Abstract
Experimental viscosity values of sucrose, glucose, and fructose aqueous solutions in a large range of temperatures (0 to 85°C) and concentrations (10 to 60% w/w) that might be encountered in food processes were obtained in order to contribute to extending the available database of food properties. The temperature dependence of viscosity could be adequately described by the Arrhenius model, and the activation energy was well represented by a unique function of the solute volume fraction, valid for sucrose, glucose, and fructose solutions.
INTRODUCTION
Viscosity is a relevant property of fluids when designing flow systems and industrial processes involving heat or mass transfer. Aqueous solutions of sucrose, glucose, or fructose at different temperatures and concentrations are found in several food processes, such as in crystallization and osmotic dehydration, as well as being the basis to formulation of a number of food products or ingredients in the bakery, ice cream, and confectionary industries. There is not a unique source of data that reports sugar solutions viscosities, and their dependence on temperature and concentration in a wide range of these variables. We found a relatively small number of works reporting sugar solutions viscosities in limited ranges of temperature and concentration. Kinematic viscosities of glucose aqueous solutions in the range of 20 to 50°C and molalities between 1.0 and 5.0 moles/kg (15.27 to 47.39% w/w) were measured with the objective of being applicable in osmotic dehydration.[Citation1] A similar work was carried out with sucrose solutions and kinematic viscosity data were reported from 20 to 50°C and molalities in the range of 0.5 to 4.5 moles/kg (14.61 to 60.64% w/w).[Citation2]
Based on the fact that both viscosity and water activity may provide important information about the state and behavior of water in food products, Mazurkiewicz et al.[Citation3] investigated the relationship between these properties in aqueous solutions of sucrose or glucose. Viscosities were determined at 25°C in the molality range of 0.1 to 1.8 moles/kg (3.4 to 61.6% w/w) for sucrose, and in the range of 1.2 to 7.5 moles/kg (21.6 to 135.1% w/w) for glucose. Bui and Nguyen[Citation4] adjusted an empirical equation to viscosities of glucose aqueous solutions as a function of temperature. The temperature varied from 25 to 75°C, and the concentration between 1.5 to 10.5 mol/L. Rampp et al.[Citation5] investigated the dependence of viscosity and self-diffusion coefficients as functions of temperature and concentration of fructose solutions between 0 and 50°C and 30 to 85% w/w.
The rheological behavior of supersaturated solutions of glucose and/or fructose solutions[Citation6] was investigated in a range of temperatures and concentrations selected to result in high viscosity systems with the objective of comparing different models—Arrhenius, Williams-Landel-Ferry (WLF), Vogel-Taumman-Fulcher (VTF), and power law — to fit experimental data. In the studied range of temperature and concentration, the VTF model resulted in the best fitting. Quintas et al.[Citation7] studied the rheology of sucrose supersaturated solutions with concentrations between 70 and 85% (w/w) and temperatures from 0 to 90°C. We concluded that, at sucrose concentrations lower than saturation, the Arrhenius model is satisfactory to reproduce experimental data, whereas at higher concentrations, the WLF model is suited to describe the observed behavior. The rheology of inverted liquid sugar at different percentages of inversion (59.68 and 89.88%) and at different temperatures was evaluated by Gratão et al..[Citation8] The temperature dependence was described by the Arrhenius model, and the activation energy was higher for the sugar with higher inversion degree. The aim of the present article was to determine experimental values of viscosities for sucrose, glucose and fructose aqueous solutions as a function of temperature and concentration. The presentation of a viscosity dataset that includes solutions of these three commonly used sugars in tabular form, covering a wide range of temperatures and concentrations that might be found in food processing, could contribute to extend the available database of food properties and constitute an useful tool for engineers and researchers dealing with sugar solutions.
MATERIAL AND METHODS
Sample Preparation
Sucrose, D-glucose, and D-fructose of analytical grade (Sigma, Germany) were dissolved in distilled water. Solutes and water were weighted in the desired proportion in a precision balance (model BG2000, Gehaka, Brazil) with a resolution of ± 0.01 g, in order to prepare a total volume of 1 liter of each solution. After mixing, the solutions were heated to 60°C, in a closed flask, during 1 hour to assure the complete dissolution of sugars.
Experimental Apparatus and Measurement Procedure
With the purpose of reducing errors associated with the equipment employed to measure viscosities, two apparatus were used independently. Viscosities of sucrose, glucose, and fructose solutions, in concentrations of 10, 50, and 60% (w/w), at temperatures in the range of 0 to 50°C, were measured in a Brookfield digital viscometer, model HADV – I+ (Brookfield Engineering Labs., Inc., USA) fitted with a Spiral Adapter SA70 spindle, rotating at 20 rpm Additional viscosity data of sucrose, glucose and fructose solutions, in concentrations of 10, 20, 30, 40, 50, and 60% (w/w), at temperatures in the range of 0 to 85°C, were obtained from flow curves (shear stress versus shear rate) determined using a Rheotest 2.1 (MLW, Germany) rheometer, Searle type, equipped with a coaxial cylinder sensor system (radii ratio, ). A thermostatic bath was used to control the working temperature. The speed of the rotating cylinder varied from 0.028 to 243 rpm. The instrument was operated at 44 different speeds, which were changed stepwise with a selector switch. Shear stress (σ) were obtained by multiplying torque readings by the viscometer constant, whereas shear rate (γ) were obtained according Krieger and Elrod.[Citation9]
RESULTS AND DISCUSSION
Rheological Behavior
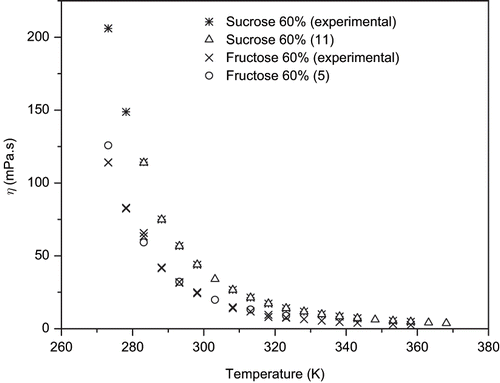
Flow curves were obtained for sucrose, glucose and fructose aqueous solutions at concentrations of 10, 20, 30, 40, 50, and 60% (w/w), and at temperatures of 0, 5, 10, 15, 20, 25, 35, 40, 45, 50, 55, 60, 65, 70, 80, and 85°C in the Rheotest 2.1 rheometer. All the solutions analyzed, in the whole domain of temperature, presented Newtonian behavior. Lazaridou et al.[Citation10] and Gratão et al.[Citation8] also observed Newtonian behavior for honey and inverted liquid sugar, respectively. to present sucrose, glucose and fructose solutions viscosities and, as shown in , the obtained data are in a good agreement with values reported by other authors, such as Perry and Chilton[Citation11] that presented data for sucrose solutions at 20, 40, and 60% (w/w), in the range of 0 to 95°C, and Rampp et al.[Citation5] that reported viscosities for fructose solutions with concentrations from 30 to 85% (w/w) and temperatures between −15 and 50°C.
Table 1 Viscosities of sucrose solutions
Table 2 Viscosities of glucose solutions
Table 3 Viscosities of fructose solutions
Comparison with data presented by other authors is difficult due to the different values of temperature and concentration at which measurements were done. In addition, some papers did not present the data in form of tables, only as plots or correlations. Chenlo et al.[Citation2] and Moreira et al.[Citation1] presented kinematic viscosity data against molality of sucrose and glucose solutions, respectively. Although data were presented in table form, density values — not presented — are necessary to convert kinematic viscosity into dynamic viscosity. Mazurkiewicz et al.[Citation3] presented data of dynamic viscosity as function of water activity of sucrose and glucose solutions. In this case it is necessary to use an empirical correlation to obtain the solution water activity in a determined concentration value. This procedure has the inconvenience of introducing rounding errors.
In to , the viscosities determined in the two tested equipments were presented: the upper lines, with temperature varying from 0 to 85°C, present data measured in the Rheotest 2.1 rheometer, whereas the lower lines, with temperature ranging from 0 to 50°C, contains data obtained in the Brookfield viscometer. Most of the values obtained with the 2 instruments, but corresponding to the same sample at the same conditions were in good agreement, except for the upper region of concentration at the lower temperatures, where some deviations were very high for sucrose and glucose solutions. The main difference between these two sets of data is that the viscosities determined in the rheometer were calculated from the slope of shear stress versus shear rate plots, with the internal cylinder rotating at 44 different speeds in the range of 0.028 to 243 rpm. On the other hand, measurements carried out in the in the viscometer were determined at only one rotating speed (20 rpm). The longer measurement time elapsed when using the rheometer could have resulted in some degree of sugar crystallization in the concentrated solutions exposed for a long time at low temperatures, decreasing solution concentration and leading to lower viscosities. In the case of fructose, which has a high solubility, there was not great differences between the two sets of data. The viscosities of sugar solutions — when compared at the same temperature and concentration — decreased in the following order of solutes: sucrose, glucose, and fructose. These differences, however, were reduced with increasing temperature and decreasing solution concentration.
Effect of Temperature and Concentration on Sugar Viscosities
As expected, sugar solutions viscosities decreased with increasing temperature. The Arrhenius model,[Citation6,Citation7] written as:
where η is the viscosity; T is the temperature; Ea the activation energy; R the gas constant; and, ηref the fluid viscosity at a reference temperature Tref, was fitted to experimental data to describe viscosity dependence of temperature. The model fitting was carried out by non-linear regression. The Arrhenius model is quite applicable to non polar liquids. Nevertheless, in fluids constituted of molecules that interact through hydrogen bridges, dipoles or covalent bonds, for instance, a deviation from this model may occur, mainly, due to temperature influence.[Citation12] Although fluid foods could hardly be considered as constituted by non polar molecules, the Arrhenius model has been often and successfully applied to describe the temperature dependence of rheological properties of fluid foods, such as fruit juices,[Citation13–15] coffee extract,[Citation16] and honey.[Citation10,Citation17]
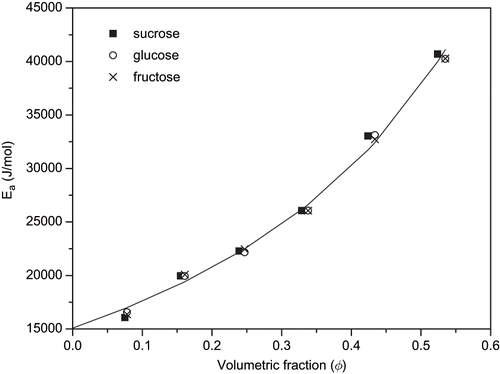
Values of the parameter Ea, corresponding to the fitting of EquationEq. (1) for all tested solutions are presented in . The selected reference temperature was 45°C (318.15 K), since the use of a middle temperature in the studied range has been recommended.[Citation7] It is observed that the activation energy, Ea, increased with increasing concentration as reported by other authors.[Citation18] The same trend and order of magnitude was observed by Zuritz et al.[Citation13] when studying the dependence of clarified grape juice activation energy as function of the juice soluble solids content (about 93% of reducing sugars). The increase in activation energy with solute concentration indicates that the temperature influence on the viscosity is higher as the solution concentration increases.[Citation19]
Table 4 Activation energy for sucrose, glucose, and fructose solutions at different concentrations
shows that there was a high degree of agreement between values of activation energy, Ea, calculated for the different solutes in solutions with same concentration. Based on this observation it was possible to describe the activation energy dependence on concentration as a function only of the solute content, regardless of the type of sugar present in the solution. This was possible by expressing the solute content as an effective volumetric fraction of solute, [Citation20] which depends on the solute type and solution concentration, and was calculated as:
where
and φsf = solute free volumetric fraction; w = mass solute fraction (w/w); M = molar mass (g/mol); and,
= Van der Waals molar volume (cm3/mol). The adjusted model to express activation energy is given by:
where Ea0 is an adjustment constant. A common value of Ea0 = 15080.24 ± 86.12 J/mol — with a correlation coefficient, r, of .997 — was obtained when correlating activation energies against volumetric fractions of all the three considered solutes. EquationEq. (4) is similar to the Einstein's equation that predicts the viscosity of a solution or suspension of spherical particles,[Citation20] differing only by the power in the denominator that in the Einstein's equation is of second order. Values of the effective volumetric fraction corresponding to different solutes and concentrations are included in , whereas the values of Van der Waals molar volumes and molar mass of sucrose, glucose, fructose and water used in EquationEq. (3) are given in . The Van der Waals molecular volume is determined by adding up the individual volumes of each fragment that constitute the molecule.[Citation21] The molar volumes presented in were obtained from Peres and Macedo.[Citation22]
Table 5 Van der Waals molar volume for sucrose, glucose, fructose and water
A good agreement was observed between activation energy values predicted by EquationEq. (4) and those calculated by fitting viscosity data to the Arrhenius model () regardless of the considered solute. The quality of the adjustment was evaluated by the distributions of residuals and by the root mean square, RMS,[Citation23] presented in and given by:
where ηobs and ηpred are, respectively, the experimental and predicted viscosities; and, N is the number of available experimental points. Values of RMS were lower than 17% for all the tested solutions. Lewicki[Citation23] considered RMS = 25% as the maximum limit for acceptance of the adjustment of theoretical or empirical models to sorption isotherms experimental data. EquationEq. (4) has also the advantage of being a correlation with a unique adjustment parameter and valid for the three types of sugars studied.
CONCLUSIONS
Experimental viscosity values for aqueous solutions of sucrose, glucose, and fructose were obtained in a wide range of temperatures and solute concentrations and Newtonian behavior was observed for all tested samples. The viscosities of sugar solutions — when compared at the same temperature and concentration — decreased in the following order of solutes: sucrose, glucose, and fructose. These differences, however, were reduced with increasing temperature and decreasing solution concentration. The Arrhenius model satisfactorily described the temperature dependence of viscosity and the activation energy — when the model was expressed in terms of a reference temperature — could be correlated with the solute content by a unique equation in function of an effective volumetric fraction of solute. This correlation was valid for the three considered sugars with the same value of the fitting parameter, Ea0.
NOMENCLATURE
| Ea | = |
Activation energy (J/mol) |
| Ea0 | = |
Constant of Equationequation 4 (J/mol) |
| M | = |
Molar mass (g/mol) |
| N | = |
Number of experimental points |
| R | = |
Gas constant (J/mol.K) |
| Rb | = |
Radio of internal cylinder (m) |
| Rc | = |
Radio of external cylinder (m) |
| RMS | = |
Root mean square (%) |
| T | = |
Temperature (K or °C) |
| Tref | = |
Reference temperature (K or °C) |
|
| = |
Van de Waals molar volume (cm3/mol) |
| w | = |
Solute mass fraction |
| Greek Letters | ||
| φ | = |
Solute volumetric fraction |
| φsf | = |
Solute free volumetric fraction |
|
| = |
Shear rate (1/s) |
| η | = |
Viscosity (Pa.s) |
| ηobs | = |
Experimental viscosity (Pa.s) |
| ηpred | = |
Predicted viscosity (Pa.s) |
| ηref | = |
Viscosity at the reference temperature (Pa.s) |
| σ | = |
Shear stress (Pa) |
REFERENCES
- Moreira , R. , Chenlo , F. and Pereira , G. 2003 . Viscosities of Ternary Aqueous Solutions with Glucose and Sodium Chloride Employed in Osmotic Dehydration Operation . J. Food Eng. , 57 : 173 – 177 .
- Chenlo , F. , Moreira , R. , Pereira , G. and Ampudia , A. 2002 . Viscosities of Aqueous Solutions of Sucrose and Sodium Chloride of Interest in Osmotic Dehydration Processes . J. Food Eng. , 54 : 347 – 352 .
- Mazurkiewicz , J. , Tomasik , P. and Zaplotny , J. 2001 . Relationships Between Water Activity and Viscosity of Solutions . Food Hydrocolloids , 15 : 43 – 46 .
- Bui , A.V. and Nguyen , M.H. 2004 . Prediction of Viscosity of Glucose and Calcium Chloride Solutions . J. Food Eng. , 62 ( 4 ) : 345 – 349 .
- Rampp , M. , Buttersack , C. and Lüdemann , H.-D. 2000 . c, T-Dependence of the Viscosity and the Self-Diffusion Coefficients in Some Aqueous Carbohydrate Solutions . Carbohydrate Research , 328 : 561 – 572 .
- Recondo , M.P. , Elizalde , B.E. and Buera , M.P. 2006 . Modeling Temperature Dependence of Honey Viscosity and of Related Supersaturated Model Carbohydrate Systems . J. Food Eng. , 77 ( 1 ) : 126 – 134 .
- Quintas , M. , Brandão , T.R.S. , Silva , C.L.M. and Cunha , R.L. 2006 . Rheology of Supersaturated Sucrose Solutions . J. of Food Eng. , 77 ( 4 ) : 844 – 852 .
- Gratão , A.C. , Berto , M.I. and Silveira Júnior , V. 2004 . Reologia de Açúcar Líquido Invertido: Influência da Temperatura na Viscosidade . Ciência e Tecnologia de Alimentos , 24 ( 4 ) : 652 – 656 .
- Krieger , I.M. and Elrod , H. 1953 . Direct Determination of the Flow Curves of Non-Newtonian Fluids. B. Shearing Rate in the Concentric Cylinder Viscometer . J. Appl. Phy. , 24 : 134 – 136 .
- Lazaridou , A. , Biliaderis , C.G. , Bacandritsos , N. and Sabatini , A.G. 2004 . Composition, Thermal and Rheological Behaviour of Selected Greek Honeys . J. of Food Eng. , 64 : 9 – 21 .
- Perry , R. and Chilton , C. 1986 . Manual de Engenharia Química , 5a , Rio de Janeiro : Guanabara Dois .
- Moore , W.J. 1972 . Physical Chemistry , 4th , Englewood Cliffs : Prentice Hall, Inc. .
- Zuritz , C.A. , Muñoz Puntes , E. , Mathey , H.H. , Pérez , E.H. , Gascón , A. , Rubio , L.A. , Carullo , C.A. , Chernikoff , R.E. and Cabeza , M.S. 2005 . Density, Viscosity and Coefficient of Thermal Expansion of Clear Grape Juice at Different Soluble Solid Concentrations and Temperatures . J. Food Eng. , 71 ( 2 ) : 143 – 149 .
- Zainal , B.S. , Rahman , R.A. , Ariff , A.B. , Saari , B.N. and Asbi , B.A. 2000 . Effects of Temperature on the Physical Properties of Pink Guava Juice at Two Different Concentrations . J. Food Eng. , 43 ( 1 ) : 55 – 59 .
- Telis-Romero , J. , Telis , V.R.N. and Yamashita , F. 1999 . Friction Factors and Rheological Properties of Orange Juice . J. Food Eng. , 40 ( 1–2 ) : 101 – 106 .
- Telis-Romero , J. , Cabral , R.A.F. , Gabas , A.L. and Telis , V.R.N. 2001 . Rheological Properties and Fluid Dynamics of Coffee Extract . J. Food Eng. , 24 ( 4 ) : 217 – 230 .
- Yanniotis , S. , Skaltsi , S. and Karaburnioti , S. 2006 . Effect of Moisture Content on the Viscosity of Honey at Different Temperatures . J. Food Eng. , 72 : 372 – 377 .
- Rao , M.A. 1986 . “ Rheological Properties of Fluid Foods ” . In Engineering Properties of Foods , Edited by: Rao , M.A. and Rizvi , S.S.H. 1 – 47 . New York : Marcel Dekker .
- Holdsworth , S.D. 1971 . Applicability of Rheological Models to the Interpretation of Flow and Processing Behavior of Fluid Food Products . J. Text. Stud. , 2 : 393 – 418 .
- Morison , K.R. and Mackay , F.M. 2001 . Viscosity of Lactose and Whey Protein Solutions . Intl J. Food Prop. , 4 ( 3 ) : 441 – 454 .
- Kodaka , M. 2004 . Correlation between Molecular Size and Packing Density of Solvents . J. Phys. Chem. B , 108 : 1160 – 1164 .
- Peres , A.M. and Macedo , E.A. 1997 . Phase Equilibria of D-Glucose and Sucrose in Mixed Solvent Mixtures: Comparison of UNIQUAC–Based Models . Carbohydrate Research , 303 : 135 – 151 .
- Lewicki , P.P. 2000 . Raoult´s Law Based Food Water Sorption Isotherm . J. Food Eng. , 43 : 31 – 40 .