Abstract
To minimize eggshell waste, calcium in eggshells was extracted as calcium chloride using 4% (w/v) HCl solution for an extraction period of 3 hs with the ratio of eggshell to HCl being 1:15 (w/v). After hydrolysis, the residues were removed by centrifugation at 1774 × g for 10 min, and the solution was heated to 110–115°C until dried, this gave an eggshell calcium chloride at a yield of 87.38% (w/w). The calcium chloride powder in this study was composed of 0.3% protein and 94.37% ash, with pH 5.27 and showed high solubility. It contained minute amount of heavy metal constituents within the specification of the Thai Food Act. X-ray diffraction analysis indicated that eggshell calcium chloride powder thus prepared was composed mainly of CaCl2.2H2O. The eggshell CaCl2 was also tested for its functional property as a firming agent in canned rambutan. The results showed that both eggshell and commercial calcium chloride gave a firm texture to canned rambutan, therefore eggshell CaCl2 can be prepared and used as food processing aids.
INTRODUCTION
Eggs weighing 100 kg consist of approximately 8–11 kg shell—the shell consists of 96 kg inorganic matter and only 4 kg organic matter per 100 kg shell solid.[Citation1] According to Stadelman,[Citation1] the composition of hen eggshell has been reported to be 94 kg calcium carbonate, 1 kg magnesium carbonate, and 1 kg calcium phosphate per 100 kg eggshell. In Thailand, ground eggshell is used in poultry feed as a substitute for limestone, and it may also be used as an ingredient in fertilizer. However, large quantities of eggshell from the hatchery and food industries are disposed of as solid waste every day. Eggshell has recently been used as human food, but the process requires certain steps in order to eliminate pathogenic microorganisms.[Citation2,Citation3] Sugoro et al.[Citation2] produced eggshell calcium using a drying temperature of 350°C and claimed that it was a good source of dietary calcium. Daengprok et al.[Citation3] fortified fermented pork sausages with calcium lactate made from eggshell. Omi and Ezawa[Citation4] reported that calcium from crushed eggshell powder was absorbed easier than commercial calcium carbonate in the small intestine of rats. Chicken eggshell matrix proteins were isolated and demonstrated their enhancement of calcium transport in human intestinal epithelial cells using Caco-2 cell line.[Citation5]
Other forms of calcium salts, however, beside calcium carbonate have been used as processing aids in food products manufacturing. These calcium salts include calcium chloride, calcium citrate, and calcium lactate. In particular, calcium chloride has been widely used as a thickening agent in dairy products and as a firming agent in some fruit and vegetable products. Injection and marination of calcium chloride solutions increase postmortem tenderization of beef steak[Citation6] and increase in water holding capacity of horse meat.[Citation7] It is known as a general purpose food additive. Therefore, the production of calcium chloride from eggshell could provide an opportunity for its utilization, adding more value to a natural resource which is normally disposed as waste.
The specification of the form of calcium chloride that can be used in food products has been delineated in the Code of Federal Regulations authorized by Thai FDA. For consumer safety, calcium chloride salt used as food additive shall contain only a minute amount of heavy metal constituents. Also, the calcium chloride solution obtained from dissolving calcium chloride in water at a ratio of 1:20 (salt:water) shall have a pH of 4.5–8.5.[Citation8] This experiment was designed to develop a laboratory process for the production of eggshell calcium chloride. The characteristics of eggshell calcium chloride specified in Thai Food Act were studied and tested its functional properties as a firming agent in canned rambutan.
MATERIALS AND METHODS
Materials
Hen eggshells from a small bakery shop were collected and used in the experiment. Eggshells were washed both inside and outside to get rid of dirt and other organic materials, and then sun dried and crushed to small pieces (approximately 0.25 cm). Crushed eggshells were dried in a tray dryer at 85°C for 4 h, and then ground to a particle size of around 0.2 mm. The proximate analysis of dried eggshell was carried out according to AOAC.[Citation9] The crushed and dried eggshells were then packed in plastic bags and stored in a desiccator at room temperature and used as raw material for the entire experiment .
Concentrated analytical reagent grade hydrochloric acid was used to prepare the acid solution in these studies. The commercial calcium chloride salt that was used as a firming agent in canned rambutan was obtained from Dole Thailand Co. Ltd. It is specified as 74–76% CaCl2.2H2O, pH 5.6, one gram of calcium chloride dissolves in 1.2 ml distilled water. Rambutan fruits (average size 15 gm/fruit without seed) were also provided by the same factory where the entire canning process was performed using the standard commercial production. The commercial calcium chloride salt used as a sample for X-ray diffraction analysis was supplied by Witcorp Chemicals Ltd.,Thailand. It is specified as calcium chloride (flake) consisted of 74.83% CaCl2, 3.01% NaCl, and 20 ppm iron, no indication of the existing form of CaCl2
Eggshell Calcium Chloride Preparation
shows the extraction process for eggshell calcium chloride. In order to prepare eggshell calcium chloride, crushed eggshells were mixed with a hydrochloric acid solution and stirred occasionally until no gas bubbles were observed (3 h). The mixture was centrifuged at 1774 × g for 10 min, the supernatant was separated and then heated to 110–115°C until dried, this yielded calcium chloride crystals or eggshell calcium chloride. Two factors that affected the yield of calcium chloride in the extraction process were studied. First, the concentration of hydrochloric acid was studied at 3 levels: 3%, 4%, and 5% (w/v). Second the ratio of eggshell to acid solution was also studied at 3 levels: 1:5, 1:10, and 1:15 (w/v). After the percent yield of eggshell calcium chloride was measured for each of these variables, the condition that gave the highest yield was chosen. The calculation of percent yield is shown in equation below. Two replicates were done for each treatment. The experimental design was 3 × 3 factorial, where the samples were assigned randomly. Data were analyzed by Analysis of Variance and means were compared by the Least Significance Difference. Significance was defined at P < 0.01.
Properties of Calcium Chloride Derived from Eggshells
Eggshell CaCl2 was analyzed for protein, lipid, moisture, and ash content following the method of AOAC.[Citation9] The methods used to characterize CaCl2 were recommended by Thai Food Act.[Citation8] The pH was measured using the method designated by the ASTM.[Citation10] X‐ray diffraction pattern of CaCl2 was obtained by an X-ray diffraction unit equipped with a copper tube X-ray generator and a nickel X-ray filter (Philips: X'Pert, serviced at the Department of Materials Engineering, Kasetsart University), and identified by comparing with database in ICDD powder diffraction file, release 1998. Quantitative analysis of CaCl2 was carried out after completely complexing it with EDTA and the content of CaCl2 was calculated using factor 7.35 for CaCl2.2H2O.[Citation11] Metal impurities were analyzed according to the method recommended by Thai Food Act.[Citation8] Samples of the extracted CaCl2 were sent for impurities analysis to the Department of Science Service, Bangkok, Thailand.
Application of CaCl2 in Food Products
In order to study its property as a firming agent, commercial CaCl2 was compared with eggshell CaCl2. The CaCl2 solution was prepared by mixing 0.7 g of CaCl2 with 100 ml distilled water. Canned rambutan was prepared from 1.5 kg rambutan fruits (seed excluded), which were soaked in 3 L of CaCl2 solution for 20 min and processed into canned fruit at Dole Thailand Co. Ltd., Chumporn. Products were kept for 14 days and then the instrumental textures of these fruits prepared using the following treatments were compared: rambutan without CaCl2, rambutan with commercial CaCl2 and rambutan with eggshell CaCl2. Textural differences were measured using a TA analyzer model TA XT 2 equipped with a P/2 cylinder probe and sample stage HDP/CRS. A compression test with the speed of 1.5 mm/sec and the compression distance of 20 mm was used. During the texture measurement, the firmness of the rambutan was measured at the peak force of the force–deformation curve obtained. Seven rambutans were tested for each treatment.
RESULTS AND DISCUSSION
Eggshell Calcium Chloride Preparation
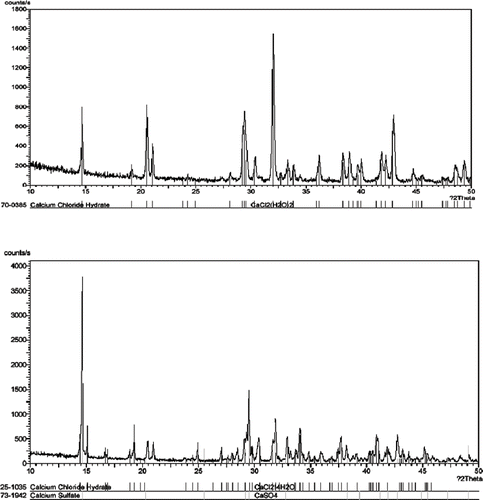
Crushed and dried eggshells weighted 100 kg are composed of 3.22 kg protein, 0.03 kg fat, 0.98 kg moisture, and 95.74 kg ash, mainly CaCO3. In preparation of eggshell calcium chloride (), the concentration of HCl, the ration of eggshell to HCl and their interaction showed significant effects on percent yield and purity of calcium chloride obtained. When crushed eggshells were mixed with a hydrochloric acid solution, bubbles were generated continuously for 3 h, after which there was no further gas production and only a small amount of eggshells remained in the mixture. In this experiment, the CaCl2 solution was then dried by heating the solution on a hot plate at 110–115°C, the crystals thus obtained were analyzed by X-ray diffraction. The X-ray diffraction patterns of eggshell and commercial calcium chloride were shown in which corresponding to the XRD pattern of CaCl2.2H2O (database # 70-0385), and of CaCl2.4H2O (database # 25-1035), respectively. The X-ray diffraction pattern of commercial CaCl2 also showed the existing of CaSO4 (database # 73-1942). Since only 74% CaCl2 salt was specified in commercial CaCl2 thus it may contain other salts like CaSO4. The drying temperature may affect the forms of CaCl2 obtained as suggested by Bowden and Terry[Citation12] that, drying CaCl2 solution in a spray dryer with the control of the drying temperature at 204.4°C will yield hydrated calcium chloride and at 371.1°C will yield anhydrous calcium chloride.
Figure 2 X-ray diffraction patterns of eggshell calcium chloride (top figure) and commercial calcium chloride (bottom figure). For comparison, the X-ray diffraction data of CaCl2.2H2O (#70-0385), CaCl2.4H2O (#25-1035) and CaSO4 (#73-1942) are shown below the corresponding diffractogram.
showed the yield of eggshell CaCl2 obtained from different treatments. The highest yield (90.80% w/w ) was obtained from treatment 9 where a 1:15 crushed eggshell to acid solution (5% hydrochloric acid solution) was used. In terms of purity, quantitative analysis of CaCl2 showed that eggshell CaCl2 from treatment 9 was 119.76% when calculated as CaCl2. 2H2O. The resulting percentages of CaCl2 were calculated using the factor 7.35 for CaCl2.2H2O.[Citation11] Due to the important of the purity of CaCl2, the conditions for treatment 6 were chosen for the extraction process because the percent yield of eggshell CaCl2 was second from the highest (87.38%) but the purity of CaCl2 was the highest at 121.55% (calculated as CaCl2.2H2O). Therefore, eggshell CaCl2 was prepared according to the condition of treatment 6 using 4% HCl solution with 1:15 (crushed eggshell : acid solution, w/v). The calcium chloride powder obtained composed mainly of calcium chloride di-hydrate as shown by X-ray diffraction analysis.
Table 1 % Yield of eggshell CaCl2 from different treatment
Properties of Eggshell CaCl2
The analysis of eggshell CaCl2 showed the presence of 1.75% Mg and its alkali salt, 0.18 mg/kg As, not more than 20 mg/kg heavy metal calculated as Pb, and 15.9 mg/kg fluoride (). When compared with specifications for CaCl2 as stated in Thai Food Act,[Citation8] only the content of Mg and its alkali salt was higher than the allowable level. However, this level is within the safety limit as stated by Food and Nutrition Board Division of Biological Science.[Citation11] The high content of Mg resulted from the MgCO3 present in eggshell. Composition of eggshell CaCl2 were 0.29% protein, 94.37% ash, and 0.49% moisture and no lipid. The pH of the eggshell CaCl2 was 5.27.[Citation10] It is possible to dissolve 1 g of eggshell CaCl2 in 1 ml water comparable to commercial CaCl2. However, the solution of eggshell calcium chloride was not as clear as commercial calcium chloride.
Table 2 Analysis of metal impurities in eggshell CaCl2 compared with specification stated in Thai Food Act[Citation8]
Canned rambutans produced with and without soaking in CaCl2 solution were kept for 14 days after production. The instrumental texture of rambutans were measured at peak force and compared. Canned rambutans with no CaCl2 treatment showed the lowest peak force () Canned rambutans produced using a CaCl2 solution prepared from commercial CaCl2 or eggshell CaCl2 had a firmer texture than samples prepared without using CaCl2.
Table 3 Peak force of canned rambutan measured by TA Analyzer
CONCLUSION
Crushed and dried eggshell weighted 100 kg was composed of 95.74 kg ash, mainly calcium carbonate. Eggshell calcium chloride could be prepared by hydrolysis of eggshells with HCl providing enough acid to react with CaCO3 in eggshell. The reaction ended when no gas bubbles were observed in the mixture. The liquid portion was separated followed by heating at 110–115°C until crystal formed. Eggshell calcium chloride in this study was produced as calcium chloride dihydrate, shown by X-ray diffraction, the solubility is high, pH 5.27, and contained only a minute amount of heavy metal constituents within the specification of the Thai food Act. Its functional property as a firming agent was comparable to commercial calcium chloride when tested in canned rambutans. The study indicates that eggshell, which has been normally disposed as waste, is an alternative source for the production of calcium chloride which can be used as a firming agent in foods.
REFERENCES
- Stadelman , W.J. 1977 . “ Quality Identification of Shell Eggs ” . In Egg Science and Technology , Edited by: Stadelman , W.J. and Cotterill , O.J. 29 – 39 . Westport, CT : AVI .
- Sugoro , N.S. , Horiike , V. , Kunou , M. and Kokubu , T. 2000 . “ Bioavailability and Commercial Use of Eggshell Calcium, Membrane Proteins and Yolk Lecithin Products ” . In Egg Nutrition and Biotechnology , Edited by: Sim , J.S. , Nakai , S. and Guenter , W. 219 – 232 . New York : CABI .
- Daengprok , W. , Garnjanagoonchorn , W. and Mine , Y. 2002 . Fermented Pork Sausage Fortified with Commercial or Hen Eggshell Calcium Lactate . Meat Sci. , 62 : 199 – 204 .
- Omi , N. and Ezawa , I. 1998 . Effects of Eggshell Ca on Preventing of Bone Loss After Ovarietomy . Journal of Home Economics Japan. , 49 : 277 – 282 .
- Daengprok , W. , Garnjanagoonchorn , W. , Naivikul , O. , Pornsinlpatip , P. , Issigonis , K. and Mine , Y. 2003 . Chicken Eggshell Matrix Proteins Enhance Calcium Transport in the Human Intestinal Epithelial Cells, Caco-2 . J. Agric. Food Chem. , 51 : 6056 – 6061 .
- Whipple , G. and Koohmaraie , M. 1993 . Calcium Chloride Marination Effects on Beef Steak Tenderness and Calpain Proteolytic Activity . Meat Sci. , 33 : 265 – 269 .
- Pe'rez-Chabela , Maria , Escalona-Buendia , He'ctor and Guerrero-Legarreta , Isabel . 2003 . Physicochemical and Sensory Characteristics of Calcium Chloride-treated Horse Meat . Int. J. Food Prop. , 6 ( 1 ) : 73 – 85 .
- Ministry of Health and Welfare . 1979 . Food Act Bangkok, , Thailand
- Association Official Analytical Chemists . 1980 . Official Methods of Analysis , 10th , 957 Arlington, VA : AOAC .
- 1996 . American Society for Testing and Materials , 456 Easton, MD : ASTM . ASTM. 15.05 (E 200:95.2),
- Food and Nutrition Board Division of Biological Science . 1981 . Food Chemical Codex , 3rd , 735 Washington, D.C. : National Academy Press .
- Bowden , J.H. and Terry , C.T. Process for the Preparation of Granular Calcium Chloride . U.S. Patent 3,433,863 . 1969 .