Abstract
The effect of washing and refrigeration on the quality of sea bream (Sparus aurata) was studied by monitoring the microbiological, chemical, and sensory changes of washed samples with tap water and non-washed samples. Washing dramatically reduced populations of bacteria, namely aerobic mesophilic bacteria (6.4–6.2 log cfu/g for gutted sea bream and 6.6–6.1 log cfu/g for whole gutted sea bream), psychrotrophic bacteria (6.7–6.0 log cfu/g and 6.8–6.1 log cfu/g), and H2S-producing bacteria (6.4–5.4 log cfu/g and 6.7–5.5 log cfu/g) after 13 days of storage. Total volatile basic nitrogen (TVB-N) values showed no significant increase for all group of sea bream samples during storage. Of the chemical indicators of spoilage, trimethylamine (TMA) values of non-washed and washed sea bream increased very slowly and reached a final value of 1.35–1.47 mg/100 g (gutted fish) and 1.36–1.38 mg/100 g (whole fish), respectively (day 13). Sensory evaluation showed a good correlation with bacterial populations. On the basis of overall acceptability scores (sensory evaluation) a shelf-life of 12 days (washed and non-washed samples) was obtained for gutted and whole sea bream.
INTRODUCTION
Fish is known to be a high protein and low fat meat.[Citation1,Citation2] Fish is one of the most highly perishable food products. During handling and storage, quality deterioration of fresh fish rapidly occurs and limits the shelf life of the product. Shelf life is defined as the period of time, under defined conditions of storage, for which a food product remains safe and fit for consumption. In other words, during this period, it should retain its desired sensory, chemical, physical, functional, or microbiological characteristics. The quality of fish degrades due to a complex process in which physical, chemical, and microbiological forms of deterioration are implicated. Enzymatic and chemical reactions are usually responsible for the initial loss of freshness, whereas microbial activity is responsible for the obvious spoilage and thereby establishes product shelf life.[Citation3,Citation4,Citation5,Citation6] In fact, the hygienic quality of fish and fishery products rapidly declines because of a cross-contamination from various sources. Bacterial spoilage of saltwater fish is caused by several Gram-negative bacteria; in particular, Shewanella putrefaciens was found to be very important for spoilage of packed cod, as well as Photobacterium phosphoreum, Pseudomonas spp., Achromobacter spp., Acinetobacter spp., Flavobacterium spp., and Aeromonas spp.[Citation7,Citation8] The application of low-dose irradiation, microwave cooking, high power ultrasound waves, by the addition of chemical preservatives, salting, curing has been previously reported to extend the shelf life of sea foods.[Citation9,Citation10,Citation11,Citation12,Citation13,Citation14,Citation15] Washing has been employed for decontamination of fresh fish and then to prolong the storage period of fresh fish. Washing in combination with refrigeration has proved to be an effective preservation method for the extension of shelf-life and quality retention of a wide variety of fresh chilled food products (fruits, vegetables, and fish).[Citation16] Pouring sea water or tap water on fish that are on the sales stands is a common application throughout the world. In some countries like Portugal it is an obligation to do so. In European countries, sea water is filtered and processed with ultra-violet light and then chlorinated (1 mg/l) before it is released to the water pipe system for the fish stands.[Citation16] This type of water is used to decrease the bacterial load and inhibit bacterial growth without the development of unwanted taste and odour caused by the chlorine in fish. In our country, seawater or tap water is applied directly. It is not a legitimate obligation, but it is common and the aim is to keep the fish presentable and clean. Washing, in addition, is an application which is used to decrease the microbial flora. Fish as whole or gutted are ready for sale at the fish official markets after washing and stored in ice. The aim of this article was to determine the effects of washing with tap water on the quality of iced whole and gutted sea bream using sensory, chemical, and microbiological assessments.
MATERIALS AND METHODS
Fish Samples and Storage Conditions
Aqua-cultured fresh sea bream (Sparus aurata) of total 20 kg were cultivated in net cages in a Turkish fish farm and harvested during the period of June 2004. The fish were killed by immersion in ice slurry (hypothermia) and delivered to the laboratory as whole within 12 h of harvesting, packed in separate insulated polystyrene boxes with ice. The mean and standard deviations of the weight and length of the fish studied were 260 g (± 30) and cm 26.2 cm (± 0.6). Fish was washed once, after landing with running tap water. The fish were divided into four lots. Gutted control (AC), gutted washed (AW), whole control (BC), and whole-washed (BW). Gutting was carried out in the fish processing plant manually. In the washed lot, the fish was daily washed with tap water, and then stored again in the same conditions as the unwashed lot. During storage, ice was replenished when necessary. Boxes had perforated bottoms to allow drainage of melted crushed ice. Boxed fish were stored at 4°C.
Sensory Evaluation
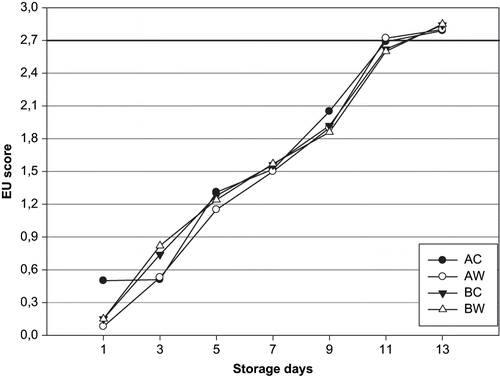
Five experienced panellists analyzed all fish during days 1, 3, 5, 7, 9, 11, and 13 according to EU fish sensory schema () for white fish.[Citation17] The evaluation depends on the table created because white fish concept include haddock, cod, saithe, pollack, redfish, whiting, ling, hake, bream, anglerfish, pouting and poor cod, bogue, picarel, conger, gurnard, mullet, plaice, megrim, sole, dab, lemon sole, flounder, and scabbard fish. For the EU scheme the mean points of each fish were calculated and the fish were classified according to the following the quality bands: E<1, 1≤A<2, 2 ≤B<2.7, and 2.7≥ C points.[Citation18]
Table 1 EU fish sensory scheme
Cooked Fish
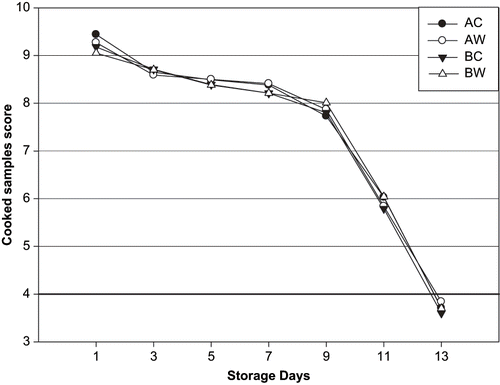
The cooked fish were assessed using the simplified Torry Sensory Scheme for cooked white fish.[Citation19] Panellists were asked to score odours, taste, and texture of fish using a 0–10 acceptability scale. Scale 10–9 is excellent, 8–7 is very good, 6–5 is acceptable, and <4 is unacceptable.
pH
Aliquots of 10 sardines were filleted from each sampling lot and homogenized using a food processor. Samples were prepared according to Vyncke,[Citation20] by blending 10 g of the homogenate with 100 ml distilled water for 1 min at room temperature in a Ultra-Turrax (IKA T 25 Basic, Staufen, Germany). pH was monitored using a WTW-pH Meter (inoLab pH Level 1 model, Weilheim, Germany). Determinations were carried out in triplicate at days 1, 3, 5, 7, 9, 11, and 13 during the storage period.
Determination of Total Volatile Basic Nitrogen (TVB-N)
Total volatile basic nitrogen was determined according to the Antonacopoulos and Vyncke[Citation21] method. For total volatile basic nitrogen (TVB-N), fish muscle (10 g) was homogenized with 6% perchloric acid (90 ml) for 1 min in an Ultra-Turrax. The homogenates were filtered through a filter paper (Whatman no. 1) and filtrates alkalized by 20% NaOH before distillation duplicate filtrates were distilled in a Velp Marka (Model UDK 140, Milan, İtaly) apparatus. The distillate titrated with 0.01 N HCl.
Determination of trimethylamin nitrogen (TMA-N)
This was determined by the method of AOAC.[Citation22] Ten gram homogenized samples were weighed, blended with 90 ml of 7.5% trichloracetic acid solution and filtrated. Blended solution was fixed with formaldehyde (20%). Four ml extract was transferred into test tubes and 1 ml formaldehyde, 10 ml anhydrous toluene, and 3 ml K2CO3 solution were added. The tubes were shaken and 5 ml toluene layer was taken out by pipet. Five ml picric acid working solution (0.02%) was added. The contents were mixed and transferred to a spectrophotometric cell. Absorbance at 410 nm against the blank was measured. At the same time, standards were prepared and measured.
Microbiological Analyses
Three fish of each batch were randomly chosen. An area of approximately 10 cm2 of the anterior-dorsal region and abdominal cavity of the right side of the fish was swabbed with a sterile swab, previously wetted with sterile peptone water (Merck, Kat No: 107228). Appropriate series of decimal dilutions were performed, from which surface inoculation was made using the 10 μl drop method in four solid medium. The media and incubations were: for aerobic plate count PCA agar (PCA, Merck, Kat No: 105463), 37°C, 1–2 days; for psychrotrophic bacteria PCA agar (PCA, Merck, Kat No: 105463), 7°C;[Citation23] for H2S-producing bacteria Iron agar 25°C, 2–3 days.[Citation24] Counts were performed in duplicate and expressed as log cfu/cm2.
Statistical Analysis
Significant differences between the samples were calculated by Excel XP 2003 by one –way analysis of variance (ANOVA) using a significance level of p < 0.05 by Tukey's Honestly Significant Difference test. A time dependent linear regression analysis was performed for the results obtained for sensory and microbiological analyses. Sperman's (rs) (rank) and Pearson correlation (r) coefficients were used to compare physical, chemical and microbiological measurements to sensory panel results.[Citation25]
RESULTS AND DISCUSSION
Sensory Analysis
In it can be seen that excellent and very good grades (E and A) were scored during the first 9 days storage for unwashed and washed fish. Moderate grades (B) were obtained between days 11 and 13 of storage for all groups. Unfit for sale of raw fish samples (C) were obtained 13 days of storage for unwashed and washed fish raw sea bream samples. Sensory results of washed and unwashed sea bream showed that day 9 was the beginning of spoilage. The samples were unfit for human consumption after 12 days. In ice with tap water washing samples, appearances of the gills, eyes, and mucus were better than in unwashed samples during the storage. Samples ice stored combined with tap water, had lower levels of intensive smell of gills and abdominal cavity than the gutted-unwashed samples after 9 days of storage. The application of washing with tap water decreased the formation of mucus and off-odour. Acceptability scores of all groups decreased significantly (p < 0.05) throughout storage (). The samples had “very good” quality up to 9 days and “good” quality up to 11 days. The samples were unfit for human consumption at 12 days. The changes in sensory data were found to be insignificant (P > 0.05) in washed and unwashed sea bream. The storage life of whole, iced sea bream evaluated by sensory assessment of the raw fish has been reported to be 15–17 days.[Citation26,Citation27] The shelf life of whole ungutted sea bream stored in ice was found to be 17–18 days at 2 ± 2°C.[Citation28] Lougovois et al.[Citation29] concluded that shelf life of whole sea bream in ice is extremely limited, typical 15 days after catch. Poli et al.[Citation30] reported that the limit of acceptability for cooked sea bass was 10 days. Erkan and Ozden[Citation31] reported that the limit of acceptability for gutted and ungutted sea bass was 11 days. Huidobro et al.[Citation32] found that the sensory evaluation scores of the cooked fillets (sea bream) had high scores for flavour, odour at the beginning of the storage period, but its score decreased at the end—unwashed samples (15 days) and washed samples (17 days). These findings are in agreement with the results described by Inácio et al.[Citation16] They reported the demerit point of washed fish was lower than unwashed fish samples during the storage in ice.
Chemical Analysis
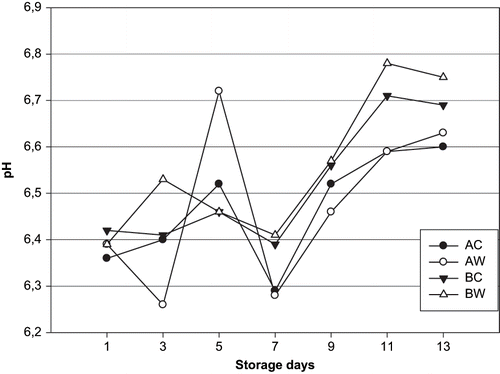
The changes in pH, TVB-N, and TMA-N for unwashed and washed sea bream during the 13-day storage period in ice are shown in the , , and . The values of pH ranged from 6.36 to 6.60 for AC groups, from 6.39 to 6.63 for AW, and from 6.55 to 6.67 for BC, from 6.39 to 6.75 for BW groups, respectively, during the 13-day storage period. Values of pH showed statistically significant (p < 0.05) changes for washed and unwashed (whole and gutted) sea bream samples during the entire period of storage. The pH was according to literature about 6.0–6.5 for fresh fish, and it increases during storage. The limit of acceptability is usually 6.8–7.0.[Citation33] The pH increases are in agreement with the findings of Kyrana et al.;[Citation27] Kyrana und Lougovois,[Citation34]; Masyinom et al.;[Citation35] Tejada and Huidobro;[Citation36] Papadopoulus et al.;[Citation37] Grigorakis et al.;[Citation38] Taliadourou et al.[Citation39] for sea bream species stored in ice.
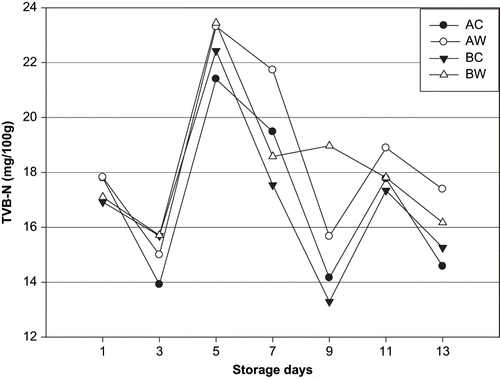
The TVB-N content ranged from 17 mg/100 g to 15 mg/100 g flesh for unwashed sea bream groups and from 17 mg/100 g for washed samples groups, respectively, during the 13-day period of storage in ice. TVB-N values showed no significant increase for unwashed and washed sea bream during storage. TVB-N values showed significant fluctuation for all fish samples as a function of storage period indicating that TVB-N is a poor indicator of fish freshness, as also proposed by.[Citation27,Citation40] Similar TVB-N values were reported for whole fish[Citation34,Citation40] as well as for gutted fish..[Citation40,Citation41] For these species fish (Sparidae) a value of 25 mg/100 g has been designated as the limit acceptability.[Citation42]
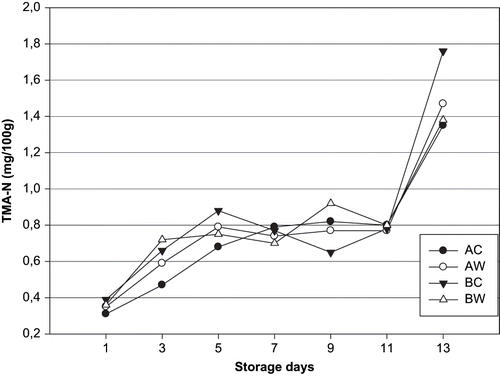
Microorganisms' activities are also related to chemical compounds (TVB, TMA, etc.), which are suggested as indicators of fish quality.[Citation43] TMA is formed from bacterial use of TMAO, naturally occurring osmoregulating substance found in most marine fish species.[Citation44] The quantity and presence of this compound depends on the species, size, sex, station of year, and so on.[Citation45] Meyer[Citation46] reported that H2S- producing bacteria were both an important spoiler and TMAO reducer. Initial TMA-N values of unwashed and washed sea bream (groups AC-BC and AW-BW) samples were 0.31, 0.39–0.35, 0.36 mg/100 g, respectively. A content of 1.35, 1.76 mg/100 g was reached for the AC-BC samples after 13 days of storage whereas the AW-BW samples reached this content 1.47, 1.38 mg/100 g after 13 days of ice storage, respectively. Values of TMA-N showed not significant (p > 0.05) between washed and unwashed sea bream in both series during the entire period of storage. The concentrations of TMA-N in numerous fatty fish never reached the limit of 5 mg TMA-N/100 g, although the rejection limit in fish flesh is usually 5–10 mg TMA-N/100 g.[Citation47] The level of TMA was typically around 0.07 mg/100 g in ice stored whole fish (sea bass) rejected by sensory panels.[Citation37] Dalgaard et al.[Citation48] reported that a population of 108–109 cfu/g of S. putrefaciens was considered crucial for TMA production. H2S- producing bacteria (including S. putrefaciens) in this study reached at the end storage 5.04 log cfu/cm2 (AC), 4.4 log cfu/cm2 (AW), 5.7 log cfu/cm2 (BC), 4.5 log cfu/cm2 (BW) for sea bream samples, respectively. A highly significant correlation was found between TMA-N and H2S- producing bacteria counts (AC, r = 0.841; AW, r = 0.779; BC, r = 0.753; BW, r = 0.874). Thus, TMA is not is not a particularly useful indicator of sea bream freshness. Similarly, low TMA values have been reported for whole fresh sea bass, trout, and sea bream.[Citation27,Citation36,Citation49]
Table 2 Correlation coefficients between sensory score and microbial counts in whole and gutted sea bass
Microbiological Analysis
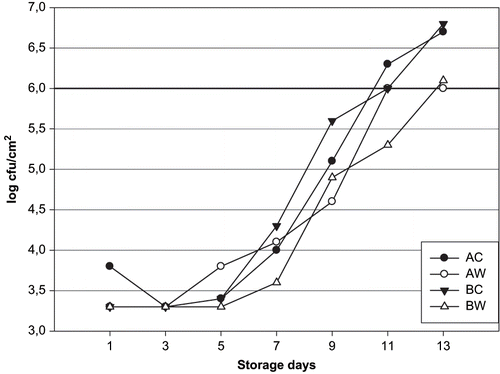
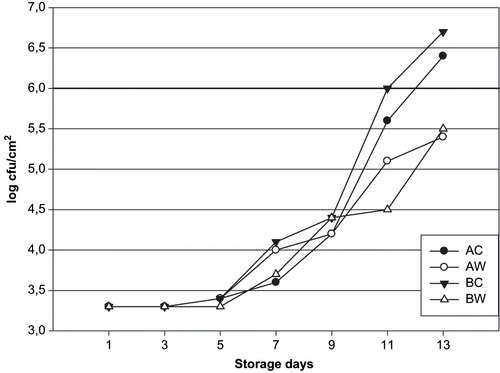
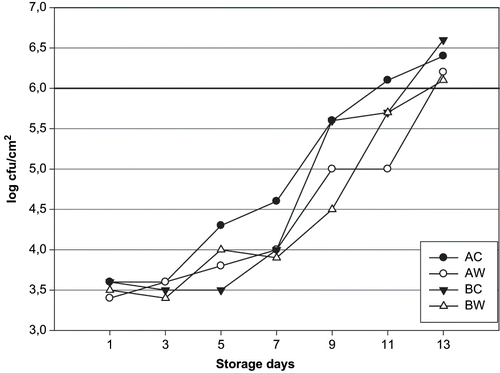
Bacterial spoilage in refrigerated fish and fish products under aerobic storage conditions is caused by Gram-negative psychrotrophic organisms such as Pseudomonas, Alteromonas, Flavobacterium spp., and H2S-producing bacteria (including Shewanella putrefaciens).[Citation50] These bacteria have been reported to be the specific spoilage bacteria in fish from temperate and tropical waters and in fresh Mediterranean fish stored aerobically under refrigeration or ice storage.[Citation4] Microbiological results are shown in , , and . The sea bream initial mesophilic aerobic bacteria counts of 3.6, 3.4, 3.6, and 3.5 log cfu/cm2 AC, AW, BC, and BW groups, respectively. These bacteria counts reached 6.1 log cfu/cm2 (in AC) 5 log cfu/cm2 (AW) (p < 0.05 significant), 5.7 log cfu/cm2 (BC and BW) (p > 0.05, not significant), respectively after 11 days of storage. The evaluation of the mesophilic aerobic bacteria count was highly linear throughout storage in all groups [AC, R2 = 0.955, y = 0.2x+2.10; AW,. Psychrotrophic bacteria counts for all groups increased with increasing time of ice storage (p < 0.05), (AC, R2 = 0.79, y = 0.28x+1.60; AW, R2 = 0.9151, y = 0.27x+1.64; BC, R2 = 0.9376, y = 0.26x+1.60; BW, R2 = 0.7939, y = 0.31x+1.36]. Initial psychrotrophic bacteria counts for AC, AW, BC, and BW groups were 3.8–3.5 log cfu/cm2, respectively. Psychrotrophic bacteria counts reached 6.7–6.0 log cfu/cm2 for AC and AW groups, and 6.8–6.1 log cfu/cm2 for BC and BW groups' sea bream, respectively, after 13 days of storage. Initial H2S-producing bacteria counts were 3.3 log cfu/cm2 for all group fish sampling periods, respectively, with final counts of 6.4 log cfu/cm2 (AC), 5.4 log cfu/cm2 (AW), 6.7 log cfu/g (BC) and 5.5 log cfu/cm2 (BW). The evaluation of the H2S-producing bacteria counts was highly linear throughout storage in all groups (AC, R2 = 0.8226, y = 0.26x+1.4; AW, R2 = 0.8127, y = 0.19x+1.76; BC, R2 = 0.8681, y = 0.30x+1.38; BW, R2 = 0.8633, y = 0.18x+1.74). Statistical analysis showed significant differences were found only for the mesophilic aerobic, psychrotrophic, and H2S-producing bacteria counts results in between the unwashed and washed groups (p < 0.05). (R2 = 0.882, y = 0.23x+1.83; BC, R2 = 0.860, y = 0.28x+1.71; BW, R2 = 0.874, y = 0.23x+1.83). Positive and significant correlations between the increase of bacterial counts and the sensory score were detected only in the all sea bream samples. Zhuang et al.[Citation51] have accepted the aerobic plate count limit of spoilage as 106–107 cfu/g. For fresh and refrigerated squid establish the microbial limit of acceptability at 6 cfu/g for psychrotropic bacteria.[Citation52] Huidobro et al.[Citation32] reported that mesophilic aerobic counts showed a slight increase for unwashed sea bream during storage, reaching a value of >7 log cfu/g (day 15), whereas for washing sea bream a corresponding value of >7 log cfu/g was recorded (day 17). Inacio et al.[Citation16] found that mesophilic aerobic bacteria counts was >5 log cfu/cm2 at the beginning of the storage period and its counts increased to over >7 log cfu/cm2 and <7 cfu/cm2 at the end of unwashed and washed whole scads (Trachurus trachurus) (12 days). They found initial H2S-producing bacteria counts of 4 log cfu/cm2 - 2 log cfu/cm2 in case of unwashed and washed samples, respectively. They also stated that the H2S-producing bacteria counts increased to counts of > 5 log cfu/cm2 and 5 log cfu/cm2 unwashed and washed fish days in ice. These findings are in agreement with the results described by Inacio et al.[Citation16] and Huidobro et al.[Citation32] Kyrana und Lougovois[Citation34] reported for sea bass after 1-day ice storage mesophilic counts of 3 log cfu/g, H2S-producing bacteria counts of 1.5 log cfu/g muscle with a maximum of 7 log cfu/g mesophilic count, 5 log cfu/g H2S-producing bacteria counts after 14–15 day (rejection time). Taliadourou et al.[Citation39] found initial of 3.5 log cfu/g in whole ungutted sea bass, respectively. They also stated that the total mesophilic counts increased to value of 5.5 log cfu/g in 9 days at 2 ± 2°C. These authors reported H2S-producing bacteria (including S putrefaciens) of 6 log cfu/g 13 days of storage of whole ungutted sea bass in ice. Lougovois et al.[Citation29] reported that the amount of mesophilic count and H2S-producing bacteria in sea bream flesh at the time of rejection (15 days) in ice was 6.3 log cfu/g and 5 log cfu/g. Paleologos et al.[Citation53] found a similar of mesophilic count and H2S-producing bacteria count (6 log cfu/g and 5.8 log cfu/g) in ungutted sea bass muscle and, in gutted sea bass muscle 7.5 log cfu/g- 6.6 log cfu/g in ice storage day 11.
Figure 6 Changes in mesophilic counts of aqua-cultured sea bream unwashed and washed with tap water.
CONCLUSIONS
The effects of washing with tap water on microbiological, chemical, and sensory properties of sea bream (Sparus aurata) stored in cold storage (+4°C) were studied. Sea bream stored in ice as sensory, chemical, and microbiological results for washed and unwashed sea bream showed that day 9 was the beginning of spoilage. The samples were unfit for human consumption after 12 days. In this study, it was found the microbiological count of washed sea bream samples lower (p < 0.05) than unwashed samples during the storage. Ice storage combined with tap water washing was also found to have the most inhibiting effects on microbial count during the storage.
ACKNOWLEDGMENTS
This study was supported by the Research Fund of the University of Istanbul (Project No: UDP-591/12072005).
Notes
17. EC Regulation. Council Regulation (EC) N 2406/96 of 26 November 1996 Laying Down Common Marketing Standards for Certain Fishery Products. (OJ L 334, 23.12.1996) 1996; 1–15
22. Hungerford, J.M., Ed. AOAC Official Method 971.14 Trimethylamine Nitrogen in Seafood Colorimetric Method. Fish and Other Marine Products. In Official Methods of Analysis of AOAC International; Cunniff, P.; Ed.; 1998; 7.
42. 95/149/EG Entscheidung der Kommission vom 8. März 1995 über TVB- Grenzewerte für bestimmte Kategorien von Fischereierzeugnissen und die anzuwenden Analysemethoden. Amtsblatt nr. L 097 vom 29/04/1995, S.0084–0087.
REFERENCES
- Chuapoehuk , P. , Raksakulthai , N. and Worawattanamateekul , W. 2001 . Process Development of Fish Sausage . Int. J. Food Proper , 4 ( 3 ) : 523 – 529 .
- Kasapis , S. , Al-Oufi , H.S. , Al-Maamari , S. , Al-Bulushi , I.M. and Goddard , S. 2004 . Scientific and Technological Aspects of Fish Product Development. Part I: Handshaking Instrumental Texture with Consumer Preference in Burgers . Int. J. Food Proper , 7 ( 3 ) : 449 – 462 .
- Gram , L. 1995 . “ Bacteriological changes. Paper No. 348 ” . In Quality and Quality Changes in Fresh Fish , Edited by: Huss , H. H. 51 – 64 . Rome : FAO Fisheries Technical Papers .
- Gram , L. and Huss , H. 1996 . H. Microbiological Spoilage of Fish and Fish Products . Int. J. Food Microbiol , 33 : 121 – 137 .
- Guillén-Velasro , S. , Ponce,-Alquiciro , E. , Forrés-González Soravia , A. and Guerrero-Legarreto , I. 2004 . Histamine Production by Two Enterobacteriaceae Strains Isolated from Tuna (Thunnus thynnus) and Jack Mackerel (Trachurus murphyii) . Int. J. Food Proper , 7 ( 1 ) : 91 – 103 .
- de Koning , A.J. 2004 . Rates of Cholesterol Ester Formation During Storage of Anchovy (Engraulis capensis) at Various Temperatures . Int. J. Food Proper , 7 ( 2 ) : 321 – 327 .
- Gram , L. and Dalgaard , P. 2002 . Fish Spoilage Bacteria—Problems and Solutions . Curr. Op. Biotechnol , 13 : 262 – 266 .
- Gram , L. and Melchiorsen , J. 1996 . Interaction between Fish Spoilage Bacteria PseudomonasSp. and Shewanella Putrefaciensin Fish Extract and on Fish Tissue . J. Appl. Bacteriol , 80 : 589 – 595 .
- Lakshmanan , R. , Venugopal , V. , Venketashvaran , K. and Bongiwar , D.R. 1999 . Bulk Preservation of Small Pelagic Fish by Gamma Irradiation: Studies on a Model Storage System using Anchovies . Food Res. Int , 32 : 707 – 713 .
- Sahin , S. and Sumnu , G. 2001 . Effects of Mircowave Cooking on Fish Quality . Int. J. Food Proper , 4 ( 3 ) : 501 – 512 .
- Jayasooriya , S.D. , Bhandari , B.R. , Torley , P. and Arcy , B.R.D. 2004 . Effect of High Power Ultrasound Waves on Properties of Meat: Review . Int. J. Food Proper , 7 ( 2 ) : 301 – 319 .
- Signorini , M. , Ponce-Alquicira , E. and Guerrero-Legarreto , I. 2003 . Proteolytic and Lipolytic Changes in Beef Inoculated with Spoilage Microorganisms and Bioprotective Lactic Acid Bacteria . Int. J. Food Proper , 6 ( 1 ) : 147 – 163 .
- Metin , S. , Erkan , N. , Varlik , C. and Özden , Ö. 2002 . Effect of Potassium Lactate on the Quality and Shelf-life of Chub Mackerel Scomber Japonicus . Fisheries Sci , 68 ( 1 ) : 210 – 214 .
- Erkan , N. 2003 . Tauchen von Fischfilets (Scomber colias und Mugil cephalus) in Natriumlactat und Propylgallat: Einfluss auf die Haltbarkeit und Qualität . Archiv Lebensmittelhyg , 54 ( 3 ) : 60 – 64 .
- Ahmad , S. , Anzar , A. , Srivastava , A.K. and Srivastava , P.K. 2005 . Effect of Curing, Antioxidant Treatment, and Smoking of Buffalo Meat on pH, Total Plate Count, Sensory Characteristics, and Shelf life during Refrigerated Storage . Int. J. Food Proper , 8 : 139 – 150 .
- Inácio , P. , Bernardo , F. and Vaz-Pirez , P. 2003 . Effect of Washing with Tap and Treated Seawater on the Quality of Whole Scad (Trachurus Trachurus) . Eur. Food Res. Technol , 217 : 416 – 411 .
- 17. EC Regulation. Council Regulation (EC) N 2406/96 of 26 November 1996 Laying Down Common Marketing Standards for Certain Fishery Products. (OJ L 334, 23.12.1996) 1996; 1–15
- Huss , H.H. Quality and Quality Changes in Fresh Fish . FAO Fisheries Technical paper, No: 348 . 1998 . FAO: Roma,
- Karl , H. , Meyer , C. and Münkner , W. 2001 . Vergleichende Eislagerung von ausgenommenen und unausgenommenen Schleien . Inf. Fisch Fischereifor , 48 : 3 – 139 .
- Vyncke , W. . pHof fish muscle: Comparison of methods 11th Western European Fish Technologists' Association (WEFTA) Meeting . Copenhagen, Denmark.
- Antonocoupoulos , N. and Vyncke , W. 1989 . Determination of Volatile Basic Nitrogen in Fish . Z Lebensm Unters, , 189 : 309 – 316 .
- 22. Hungerford, J.M., Ed. AOAC Official Method 971.14 Trimethylamine Nitrogen in Seafood Colorimetric Method. Fish and Other Marine Products. In Official Methods of Analysis of AOAC International; Cunniff, P.; Ed.; 1998; 7.
- Baumgard , J. 1986 . “ Lebensmittel tierischer Herkunft, Feinkosterzeugnisse, gefrorene, tiefgefrorene und getrocknete Lebensmittel, Fertiggerichte, hitzekonservierte Lebensmittel, Speiseeis, Zucker, Kakao, Zuckerwaren, Rohmassen ” . In Mikrobiologische Untersuchung von Lebensmitteln , Edited by: Baumgart , J. , von Jürgen Firnhaber , M. and Spicher , G. 25 Hamburg : Behr's Verlag .
- Lagrange , F. , Jark , U. , Etzel , V. and Feldhusen , F. 2003 . Einfluss des Ausnehmens auf die Qualität von frischem Seefisch . Arch für Lebensmittelhyg , 54 : 3 – 65 .
- Sümbüloğlu , K. and Sümbüloğlu , V. 2002 . Biyoistatistik. , Ankara : Tic. Ltd. Şti; Baski . Hatipoğlu Basim ve Yayim San.
- Huidobro , A. , Pastor , A. and Tejada , M. 2000 . Quality Index Method Developed for Raw Gilthead Seabream (Sparus Aurata) . J. Food Sci , 65 ( 7 ) : 1202 – 1205 .
- Kyrana , V.R. , Lougovois , V.P. and Valsamis , D.S. 1997 . Assessment of Shelf-life of Maricultured Gilthead Sea Bream (Sparus Aurata) Stored in Ice . Int. J. Food Sci. Technol , 32 : 339 – 347 .
- Alasalvar , C. , Taylor , K.D. , Öksüz , A. , Garthwaite , T. , Alexis , M.N. and Grigorakis , K. 2001 . Freshness Assessment of Cultured Sea Bream (Sparus Aurata) by Chemical, Physical and Sensory Methods . Food Chem , 72 ( 1 ) : 33 – 40 .
- Lougovois , V.P. , Kyranas , E.R. and Kyrana , V.R. 2003 . Comporasion of Selected Methods of Assessing Freshness Quality and Remaining Storage Life of Iced Gilthead Sea Bream (Sparus Aurata) . Food Res. Int , 36 : 551 – 560 .
- Poli , M.B. , Parisi , G. , Zambacavallo , G. , Mecatti , M. , Lupi , P. , Gualtieri , M. and Franci , O. 2001 . Quality Outline of European Sea Bass (Dicentrarchus labrax) Reared in İtaly: Shelf Life, Edible Yield, Nutritional and Dietetic Traits . Aquaculture , 202 : 303 – 313 .
- Erkan , N. and Özden , Ö. 2006 . Gutted and Ungutted Sea Bass (Dicentrarchus Labrax) Stored in Ice: Influence on Fish Quality and Shelf Life . Int. J. Food Proper , 9 ( 2 ) : 331 – 345 .
- Huidobro , A. , Mendes , R. and Nunes , M.L. 2001 . Washing effect on the quality index method (QIM) developed for raw gilthead seabream ( Sparus aurata) . Eur Food Res Technol, , 212 : 408 – 412 .
- Ludorff , W. and Meyer , V. 1973 . Fische und Fischerzeugnisse , Hamburg und Berlin : Verlag Paul Parey .
- Kyrana , V.R. and Lougovois , V.P. 2002 . Sensory, Chemical and Microbiological Assessment of Farm-raised European Sea Bass (Dicentrarchus Labrax) Stored in Melting Ice . Int. J. Food Sci. Technol. , 37 : 319 – 328 .
- Masniyom , P. and Benjakul , S. 2002 . and Visessanguan, W. Shelf- life Extension of Refrigerated Sea Bass Slices under Modified Atmosphere Packaging . J. Sci. Food Agric , 82 : 873 – 880 .
- Tejada , M. and Huidobro , A. 2002 . Quality of Farmed Gilthead Seabream (Sparus Aurata) during Ice Storage Related to the Slaughter Method and Gutting . Eur. Food Res. Technol , 215 : 1 – 7 .
- Papadopoulos , V. , Chouliara , I. , Badeka , A. , Savvaidis , I.N. and Kontominas , M.G. 2003 . Effect of Gutting on Microbiological, Chemical, and Sensory Properties, of Aqua-cultured Sea Bass (Dicentrarchus Labrax) Stored in Ice . Food Microbiol , 20 : 414 – 420 .
- Grigorakis , K. , Taylor , K.D.A. and Alexis , M. 2003 . Seasonal Patterns of Spoilage of Ice-stored Cultured Gilthead Sea Bream (Sparus Aurata) . Food Chem , 81 : 263 – 268 .
- Taliadourou , D. , Papadopoulos , V. , Domvridou , E. , Savvaidis , I. and Kontominas , G.M. 2003 . Microbiological, Chemical and Sensory Changes of Whole and Filleted Mediterranean Aquacultured Sea Bass (Dicentrarchus Labrax) Stored in Ice . J. Sci. Food Agric , 83 : 1373 – 1379 .
- Koutsoumanis , K. and Nychas , G.J.E. 2000 . Application of a Systematic Procedure to Develop a Microbial Model for Rapid Fish Shelf-life Predictions . Int. J. Food Microbiol , 60 : 171 – 184 .
- Huidobro , A. , Mendes , R. and Nunes , M.L. 2001 . Slaughtering of Gilthead Sea Bream (Sparus Aurata) in Liquid Ice: Influence on Fish Quality . Eur. Food Res. Technol , 213 : 267 – 272 .
- 42. 95/149/EG Entscheidung der Kommission vom 8. März 1995 über TVB- Grenzewerte für bestimmte Kategorien von Fischereierzeugnissen und die anzuwenden Analysemethoden. Amtsblatt nr. L 097 vom 29/04/1995, S.0084–0087.
- de Koning , A.J. 2002 . Quantitative Quality Tests for Fish Meal. II. An Investigation of the Quality of South African Fish Meals and the Validity of a Number of Chemical Quality Indices . Int. J. Food Proper , 5 ( 3 ) : 495 – 507 .
- Koutsoumanis , K. and Nychas , G.J.E. 1999 . Chemical and Sensory Changes Associated with Microbial Flora of Mediterranean Boque (Boops Boops) Aerobically at 1°C, 3°C, 7°C and 10°C . Appl. Environ. Microbiol , 65 : 698 – 706 .
- Erkan , N. 2003 . Verderb in Fisch und Fischwaren, Chemische und Mikrobiologische Veränderungen und Gefahren . Rund Fleischhyg Lebensmittelüber , 55 ( 11 ) : 254 – 258 .
- Meyer , C. 1997 . Bedeutung von Shewanella putrefaciens bei der Bestimmung der Frische von Fisch . Schriften der Bundesforschungsanstalt für Fischerei , Nr.24 : 72 – 77 .
- Sikorski , Z.E. , Kolakowska , A. and Burt , J.R. 1990 . Post Harvest Biochemical and Microbial Changes Seafood; Resources Nutritional Composition and Preservation , Edited by: Sikorski , Z.E. 55 – 77 . Boca Raton, FL : CRC Press-Inc .
- Dalgaard , P. , Gram , L. and Huss , H. 1993 . Spoilage and Shelf Life of Cod Fillets Packed in Vacuum or Modified Atmospheres . Int. J. Food Microbiol , 19 : 283 – 294 .
- Rodriguez , C.J. , Besteiro , I. and Pascual , C. 1999 . Biochemical Changes in Freshwater Rainbow Trout (Oncorhynchus Mykiss) during Chilled Storage . J. Sci. Food Agric , 79 ( 11 ) : 1473 – 1480 .
- Huss , H.H. Assurance of Seafood Quality . FAO Fisheries Technical paper No: 334 . 1994 . FAO: Roma,
- Zhuang , R.Y. , Huang , Y.W. and Beuchat , L.R. 1996 . Quality Changes during Refrigerated Storage of Packaged Shrimp and Catfish Fillets Treated with Sodium Acetate, Sodium Lactate or Propyl Gallate . J. Food Sci , 61 ( 1 ) : 241 – 244 .
- Lapa-Guimarães , J. , Aparecida Azavedo da Silva , M. , Eduardo de Felicio , P. and Contreras Guzman , E. 2002 . Sensory, Colour and Psychrotrophic Bacterial Analyses of Squids (Loligo Plei) during Storage in Ice . Lebensm.-Wiss. U.- Technol , 35 : 21 – 29 .
- Paleologos , E.K. , Savvaidis , I.N. and Kontominas , M.G. 2004 . Biogenic Amines Formation and Its Relation to Microbiological and Sensory Attributes in Ice-stored Whole, Gutted and Filleted Mediterranean Sea Bass (Dicentrarchus Labrax) . Food Microbiol , 21 : 549 – 557 .