Abstract
Pectic substances were extracted from Japanese pepper (Zanthoxylum piperitum DC) fruit with hot diluted HCl (90°C, pH 2.2). The yield of the pectic substances was 8.4% (w/w) based on dry material. The contents of total carbohydrate, anhydrogalacturonic acid, ash, protein, and moisture of the pectic substances were 84.1, 66.9, 3.6, 2.7, and 9.6% (w/w), respectively. The degree of methylation was estimated to be 80.3% by using alcohol oxidase test. This indicated that the pectic substances are high methoxyl pectins and have an ability to form sugar gels. The jelly grade of the pectic substances was estimated to be 130 (USA-SAG) at standard condition. Viscosity-average molecular weight was relatively low (30.3 kDa). When the molecular weight distribution was checked by gel-permeation chromatography on Sepharose CL-4B, the elution profile of the pectic substances was highly polydispersed extending from 40 kDa (or less) to 2000 kDa.
INTRODUCTION
Pectins are one of the main components of the primary cell walls of all plant. They are group of polysaccharides consisting almost of d-galacturonic acid and galacturonic acid methyl ester residues interspersed with a few (1→2)-linked l-rhamnose residues, which are linked to neutral sugar side-chains, such as l-arabinose, d-galactose, d-xylose, d-mannose, and d-glucose.[Citation1] The degree of methylation (DM) is of importance for the use of pectins in food industries. High methoxyl pectin (DM > 50%) forms gels at low pH with 50% or more sugar and low methoxyl pectin (DM < 50%) forms gels at broad range of pH with or without sugar using calcium or other polyvalent cations.
Pectins are often used as a gelling agent in jams and jellies and other food products in the food industry. Although citrus peels, apple pomace, and sugar beet pulp are good sources of pectin, recently several other sources of pectin for use in the food industry have been investigated, such as sunflower,[Citation2] Japanese quince,[Citation3] and Kureo Ma Noy.[Citation4]
Japanese pepper (Zanthoxylum piperitum DC.) that belongs to the Rutaceae family is a deciduous and shrubbery tree and is distributed in the Japanese islands, China, and the Korean peninsula. It is well known that Japanese pepper has a special aroma over the whole plant and its pericarp is commonly used as a spice. Hence, Japanese pepper has been investigated for its characteristic aroma and stimulant effect. For example, citronellal and sanshool are known to be the most important compounds.[Citation5,Citation6] In addition, it was also employed in traditional medicine,[Citation7] and it has been reported that the fruit extract of the Japanese pepper has strong antioxidant activity.[Citation8] Up to now, there have been few reports concerning saccharides of Japanese pepper fruit, although the main component of the fruit is saccharide. In this study, chemical composition and rheological properties of the pectic substance from Japanese pepper fruit were investigated, and we revealed that Japanese pepper fruit is a promising resource of the pectin. This is the first published report to study the chemical composition and some of the physicochemical properties of the pectic substances from Japanese pepper fruit.
MATERIALS AND METHODS
Materials and Chemicals
Japanese pepper fruit, which was collected at Higashi Kishuu area in Japan, was a kind gift from Isefunzai Co. (Mie, Japan). Commercial pectin from citrus peel and alcohol oxidase was bought from Wako Pure Chemical Industry (Osaka, Japan). Unless otherwise indicated, all chemical reagents were of the guaranteed reagent grade.
Extraction of the Pectic Substances from Japanese Pepper Fruit
Dried fruit of Japanese pepper (100 g) was soaked with 3 L of water at 90°C. The pH of the suspension was adjusted to 2.2 with 1 M HCl after soaking and it was gently mixing for 1h at this temperature. Then the slurry was centrifuged at 10 k × g for 10 min at room temperature. The supernatant was concentrated to approximately half of its volume in a rotary evaporator at 40°C before being precipitated with 99.5% ethanol (final ethanol concentration was 70%). The precipitate was rinsed several times with 99.5% ethanol, and finally dried-up in a vacuum oven below 10 Pa for 10 h at 40°C and weighed (8.4 g, light red flakes).
Determination of Ash, Protein and Moisture Content
Ash content was determined by incinerating 1 g of the pectic substances in a furnace at 600°C for 4 h. The subsequent ash was cooled and stored in a desiccator with P2O5 until weighing. Nitrogen (N) was determined by the Kjeldahl procedure and protein content was estimated as N × 6.25. The moisture content was calculated as the weight loss after drying at 110°C for 3 h.[Citation9]
Determination of Neutral Sugars
The pectic substances (200 μg) were hydrolyzed in 1.0 mL of 2 M trifluoroacetic acid for 2 h at 120°C. The hydrolysate was dried with a rotary evaporator at 40°C and reduced to their corresponding alditols by the addition of 500 μL of 1 M NH3 containing 10 mg of NaBH4 for 1h at room temperature. After destroying the excess of NaBH4 by the addition of glacial acetic acid in an ice bath, acetylation was performed with 2.0 mL of acetic anhydride in the presence of 250 μL of 1-methylimidazole as a catalyst for 20 min at 40°C. The acetylation was stopped by the addition of 5.0 mL of water and the alditol acetates were extracted with chloroform. The extract was then analyzed by GC-MS (5971A, Agilent Technologies, Inc., Palo Alto, CA, USA) with a DB-5 capillary column (0.25 mm × 28.5 m).
Determination of Total Neutral Sugars and Anhydrogalacturonic Acid Content
The content of total neutral sugar (galactose equivalent) was determined by the phenol-sulfuric acid method[Citation10] after correction for interference from galacturonic acid. Anhydorogalacturonic acid (AGA) content was determined according to the xylenol method.[Citation11] The pectic substances in 500 μL of 1% (w/v) NaCl was mixed with 4.0 mL of sulfuric acid in an ice bath, and heated in a boiling water bath for 10 min. Then the solution was mixed with 200 μL of glacial acetic acid containing 0.1% xylenol, and rested for 10 min at room temperature. The reaction mixture was measured at absorbance 450–400 nm, while d‐galacturonic acid solutions (0–100 μg/mL) was used to construct the standard curve for the determination of AGA content.
Determination of Degree of Methylation
Degree of methylation (DM) in the pectic substances was determined by using alcohol oxidase test.[Citation12] The pectic substances (10 mg) were suspended in 10 mL of 0.5 M KOH for 1 h at room temperature. Adjusting the pH to 7.5 by the addition of 0.5 M phosphoric acid, total volume was measured up 25.0 mL in a mass flask by the addition of 50 mM phosphate buffer (pH 7.5). Then 0.5 mL of the solution was reacted with 0.5 mL of alcohol oxidase (1.0 U/mL) at 25°C for 20 min. Two mL of coloring reagent (0.02 M acetylacetone/2 M ammonium acetate/0.05 M acetic acid) was added to the reaction mixture and incubated at 60°C for 15 min. Color of the reaction mixture developed at absorbance 412 nm was recorded, while methanol solutions (0–10 μg/mL) were used to construct the standard curve for the determination of the methyl ester linkages in the pectic substance. The degree of methylation was calculated as molar ratio of methanol to galacturonic acid.
Determination of Viscosity-average Molecular Weight
Viscosity measurement was performed by using a Brookfield viscometer (Toki Sangyo, Tokyo, Japan) with a BL-rotor at 25°C. The pectic substances were suspended in an aqueous solution of 1% (w/v) sodium meta-phosphate containing 0.15 M NaCl. Viscosity measured was converted to specific viscosity using:
where η is the viscosity of the pectic substances solution and ηs is the viscosity of solvent and ηsp is the specific viscosity. The two equations commonly employed for determining intrinsic viscosity [η] of food gums;[Citation13] Huggins EquationEq. (1) and Kraemer EquationEq. (2):
where C is concentration (g/L); ηsp/C = reduced viscosity; k h = Huggins constant.
where ηr is relative viscosity (solution to solvent); k k = Kraemer's constant. Intrinsic viscosity was calculated by extrapolating to C = 0 Huggins EquationEq. (1) and Kraemer EquationEq. (2) equations. Viscosity-average molecular weight (Mw) of the pectic substances were estimated by applying Mark Houwink EquationEq. (3), relating [η] with Mw:[Citation14]
where k is a constant and a relates to the stiffness of the polymer chain. In this work the following values were assumed k = 9.55 × 10−4 and a = 0.73,[Citation15] which is often applied to calculations of Mw of pectins.
Gel-permeation Chromatography
Sepharose CL-4B (GE Healthcare, Piscataway, NJ, USA) was used for gel-permeation chromatography. The column (47.5 × 1.1 cm) was eluted by 50 mM phosphate buffer (pH 6.8) containing 0.1 M NaCl at the flow rate of 0.15 mL/min. The column was calibrated with Dextran 2000, 500, 70, and 40 (GE Healthcare, Piscataway, NJ, USA). The pectic substance dissolved in the same buffer (up to 1.0 mg) was injected on the column and then 1.0 mL fractions were collected.
Gel Evaluation
Pectin sugar gels of the pectic substances from Japanese pepper fruit was prepared according to the I. F. T. procedure to control the SAG degree.[Citation16] The soluble solids content for all samples was adjusted to 65° Brix.
RESULTS AND DISCUSSION
Extraction of the pectic substances from Japanese pepper fruit was carried out under hot acidic condition. Pectic substances are usually obtained by a sequential extraction with water, chelating agent, and acid or alkali. In this sequential extraction, acid often gave the most efficient extracts of pectins.[Citation3] Although acid might also hydrolyze to a lesser extent some esters of galacturonic acid and some linkage between galacturonic acid residues, the acid-stability of the pectins with high gelling capacity are obtained by acid treatment from apple or citrus.[Citation17]
The pectic substances from Japanese pepper fruit obtained with hot acid represented 8.4% (w/w) based on dry material. The yield was less than sunflower head,[Citation18] but higher than those obtained from traditional pectin sources, like apple[Citation19] and citrus.[Citation20] The chemical compositions of the pectic substances both from Japanese pepper fruit and commercial citrus peel pectin are shown in . There were little differences between the pectic substance from Japanese pepper fruit and citrus peel pectin with regard to ash, protein, moisture, and total carbohydrate. These, to some extent, would reflect the common chemical composition of starting raw materials. Since total carbohydrate content of the pectic substances from Japanese pepper fruit represented 84.1% (w/w) suggesting that the carbohydrate is the main component of the pectic substances. In addition, galacturonic acid, rhamnose, arabinose, and galactose were the main sugars detected, indicating that pectin was the main polysaccharide in the pectic substances from Japanese pepper fruit. The AGA content of the pectic substance (66.9%, w/w) was lower than citrus pectin (71.4%, w/w) and more than beet pulp pectin.[Citation21] The neutral sugars content based on the total carbohydrate was 20.4 mol%, which was relatively high as compared with the citrus peel pectin (16.7 mol%). It is indicated that there are relatively high amounts of rhamnogalacturonan in the pectic substances, as is also found for sugar beet pectin.[Citation22]
Table 1 Chemical composition (%, w/w) based on dry material and jelly grade (USA-SAG) of the pectic substances from Japanese pepper fruit and commercial citrus peel pectin
The degree of methylation (DM) which was calculated both by the methoxyl contents and AGA contents are also shown in . The pectic substances from Japanese pepper fruit were highly methylated to be over 80%, indicating that the pectic substances are classified as a high methoxyl pectin which forms gels after heating in sugar solutions with concentration higher than 55% and pH lower than 3.5. The DM is rather high in comparison with pectin from citrus peel pectin () and beet pectin.[Citation21]
Intrinsic Viscosity and Molecular Weight
The viscosity of the pectic substances from Japanese pepper fruit was measured in 1% (w/v) sodium meta-phosphate solution containing 0.15 M NaCl and represented a typical result obtained by extrapolating to C = 0 Huggins (EquationEq. 1) and Kraemer (EquationEq. 2). The values of intrinsic viscosities [η], viscosity-average molecular weights (Mw), Huggins coefficient (k h), and Kraemer's coefficient (k k) are given in . Huggins and Kraemer's coefficients obtained in this study were close to 0.5 and 0.05, respectively, indicating that sodium meta-phosphate was a good solvent for this pectic substances.[Citation3] The intrinsic viscosities obtained by using Huggins (EquationEq. 1) and Kraemer (EquationEq. 2) were 1.79 and 1.78, respectively. These were nearly consistent with sunflower pectin,[Citation12] and lower than pectic substances of cherry fruit,[Citation17] and higher than pectic polysaccharide from sugar beet pulp.[Citation23] Viscosity-average Mws calculated by the Equationequation 3 based on the intrinsic viscosity of Huggins (EquationEq. 1) and Kraemer (EquationEq. 2) were 30.3 and 30.2 kDa, respectively. These were lower than sunflower pectin,[Citation13] and pectic substances of cherry fruit,[Citation17] and pectic polysaccharide from sugar beet pulp.[Citation23]
Figure 1 Intrinsic viscosity of the pectic substances from Japanese pepper fruit in an aqueous solution of 1% w/v sodium meta-phosphate containing 0.15 M NaCl, by extrapolation to zero concentration with Huggins (▪) and Kraemer's (○).
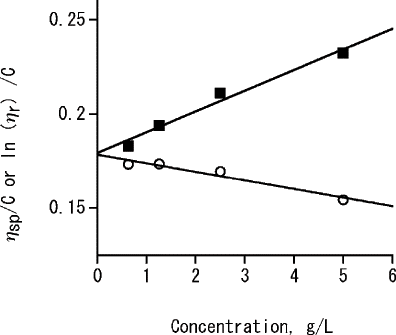
Table 2 Viscosity-average molecular weight (Mw) of the pectic substances from Japanese pepper fruit
Gel-permeation Chromatography
shows the elution profile of the pectic substances from Japanese pepper fruit obtained on Sepharose CL-4B eluted with 50 mM phosphate buffer pH 6.8 containing 0.1 M NaCl. The pectic substances were highly polydispersed extending from 40 kDa (or less) to 2000 kDa, and two populations could be recognized: a high molecular weight population with K av around 0.3, a low molecular weight population representing the most of the material with K av around 0.7. The high molecular weight population was rich in neutral sugars and the ratio neutral sugars/galacturonic acid was calculated approximately 1.0. The low molecular weight region was rich in galacturonic acid with the ratio neutral sugars/galacturonic acid of approximately 0.1, indicating that this region contained mainly homogalacturonans.[Citation23,Citation24] On the other hand, heterogeneity of the pectic substances was increasing in the lower molecular weight population since the neutral sugars population beyond the K av 1.0 was higher than galacturonic acid.
Figure 2 Gel-permeation chromatography on Sepharose CL-4B (47.5 × 1.1 cm) of the pectic substances from Japanese pepper fruit. Sample (1.0 mg) was injected on the column and eluted by 50 mM phosphate buffer (pH 6.8) containing 0.15 M NaCl. Fractions (1.0 mL) were assayed for the neutral sugars (•) and galacturonic acid (○) by the phenol sulfuric acid method and the xylenol method, respectively.
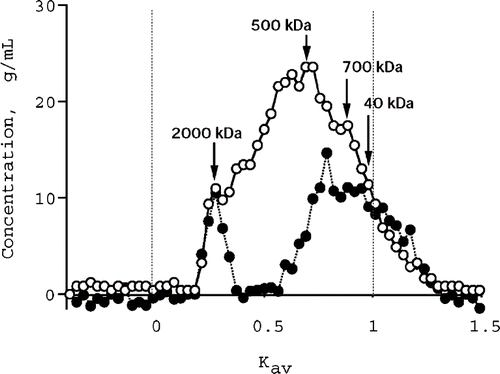
The gel-permeation chromatography pattern of the pectic substances was very similar to that of the acid extracted hop pectin,[Citation24] and the acid-soluble pectic substance of cherry fruits.[Citation17] In addition, the neutral sugar composition of the pectic substances was almost corresponding to the acid extracted hop pectin which contained arabinogalactan in the high molecular weight population.[Citation24] Arabinose and galactose are known to form side-chains with pectic substances.[Citation25]
Jelly Grade of the Pectic Substances from Japanese Pepper Fruit
Jelly grade is one of the most important parameter for pectic substance, which indicates that the number of parts of sugar required for one part of pectin to produce jelly of desirable consistency under standard condition.[Citation16] The jelly grade of the pectic substances from Japanese pepper fruit was estimated to be 130 (USA-SAG) () which is not high value for its relatively high DM. Jelly grade of pectin at the standard condition needs a high DM[Citation22,Citation26] and high Mw.[Citation27] Though the DM of the pectic substances from Japanese pepper fruit is relatively high, the viscosity-average Mw of the pectic substance is low (). Therefore it is thought that the jelly grade was 130 (USA-SAG), which was lower than our expectation.
CONCLUSION
Japanese pepper fruit is an interesting source for its pectic substances content. In addition, the high DM is suitable as a gelling agent. However, the low jelly grade which is influenced by the low Mw may limit its use. Then it is important to improve the extraction method that could prevent from degradation of the pectic substance. In this study we reported the basic chemical and physical properties of this new source. Thus, further investigation should be done to characterize their physicochemical properties and particularly their gelling properties.
ACKNOWLEDGEMENTS
Mr. Yamakawa and Mr. Hayakawa's (Science & Technology Promotion Center, Mie, Japan) help with GC/MS analysis are gratefully appreciated.
REFERENCES
- Ridley , B.I. , O'Neill , M.A. and Mohnen , D. 2001 . Pectins: Structure, Biosynthesis, and Oligogalacturonide-related Signaling . Phytochemistry , 57 : 929 – 967 .
- Kim , W.J. , Sosulski , F. and Campbell , S.J. 1978 . Formulation and Characteristics of Low-ester Gels from Sunflower Pectin . J. Food Sci. , 43 : 746 – 749 .
- Thomas , M. , Guillemin , F. , Guillon , F. and Thibault , J.F. 2003 . Pectins in the Fruits of Japanese Quince (Chaenomeles Japonica) . Carbohydr. Polym. , 53 : 361 – 372 .
- Singsong , J. , Ningsanond , S. , Cui , S.W. and Goff , H.D. 2005 . Extraction and Physicochemical Characterization of Kureo Ma Noy Pectin . Food Hydrocoll. , 19 : 793 – 801 .
- Sakai , T. , Yoshihara , K. and Hirose , Y. 1968 . Constituents of Fruit Oil from Japanese Pepper . Bull. Chem. Soc. Jpn. , 41 ( 8 ) : 1945 – 1950 .
- Sakai , T. , Yoshihara , K. and Hirose , Y. 1970 . A Comparative Study of the Constituents of Volatile Oils of Zanthoxylum . Bull. Chem. Soc. Jpn. , 43 : 484 – 487 .
- Hashimoto , K. , Satoh , K. , Kase , T. , Ishige , A. , Kubo , M. , Sasaki , H. , Nishikawa , S. , Kurosawa , S. , Yakabi , K. and Nakamura , T. 2001 . Modulatory Effect of Aliphatic Acid Amides from Zanthoxylum piperitum on Isolated Gastrointestinal Tract . Planta Med. , 67 : 179 – 181 .
- Yamazaki , E. , Inagaki , M. , Kurita , O. and Inoue , T. 2006 . Antioxidant Activity of Japanese Pepper (Zanthoxylum piperitum DC.) . Fruit. Food Chem. , 100 ( 1 ) : 171 – 177 .
- Association of Official Analytical Chemists . 1980 . Official Methods of Analysis , 13th , Washington, DC : AOAC .
- Dubois , M. , Gilles , K.A. , Hamilton , J.K. , Rebers , P.A. and Smith , F. 1956 . Colorimetric Method for Determination of Sugars and Related Substances . Anal. Chem. , 28 : 350 – 356 .
- Walter , W.M. Jr. , Fleming , H.P. and MacFeeters , R.F. 1993 . Base-Mediated Firmness Retention of Sweetpotato Products . J. Food Sci. , 58 : 813 – 816 .
- Klavons , J.A. and Benetter , D. 1986 . Determination of Methanol Using Alcohol Oxidase and Its Application to Methyl Ester Content of Pectin . J. Agric. Food Chem. , 34 : 597 – 599 .
- Iglesias , M.T. and Lozano , J.E. 2004 . Extraction and Characterization of Sunflower Pectin . J. Food Eng. , 62 : 215 – 223 .
- Arslan , N. 1995 . Extraction of Pectin from Sugar-beet Pulp and Intrinsic Viscosity-molecular Weight Relation Ship of Pectin Solutions . J. Food Sci. Technol. , 32 : 381 – 385 .
- Anger , H. and Berth , G. 1986 . Gel Permeation Chromatography and the Mark-Houwink Relation for Pectins with Different Degrees of Esterification . Carbohydr. Polym. , 6 : 193 – 202 .
- I. F. T. Committee on Pectin Standardization . 1959 . Final report of the IFT Committee . Food Technol. , 13 : 496 – 500 .
- Barbier , M. and Thibault , J.F. 1982 . Pectic Substances of Cherry Fruits . Phytochemistry , 21 : 111 – 115 .
- Sahari , M.A. , Akbarian , A.M. and Hamedi , M. 2003 . Effect of Variety and Acid Washing Method on Extraction Yield and Quality of Sunflower Head Pectin . Food Chem. , 83 : 43 – 47 .
- Massiot , P. , Baron , A. and Drilleau , J.F. 1994 . Characterization and Enzymatic Hydrolysis of Cell-wall Polysaccharide from Different Tissue Zones of Apple . Carbohydr. Polym. , 25 : 145 – 154 .
- Alarcão , E. and Silva , M.L. 1990 . Characterization of a Pectin from Sunflower Heads Residues . Acta Alimentaria , 19 : 19 – 26 .
- Mesbahi , G. , Jamalian , J. and Farahnaky , A. 2005 . A Comparative Study on Functional Properties of Beet and Citrus Pectins in Food Systems . Food Hydrocoll. , 19 : 731 – 738 .
- Oosterveld , A. , Beldman , G. , Leeuwen , S. and Voragen , A.G. 2000 . J. Effect of Deacetylation on Gelation of Sugar Beet Pectin in the Presence of Calcium . Carbohydr. Polym. , 43 : 249 – 256 .
- Oosterveld , A. , Beldman , G. and Voragen , A.G.J. 2000 . Oxidative Cross-linking of Pectic Polysaccharides from Sugar Beet Pulp . Carbohydr. Res. , 328 : 199 – 207 .
- Oosterveld , A. , Voragen , A.G.J. and Schols , A.A. 2002 . Characterization of Hop Pectins Shows the Presence of an Arabinogalactan-protein . Carbohydr. Polym. , 49 : 407 – 413 .
- Jiménez , A. , Guillén , R. , Bolaoños , F.J. and Heredia , A. 1994 . Cell Wall Composition of Olives . J. Food Sci. , 59 : 1192 – 1196 . 1201.
- Virk , B.S. and Sogi , D.S. 2004 . Extraction and Characterization of Pectin from Apple (Malus Pumila. Cv Amri) Peel Waste . Int. J. Food Prop. , 7 ( 3 ) : 693 – 703 .
- Rombouts , F.M. and Thibault , J.F. 1986 . Feruloylated Pectic Substances from Sugar-beet Pulp . Carbohydr. Res. , 154 : 177 – 187 .