : 55 – 74 .
- Riedel , L. 1977 . Calorimetric Measurements of Heats of Hydration of Foods . Chemie Microbiologie und Technologie der Lebensmittel , 5 : 97 – 101 .
- Sanjuan , R , Garcia-Reverter , J. , Bon , J. and Mulet , A. 1994 . Moisture Retention in Chufa (Cyperus esculentus, L.), Equilibrium Isotherms and Isosteric Heats . Revista Espanola de Ciencia y Tecnologia de Alimentos , 34 ( 6 ) : 653 – 662 .
- Sanchez , E.S. , Sanjuan , R. , Simal , S. and Rossello , C. 1997 . Calorimetric Techniques Applied to Determination of Isosteric Heat of Desorption for Potato . J. Sci. Food Agric. , 74 : 57 – 63 .
- Mulet , A. , Garcia-Reverter , J. , Sanjuan , R. and Bon , J. 1999 . Sorption Isosteric Heat Determination by Thermal Analysis and Sorption Isotherms . J. Food. Sci. , 64 ( 1 ) : 64 – 68 .
- McLaughlin , C.P. and Magee , T.R.A. 1998 . The Determination of Sorption Isotherm and the Isosteric Heats of Sorption for Potatoes . J. Food Eng. , 35 ( 3 ) : 267 – 280 .
- Iglesias , H.A. and Chirife , J. 1976 . Isosteric Heat of Water Sorption on Dehydrated Foods. Part I. Analysis of Differential Heat Curves . Lebensmittel Wissenchaft Technologie , 9 ( 2 ) : 116 – 122 .
- Soysal , Y. and Oztekin , S. 2001 . Sorption Isosteric Heat for Some Medicinal and Aromatic Plants . J. Agric. Engng. Res. , 78 ( 2 ) : 159 – 166 .
- Mohamed , L.A. , Kouhila , M. , Lahsasni , S. , Jamali , A. , Idlimam , A. , Rhazi , M. , Aghfir , M. and Mahrouz , M. 2005 . Equilibrium Moisture Content and Heat of Sorption of Gelidium Sesquipedale . J. Stored Products Res. , 41 ( 2 ) : 199 – 209 .
- Lahsasni , S. , Kouhila , M. and Mahrouz , M. 2004 . Adsorption–Desorption Isotherms and Heat of Sorption of Prickly Pear Fruit (Opuntia ficus indica) . Energy Conversion and Management , 45 ( 2 ) : 249 – 261 .
- Vazquez , G. , Chenlo , F. and Moreira , R. 2003 . Sorption Isotherms of Lupine at Different Temperatures . J. Food Eng. , 60 ( 4 ) : 449 – 452 .
- Hossain , M.D. , Bala , B.K. , Hossain , M.A. and Mondol , M.R.A. 2001 . Sorption Isotherms and Heat of Sorption of Pineapple . J. Food Eng. , 48 ( 2 ) : 103 – 107 .
- Oztekin , S. and Soysal , Y. 2000 . Comparison of Adsorption and Desorption Isosteric Heats for Some Grains . Agricultural Engineering International: the CIGR Journal of Scientific Research and Development , II : 1 – 17 .
- Tsami , E. , Maroulis , Z.B. , Morunos-Kouris , D. and Saravacos , G.D. 1990 . Heat of Sorption of Water in Dried Fruits . Int. J. Food Sci. Technol. , 25 : 350 – 359 .
- Sutherland , J.W. , Banks , P.J. and Griffiths , H.J. 1971 . Equilibrium Heat and Moisture Transfer in Air Flow through Grain . J. Agric. Engng. Res. , 16 ( 4 ) : 368 – 386 .
- Hunter , A.J. 1987 . An Isostere Equation for Some Common Seeds . J. Agric. Engng. Res. , 37 : 95 – 105 .
- International Organization for Standardization . 1979 . “ Recommendation ISO712–1979(E) ” . In Cereals and Cereal Products — Determination of Moisture Content , Geneva : ISO Reference Method; ISO .
- Troller , J.A. and Christian , J.H.B. 1978 . Water Activity and Food , 253 New York : Academic Press . (Japanese version)
- Haque , M.A. , Shimizu , N. and Kimura , T. Sorption Isotherms of Hybrid Rice Kernels . Poster Presented at the World Rice Research Conference . 2004 , Tsukuba, Japan. November 5–7 .
- Haque , M.A. , Shimizu , N. and Kimura , T. Evaluation of an Accelerated Method for the Determination of Equilibrium Moisture Content of Rice kernels . Proceedings of the 5th Conference of Japanese Society of Food Engineering . Poster Presented at the , Aug 3–4 . University of Tokyo .
- Tsami , E. 1991 . Net Isosteric Heat of Sorption in Dried Fruits . J. Food Eng. , 14 ( 4 ) : 327 – 335 .
- Tolaba , M.P. and Suarez , C. 1990 . Desorption Isotherms of Shelled Maize: Whole Dehulled and Hulls . Int. J. Food Sci. Technol. , 25 : 435 – 441 .
- Tolaba , M.P. , Suarez , C. and Violaz , P. 1997 . Heats and Entropies of Sorption of Cereal Grains: A Comparison between the Integral and Differential Quantities . Drying Technol. , 15 ( 1 ) : 137 – 150 .
- Okos , M.R. , Narsimhan , G. , Singh , R.K. and Weitmauuer , A.C. 1992 . “ Food Dehydration ” . In Handbook of Food Engineering , Edited by: Heldman , D.R. and Lund , D.B. New York : Marcel Dekker .
- Wang , N. and Brennan , J.G. 1991 . Moisture Sorption Isotherm Characteristics of Potatoes at Four Temperatures . J. Food Eng. , 14 ( 4 ) : 269 – 287 .
- Chen , C. and Morey , R.V. 1989 . Comparison of Four EMC/ERH Equations . Transactions of the ASAE , 32 ( 3 ) : 983 – 990 .
- Basunia , M.A. and Abe , T. 2001 . Moisture Desorption Isotherms of Medium Grain Rough Rice . J. Stored Prod. Res. , 37 ( 3 ) : 205 – 219 .
- Sopade , P.A. 2001 . Criteria for an Appropriate Sorption Model Based on Statistical Analysis . International Journal of Food Properties , 4 ( 3 ) : 405 – 418 .
- Tungsangprateep , S. and Jindal , V.K. 2004 . Sorption Isotherms and Moisture Diffusivity in Fried Cassava-Shrimp Chips . International Journal of Food Properties , 7 ( 2 ) : 215 – 227 .
- Sun , D.-W. 1999 . Comparison and Selection of EMC/ERH Isotherm Equations for Rice . Journal of Stored Products Research , 35 ( 3 ) : 249 – 264 .
- Rahman , S. 1995 . Food Properties Handbook , New York, , USA : CRC Press .
- Hunter , A. 1989 . On the Heat of Sorption of Australian Paddy Rice . Journal of Agricultural Engineering Research , 44 ( 3 ) : 237 – 239 .