Abstract
Plant phenolics have gained considerable interest in recent years for their potential effects against food related microorganisms. In the present study, phenolic extracts from the leaves of three plants namely Moringa oleifera, Morus indica, and Mentha spicata were prepared by a mixture of methanol/acetone/water. The UV spectra of extracts were recorded and contents of total phenolics determined. The extracts were incorporated in pineapple juice and their solubility and stability were studied. In addition, the acceptability of treated juice samples was sensory evaluated. The solubility of M. indica and M. oleifera extracts was found to be more than that of M. spicata. The two extracts were also stable in the pH environment of pineapple juice when stored at 4°C for two weeks. The pineapple juice treated with M. indica extract was more acceptable compared to that of M. oleifera, according to sensory evaluation. Therefore, the effect of addition of phenolic extract from M. indica on the shelf life of pineapple juice stored at 4°C was investigated by monitoring the changes in titrable acidity and sensory parameters for 8 weeks. Results indicated that the extracts of natural phenolic compounds can be used to improve the quality and safety of foods.
INTRODUCTION
Increased concerns over the possible toxicological effects of synthetic food additives,[Citation1,Citation2] as well as consumer preference for natural products have resulted in increased interest towards the search for natural substances[Citation3,Citation4,Citation5] possessing antimicrobial and antioxidant properties. Spices and herbs have been used from prehistorical times not only for flavoring foods but also for their food preserving properties due to the presence of antioxidative and antimicrobial constituents.[Citation6] Natural aromatic plants and spices have been widely used in many food products such as meat[Citation7,Citation8,Citation9] and bakery products.[Citation10,Citation11,Citation12]
Phenolic compounds constitute one of the most numerous and ubiquitous groups of plant metabolites, and therefore, are an integral part of both human and animal diet.[Citation13] They seem to be involved in the defense of plants against invading pathogens including insects, bacteria, fungi, and viruses[Citation13,Citation14] and contribute to their colour and sensory properties such as bitterness and astringency.[Citation15] Historically, phenolic compounds have been considered as anti-nutrient by nutritionists, because some, e.g. tannins, have such adverse effects as decreasing the activities of digestive enzymes, energy, protein and amino acid availability, mineral uptake, and having other toxic effects. Recent interest, however, in food phenolics has increased greatly because of the antioxidant and free radical scavenging abilities associated with some phenolics and their potential effects on human health.[Citation13] Several reviews deal with the antimicrobial activity of phenolics in defined systems and against food related microorganisms.[Citation16,Citation17,Citation18] One of the earliest studies examining the antimicrobial activity of phenolics in food was that of Nickerson and Starr.[Citation19] Phenolic compounds have been used in the food industry as natural colorants and preservatives.[Citation13,Citation20]
As part of a program designed to discover new naturally occurring preservatives as alternative to synthetic ones, we would like to incorporate such compounds into food to protect them against spoilage caused by microorganisms. The objective of this article was to evaluate the efficiency of plant phenolic extracts in retardation of spoilage in fruit juice. For this purpose, solubility, stability, and acceptability of phenolic extracts from the leaves of three plants namely, Morus indica, Moringa oleifera, and Mentha spicata were assessed in the model system of pineapple juice. Accordingly, the M.indica extract was selected to study the effect of natural phenolics on the storage stability of pineapple juice.
MATERIALS AND METHODS
Materials
M. indica (MI) leaves were collected from the Department of Sericulture, University of Mysore, Mysore, India. M. oleifera (MO) and M. spicata (MS) leaves were obtained from local market, Mysore, India. The leaves were cleaned, washed, and dried in a hot air oven (50°C). The material was ground to a fine powder, passed through a 60-mesh sieve and stored in air tight containers at 4°C until used.
Preparation of Phenolic Extracts
Phenolic extract (PE) from each plant material was prepared by the method of Jimeneze-Escrig et al.[Citation21] One gram of dried plant material was placed in a test tube; 40ml of methanol/ water (1:1;v/v) and sufficient HCl to obtain a final pH 2.0 were added. The tube was thoroughly shaken at room temperature for 1 h, centrifuged at 2500 g for 10 min, and the supernatant was recovered. Forty milliliters of acetone/water (7:3;v/v) was added to the residue and shaking and centrifugation were repeated. The extracts were mixed, methanol and acetone were evaporated in vacuum at 40°C, and the remaining aqueous solution was freeze-dried to dryness. The content of total phenolics in extracts was determined according to the Folin- Ciocalteu micro method,[Citation22] using gallic acid as standard and expressing the results as gallic acid equivalents. The UV spectra of extracts were recorded at wavelengths ranging from 200 to 400 nm using a Systronic UV-VIS spectrophotometer 117.
Solubility and Stability of Phenolic Extracts Added to Pineapple Juice
Pineapples were peeled, pulped, and squeezed to extract the juice. The extracted juice was filtered using a muslin cloth to obtain a clear juice. The solubility and storage stability of phenolic extracts in pineapple juice was determined according to the method of Friedman and Jurgens.[Citation14] Each extract (500 mg) was dissolved in 25 ml of pure juice. The suspension was stirred with constant agitation on a magnetic stirrer for 2 h. The solution obtained was used to start two series of experiments. Each series (A and B) started with 10 ml of the 20 mg/ml PE/juice mixture and 10 ml of pure juice, measured with 10 ml volume pipette. The concentrations were halved four times to give final concentrations of 20, 10, 5, 2.5, and 1.25 mg/ml. After the dilutions were completed, all solutions were diluted two times with a 1:19 juice/water mixture; the final dilutions were made immediately before each measurement. The final concentrations ranged from 1.25 × 10−2 to 2 × 10−1 mg/ml. Since the PE from MI and MO were more soluble than that of MS, they were selected for further studies.
The following experiment was carried out to assess the stability of phenolic extracts added to pineapple juice. Solutions of 100 mg of PE from MI and MO in 10 ml of juice were prepared, separately. Each solution (1 ml) was diluted with 9 ml of pure juice and stored at 4°C. The absorption of PE in each solution (at corresponding λmax) was determined at zero time and then at 48 h intervals for 2 weeks. Before each measurement, the stock solutions were diluted to 0.001% with a fresh 1:19 juice/water mixture.
Acceptability of Pineapple Juice Treated with PE
The pineapple juice was sweetened (2% sugar) according to taste. The PE from MI and MO were added to the juice separately and subjected to sensory evaluation. Results indicated that the juice treated with MI extract was more acceptable in terms of flavor, taste, color, and appearance compared to that of MO extract. Therefore, MI extract was selected for further studies. The selected extract was added to pineapple juice at different levels (0.25, 0.5, and 1%) and organoleptic characteristics were evaluated. The data indicated that the threshold level of MI extract in juice was 0.25%.
Storage Stability of Pineapple Juice
Based on the results of solubility, stability, and acceptability studies, the MI extract (at level of 0.25%) was selected to study the effect of natural phenolics from plants on the shelf life of pineapple juice. Juice was prepared to provide three variations. Control sample was prepared without addition of any preservative. The other two variations were prepared with addition of synthetic preservative (sodium benzoate, 200 ppm) as standard and natural phenolic extract (PE of MI, 0.25%). Juice variations were stored at 4°C and changes in titrable acidity and sensory parameters recorded at intervals of 2 weeks over a period of 8 weeks.
Titrable Acidity
An aliquot of sample was diluted with distilled water (1:20; v/v) and boiled to expel excess CO2. The solution (25 ml) was titrated against 0.1 N NaOH and titrable acidity was expressed as percent citric acid (anhydrous) equivalents.[Citation23] The analysis was carried out in 2 replicates.
Sensory Studies
Sensory evaluation of juices (freshly prepared and stored) was conducted to compare the acceptability of products during storage period. Six panelists were selected among the research scholars and post-graduated students in the Department of Food Science and Nutrition, on the basis of their willingness to participate and also a sweet threshold test. Panelists were presented with the product on 2 occasions to familiarize them with the quality attributes. Three differently coded samples were served to the panelists and sensory scores for different attributes like color, flavor, appearance, taste, and overall quality were obtained.
Statistical Analysis
Data were subjected to ANOVA, followed by Duncan's Multiple Range Test.[Citation24]
RESULTS
Yield of Extraction and Total Phenolics
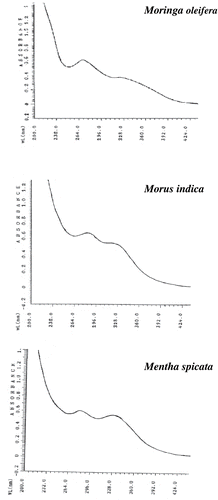
The yield of extraction and total phenolics data are presented in . The content of total phenolics (mg gallic acid per g of extract) ranged from 130.50 in MI extract to 162.50 in MO extract. The extracts of MI and MS were characterized by a higher amount of total phenolics. The UV spectra of phenolic extracts of selected plant materials are shown in . The spectrum of PE from MS was characterized by two absorption maxima at 268 and 328 nm, indicating both flavonoids and phenolic acids are predominant groups of phenolics present in this extract. Absorption maxima for UV spectra of PE from MI and MO were noted at 283 and 270 nm, respectively, which were not typical for phenolic acids, but it showed a shoulder in the range of 320–330 nm. Thus, flavonoids might be the major phenolic compounds in these extracts. These results are in good agreement with previous reports for MO [Citation25] and MI.[Citation26]
Table 1 Yield of extraction, content of total phenolics, and UV data of phenolic extracts
Solubility and Stability
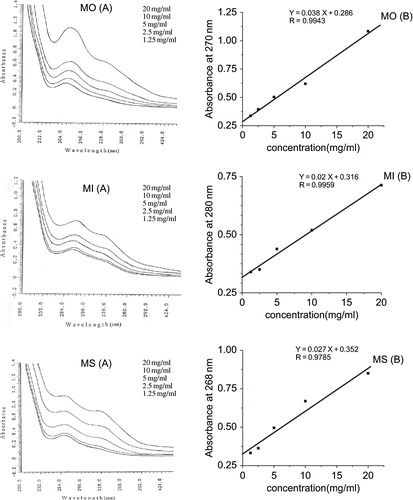
The absorption spectra of different amounts of phenolic extracts dissolved in pineapple juice are illustrated in . A plot of concentration versus absorbance maximum for each extract showed a linear fit of data with 95% confidence intervals, indicating that UV spectroscopy can be used to determine the amount of phenolics added in the juice. However, the correlation coefficient (R2) between absorbance of maximum of UV spectra (Y) and concentration of PE from MI and MO were 0.9959 and 0.9943 respectively, indicating better solubility of these extracts compared to that of MS (R2 = 0.9785). Therefore, The PE from MI and MO were selected for further studies.
Figure 2 Solubility of phenolic extracts added to pineapple juice: (A) Concentration dependence of absorption spectra; (B) linear relationship between concentration and absorbance at maximum wavelength. MO: Moringa oleifera, MI: Morus indica, MS: Mentha spicata.
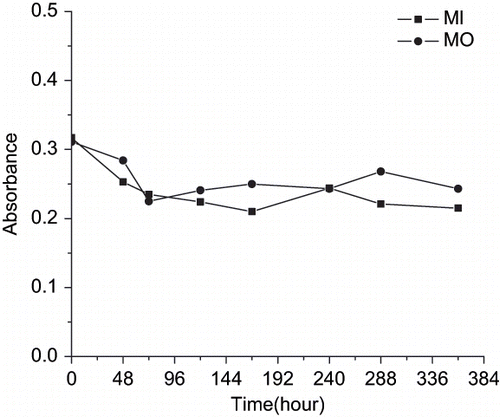
depicts the changes of absorbance of PE of MI and MO in the model solution of pineapple juice during storage at 4°C for two weeks. Results indicated that these extracts were relatively stable in the pH environment of juice, as the amount of absorbance measured in related wavelength did not change significantly (p < 0.05) during storage period.
Storage Stability of Pineapple Juice
Titrable acidity
Measurement of titrable acidity is helpful in study of preservation process and preservation action in fruit products. The changes occurring in acidity values of juice variations during storage are presented in . Titrable acidity of control, did not differ significantly (p < 0.05) in 2nd and 4th week of storage after which it started to increase gradually (p < 0.05). Acidity value of the standard sample was unchanged (p < 0.05) during storage. In the juice treated with PE from MI, acidity value did not differ significantly (p < 0.05) up to 8 weeks. This might be attributed to potency of these natural compounds to inhibit activity of microorganisms.
Table 2 Titrable acidity (% citric acid equivalents) of pineapple juice variations during storage (∼4°C, 8 weeks)
Sensory studies
summarizes the results of sensory analysis of the pineapple juice variations and gives the mean scores for overall acceptability (during the storage period and between the variations). In the control, which did not contain any preservative, the mean scores assigned by the panelists were significantly lower (p < 0.05) after 4th week, which indicates that control was not acceptable compared to zero and 2nd week of evaluation. In standard (containing 200 ppm of sodium benzoate) the mean scores did not differ during the storage period. In the sample treated with PE from MI, it was interesting to note that the overall acceptability did not differ even after 8 weeks of storage. However, the juice treated with sodium benzoate received higher (p < 0.05) scores on 6th and 8th week than control and juice treated with natural extract.
Table 3 Acceptability of pineapple juice variations during storage (∼4°C, 8 weeks)
DISCUSSION
In the present study, the leaves of three different plant materials namely, M. oleifera, M. indica, and M. spicata were used as sources of naturally occurring phenolic compounds. The samples were analyzed for the content of total phenolics. The three samples had a high content of total phenolics, however, it was higher in MS and MO compared to MI. The content of total phenolics of MO extract was higher than that of reported earlier in Moringa leaves from different agro-climatic origins.[Citation25] The content of total phenolics (mg gallic acid /g of extract) in water extract of M. spicata was reported to be 231,[Citation27] which is higher than the value obtained in the present study (158.75). These differences might be due to different procedures used for extraction of phenolic compounds as well as the growing location, stage of leaf development, genetic variability, and post harvest handling of the leaf sample. Several reports are also available on the content of total phenolics of other green vegetables.[Citation21,Citation28,Citation29]
In order to evaluate the efficiency of these natural compounds in retention of fruit juices, several points such as solubility, stability and acceptability need to be considered in their selection and use. In this study, the phenolic extracts from selected sources were added at different levels (0.1–2%) to pineapple juice as a model system and their solubility, stability and acceptability were studied. Accordingly, the PE from MI was selected for further studies.
The effect of addition of the MI extract on the storage stability of pineapple juice stored at 4°C was determined by monitoring changes in titrable acidity periodically over a period of 8 weeks. In addition, sensory evaluation of juice variations was conducted to determine their acceptability during storage period.
Shelf life is a major consideration in developing, producing and marketing of food products. It refers to the time during which a product remains acceptable to the consumer in terms of sensory properties. Many factors influence the shelf life of a product such as moisture loss, spoilage due to microorganisms, enzymatic changes, and oxidation.[Citation30] Preservatives, when added to food, help in preventing or retarding decomposition, fermentation, or acidification of food. An ideal substance should be non-toxic, economical, with a wide range of antimicrobial activity, with no effect on flavor, taste, aroma, and so on.[Citation23] Most manufacturers use heat or synthetic antimicrobial agents to extend the shelf life of fruit juices. Unlike chemically synthesized antimicrobial agents, those from natural sources are acceptable to consumers. Since heat treatment requires energy, it adds to the cost of products. Moreover, heat treatment can induce non-enzymatic browning reactions between amino acids, proteins, and carbohydrates present in some juices to form Maillard browning products.[Citation31,Citation32] Heat and storage are also reported to diminish both phenolic compounds and vitamins, especially vitamin C.[Citation33] For these reasons, a need exists to develop new preservative compounds from natural sources that will protect juices against spoilage. In this study, the pineapple juice without addition of any preservative was stable for 4 weeks, which could be attributed to the naturally occurring phenolic compounds and sugar present in the juice. However, the PE from MI was able to retard the process of spoilage, as the titrable acidity value of juice treated with this extract was significantly lower than that of control, even at the level of 0.25%. The juice treated with PE was also acceptable for up to 6 weeks. The MI extract was found to exhibit activity against both G (+) and G (−) bacteria.[Citation34] It is reported that certain naturally occurring phenolic compounds including chlorogenic acid and gallic acid and tea polyphenols possess antimicrobial activities.[Citation14] The effectiveness of such compounds against infection by human pathogens has been already demonstrated for milk[Citation35] and with unnatural additives for apple juice.[Citation36]
In conclusion, present results demonstrated that naturally occurring phenolic compounds can be used to increase the shelf life and safety of fruit juice. They are safe and impart health benefits to the consumer. However, it seems necessary to know the composition of the phenolic extracts examined and to identify the individual compounds in order to fully explain the obtained results. These studies are in progress in our laboratory.
Notes
34. Unpublished observations.
REFERENCES
- Namiki , M. 1990 . Antioxidants/Antimutagens in Foods . Crit. Rev. Food Sci. Nutr. , 20 : 273 – 300 .
- Barlow , S.M. 1990 . “ Toxicological Aspects of Antioxidants Used as Additives ” . In Food Antioxidants , Edited by: Hudson , B.J.F. 253 – 307 . London : Elsevier .
- Gazzani , G. , Papetti , A. , Massolini , G. and Daglia , M. 1998 . Antioxidant and Prooxidant Activity of Water Soluble Components of Some Common Diet Vegetables and the Effect of Thermal Treatment . Food Chem. , 6 : 4118 – 4122 .
- Cown , N.M. 1999 . Plant Products as Antimicrobial Agents . Clin. Microbiol. Rev. , 12 : 564 – 582 .
- Amakura , Y. , Umino , Y. , Tsuji , S. , Ito , H. , Hatano , T. , Yoshido , T. and Tonogai , Y. 2002 . Constituents and Their Antioxidative Effect in Eucalyptus Leaf Extract Used as Natural Food Additive . Food Chem. , 77 : 47 – 56 .
- Nakatani , N. 1997 . “ Antioxidants from Spices and Herbs ” . In Natural Antioxidants. Chemistry, Health Effects and Applications , Edited by: Shahidi , F. 64 – 73 . Champaign, IL : AOCS Press .
- Kayaardi , S. , Durak , F. , Kayacier , A. and Kayaardi , M. 2005 . Chemical Characteristics of Kavurma with Selected Condiments . Int. J. Food Prop. , 8 : 513 – 520 .
- Ahn , J. , Grün , I.U. and Fernando , L.N. 2002 . Antioxidant Properties of Plant Extracts Containing Polyphenolic Compounds in Cooked Ground Beef . J. Food Sci. , 67 : 1364 – 1369 .
- Wong , P.Y.Y. and Kitts , D.D. 2002 . The Effect of Herbal Pre-seasoning on Microbial and Oxidative Changes in Irradiated Beef Steaks . Food Chem. , 76 : 197 – 205 .
- Reddy , V. , Urooj , A. and Kumar , A. 2005 . Evaluation of Antioxidant Activity of Some Plant Extracts and Their Application in Biscuits . Food Chem. , 90 : 317 – 321 .
- Bajaj , S. , Urooj , A. and Prabhasankar , P. 2006 . Effect of Incorporation of Mint on Texture, Colour and Sensory Parameters of Biscuits . Int. J. Food Prop. , 9 : 961 – 700 .
- Bassiouny , S.S. , Hassanien , F.R. , El-Razik , A.F. and El-Kayati , S.M. 1990 . Efficiency of Antioxidants from Natural Sources in Bakery Products . Food Chem. , 37 : 297 – 305 .
- Bravo , L. 1998 . Polyphenols: Chemistry, Dietary Source, Metabolism, and Nutritional Significance . Nutr. Rev. , 56 : 317 – 333 .
- Friedman , M. and Jurgens , H.S. 2000 . Effect of pH on the Stability of Plant Phenolic Compounds . J. Agric. Food Chem. , 48 : 2101 – 2110 .
- Mozetic , B. and Trebse , P. 2004 . Identification of Sweet Cherry Anthocyanins and Hydroxycinnamic Acids Using HPLC Coupled with DAD and MS Detector . Acta. Chim. Slov. , 51 : 151 – 158 .
- Branen , A.L. , Davidson , P.M. and Katz , B. 1980 . Antimicrobial Properties of Phenolic Antioxidants and Lipids . Food Tech. , 34 : 42 – 53 . 63
- Davidson , P.M. and Branen , A.L. 1981 . Antimicrobial Activity of Non-halogenated Phenolic Compounds . J. Food. Prot. , 44 : 623 – 632 .
- Kabara , J.J. 1981 . Food Grade Chemicals for Use in Designing Food Preservative Systems . J. Food Prot. , 44 : 633 – 647 .
- Nickerson , J.T.R. and Starr , L.D. Treatment of Processed Animal Tissue . U.S. patent 2,933,399 . 1960 .
- Dillard , C.J. and German , J.B. 2000 . Phytochemicals: Nutraceuticals and Human Health . J. Sci. Food Agric. , 80 : 1744 – 1756 .
- Jimeneze-Escrig , A.J. , Dragsted , L.O. , Daneshvar , B. , Pulido , R. and Calixto , F.S. 2003 . In-vitro Antioxidant Activities of Edible Artichoke (Cynara Scolymus L.) and Effect on Biomarkers of Antioxidants in Rats . J. Agric. Food Chem. , 51 : 5540 – 5545 .
- Slinkard , K. and Singleton , V.L. 1977 . Total Phenol Analysis; Automation and Comparison with Manual Methods . American Journal of Enology and Viticulture , 28 : 49 – 55 .
- Sathe , A.Y. 1999 . A First Course in Food Analysis , Vol. 32 , 142 New Delhi : New Age International (p) Ltd., Publishers .
- Steel , R.G.D. and Torrie , J.H. 1980 . Principles and Procedures of Statistics , New York : McGraw-Hill .
- Siddhuraju , P. and Becker , K. 2003 . Antioxidant Properties of Various Solvent Extracts of Total Phenolic Constituents from Three Different Agro Climatic Origins of Drumstick Tree (Moringa Oleifera Lam) Leaves . J. Agric. Food Chem. , 51 : 2144 – 2155 .
- Zhishen , J. , Mengcheng , T. and Jianming , W. 1999 . The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals . Food Chem. , 64 : 555 – 559 .
- Dorman , D.H.J. , Kosa , M. , Kahlos , K. , Holm , Y. and Hiltunen , R. 2003 . Antioxidant Properties and Composition of Aqueous Extracts from Mentha Species, Hybrids, Varieties, and Cultivars . J. Agric. Food Chem. , 51 : 4563 – 4569 .
- Vinson , J.A. , Hao , Y. , Su , X. and Zubik , L. 1998 . Phenol Antioxidant Quality and Quantity in Foods: Vegetables . J Agric. Food Chem. , 46 : 3630 – 3634 .
- Cano , A. and Arnao , M.B. 2005 . Hydrophilic and Lipophilic Antioxidant Activity in Different Leaves of Three Lettuce Varieties . Int. J. Food Prop. , 8 : 521 – 528 .
- Adegoke , G.O. , Vijay Kumar , M. , Gopal Krishna , A.G. , Varadarage , M.C. , Sambaiah , K. and Lokesh , B.R. 1998 . Antioxidants and Lipid Oxidation in Foods: A Critical Appraisal . J. Food Sci. Tech. , 35 : 283 – 298 .
- Friedman , M. 1996 . Food Browning and Its Prevention . J. Agric. Food Chem. , 44 : 631 – 653 .
- Molnar-Perl , I. and Friedman , M. 1990 . Inhibition of Browning by Sulfur Amino Acids. 2. Fruit Juices and Protein Containing Foods . J. Agric. Food Chem. , 38 : 1648 – 1651 .
- Friedman , M. , Gumbmann , M.R. and Ziderman , I. I. 1987 . Nutritional Value and Safety in Mice of Proteins and Their Mixtures with Carbohydrates and Vitamin C After Heating . J. Nutr. , 117 : 508 – 518 .
- 34. Unpublished observations.
- Payne , K.D. , Rico-Munoz , E. and Daridso , P.M. 1989 . The Antimicrobial Activity of Phenolic Compounds Against Listeria Monocytogenes and Their Effectiveness in a Model Milk System . J. Food Prot. , 52 : 151 – 153 .
- Fisher , T.L. and Golden , D.A. 1998 . Survival of Escherichia coli 0157: H7 in Apple Cider as Affected by Dimethyl Dicarbonate, Sodium Bisulfite, and Sodium Benzoate . J. Food Sci. , 63 : 904 – 906 .