Abstract
Viscous protein was purified from yam (Dioscorea opposita Thunb.) tuber mucilage tororo by Phenyl-Toyopearl 650M, DEAE-Toyopearl 650M, and CM-Toyopearl 650M column chromatographies, and partially characterized. The molecular weight of the purified protein was estimated to be about 200 kDa, by Native-PAGE and Toyopearl HW-55F gel filtration. Moreover, it had identical subunits of molecular weight of about 32 kDa, either without or with 2-mercaptoethanol, respectively. This protein was identified as dioscorin, the major storage proteins in yam species tubers, when comparing the sequence obtained in an automatic Edman sequencer with the database using FASTA WWW System. On the other hand, the purified protein showed carbonic anhydrase activity and the activity was not inhibited by 2,6-pyridinedicarboxylic acid: zinc atoms were absent in the purified protein. The optimum pH in viscosity was about 5.8 and optimum temperature 20–30°C. Heat denaturation of the protein occurred at about 40°C, but the protein retained more than 66 % of the original viscosity at 70°C for 30 min.
INTRODUCTION
Yam species is a member of the monocotyledonous family and belongs to the order Dioscoreaceae. Yams are cultivated in all over the world, and the number of yams amounts to about six hundreds species.[Citation1] Yams (Dioscorea spp., Dioscoreacea) are the 3rd most important tropical “root” crop after cassava and sweet potato.[Citation2] The representative or commonly found yam species are Dioscorea opposita and D. japonica in Japan, and D. alata, D. bulbifera, D. esculenta, and D. trifida in other regions such as Africa, East Asia, or America. In China, yam tubers of Dioscorea spp. are widely consumed and are used as an ingredient of traditional herbal medicines.
It is known that yam tuber contains approximately 13–27% carbohydrates and most of them are starch. Starch is one of the most important natural organic compounds, abundant in nature. The most common sources of food starch are corn, potato, wheat, tapioca, and rice.[Citation3] Developed countries such as Canada, USA, Europe, and Japan have 77% of the global starch market.[Citation4] The native starches exhibit some disadvantages in certain industrial applications. Its granules hydrate easily, swell rapidly, rupture, lose viscosity, and produce weak bodied, stringy and cohesive pastes.
The Japanese consume yam tuber as yam rice boiled with barley, grated yam soup, fried food, food in sweetened vinegar, Japanese sweets, food made from fish paste, and a thickener for buckwheat flour. Among them, grated yam “tororo” is the main art of cooking in Japan. Generally speaking, many yam tuber mucilages are viscous, and it is considered that the main component producing viscosity is protein. Moreover, it is known that the viscous substance of yam mucilage is composed mainly of mannan-protein macromolecules.[Citation5,Citation6] To unveil the distinct properties of yam tuber proteins, a number of studies have focused on the sequence determination.[Citation7,Citation8] However, there is very little information about viscous protein from yam tuber mucilage to date, although there is a report about proteinase and α-amylase inhibitors in yam.[Citation9] Thus, it is of great importance to characterize the viscous protein from yam tuber mucilage. Nowadays, consumers want to see more “natural” and “healthier” industrial products manufactured “without chemical processing” on the market. If the viscous component from yam tuber mucilage tororo is isolated and characterized, yam tuber mucilage as along with its starch may be aggressively used as an ingredient in processed food such as kamaboko.
This article deals with the purification and partial characterization of viscous protein from Japanese yam (D. opposita Thunb.) tuber mucilage tororo. The present study will help to prevent the decrease of viscosity on yam mucilage tororo during storage and will be useful for application in food processing.
MATERIALS AND METHODS
Samples
Fresh yam (Dioscorea opposita Thunb.) tubers were purchased from a local wholesale market, Abashiri, Hokkaido, Japan.
Chemicals
Phenyl-Toyopearl 650M, CM-Toyopearl 650M, DEAE-Toyopearl 650M, and Toyopearl HW-55F were from Tosoh Co. (Tokyo, Japan). Marker proteins for gel chromatography were from Boehringer Mannheim Co. (Tokyo, Japan). Marker proteins for electrophoresis were from Sigma Chemical Co. (St. Louis, MO) and from Amersham Biosciences UK Ltd. (UK). All other reagents were of analytical grade.
Preparation of Viscous Protein from Yam Tuber Mucilage tororo
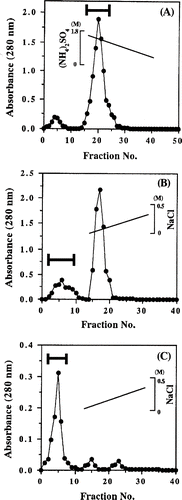
After cleaning with water, yam tubers were peeled and immediately ground using a grater. After the extraction with the gentle stirring at 4°C for 1 day, the viscous extracts were centrifuged at 50,000 × g for 1 h. The supernatants were brought to 40% solid ammonium sulfate saturation, and this solution was centrifuged at 28,500 × g for 30 min. The supernatants (302.4 mg of protein) were pooled and applied to a Phenyl-Toyopearl 650 M columns (1.0 × 5.0 cm) previously equilibrated with 10 mM sodium phosphate buffer (pH 7.0) containing 1.8 M ammonium sulfate. The column was washed with the same buffer and the viscous protein was eluted with a linear gradient of 1.8-0 M ammonium sulfate in the same buffer at a flow rate of 1.0mL/min (). 2.5 mL fractions were collected. The pool of the viscous protein (103.1 mg of protein) was dialyzed against 10 mM sodium phosphate buffer (pH 7.0) and this protein fraction was applied to DEAE-Toyopearl 650M column (1.0 × 5.0 cm) equilibrated with the same buffer. The column was washed with the same buffer and the viscous protein was eluted with a linear gradient of 0-0.5 M NaCl in the same buffer at a flow rate of 1.0 mL/min (). 2.0 mL fractions were collected. After the sample (17.8 mg of protein) was dialyzed against 10 mM sodium phosphate buffer (pH 6.0), the viscous solution was applied to a CM-Toyopearl 650 M columns (1.0 × 3.0 cm) equilibrated with the same buffer. After washing with the same buffer, the viscous protein was eluted with a linear gradient of 0–0.5 M NaCl in the same buffer at a flow rate of 1.0 mL/min (). 2.0 mL fractions were collected. The purified viscous protein was dialyzed against 10 mM sodium phosphate buffer (pH 6.0) and was used in the tests.
Figure 1 Purification of viscous protein from yam (Dioscorea opposita Thunb.) tuber mucilage tororo. (A) Phenyl-Toyopearl 650M column chromatography. (B) DEAE-Toyopearl 650M column chromatography of the Phenyl-Toyopearl 650M fraction. (C) CM-Toyopearl 650M column chromatography of the DEAE-Toyopearl 650M fraction.
Protein Concentration
Protein concentration was measured as the method of Lowry et al.[Citation10] using bovine serum albumin as standard.
Kinematic Viscosity Measurements
These were made on 5 mL sample solution in distilled water and using a Cannon-Fenske type viscometer (SIBATA Scientific Technology Ltd., Tokyo, Japan) with a viscosity constant of 2.88 × 10−3 cSt/s. The kinematic viscosity of sample solution was obtained by measuring its viscosity at 25°C. The -kinematic viscosity was calculated according to the following equation:
Molecular Weight Determination
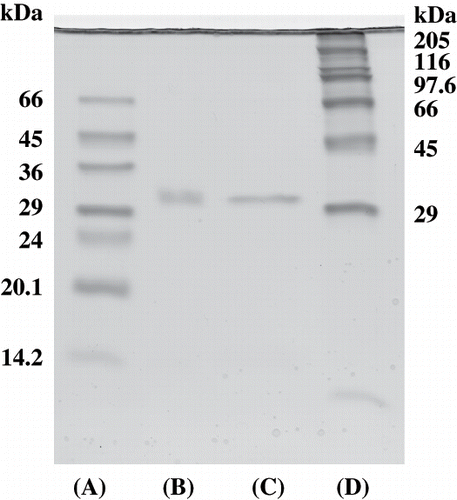
The molecular weight of the purified viscous protein was estimated using Toyopearl HW-55F (1.5 × 75 cm) gel filtration and polyacrylamide gel electrophoresis (Native-PAGE).[Citation11] Ferritin (450 kDa), catalase (240 kDa), aldolase (158 kDa), and albumin (68 kDa) were used as the standard markers for gel filtration and thyroglobulin (669 kDa), ferritin (440 kDa), catalase (232 kDa), lactate dehydrogenase (140 kDa), and albumin (66 kDa) were as the standard markers for Native-PAGE. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli[Citation12] using 12.5% gel. Myosin (205 kDa), β-galactosidase (116 kDa), phosphorylase (97.4 kDa), bovine serum albumin (66 kDa), ovalbumin (45 kDa), glyceraldehyde-3-phosphate dehydrogenase (36 kDa), carbonic anhydrase (29 kDa), bovine pancreas trypsinogen (24 kDa), soybean trypsin inhibitor (20.1 kDa), and bovine milk α-lactoalbumin (14.2 kDa) were used as standards. After electrophoresis, the gel was stained with Coomassie brilliant blue R-250.
N-Terminal Amino Acid Sequence
After SDS-PAGE, the protein was electroblotted to a polyvinylidenedifluoride (PVDF) membrane and then cut from the PVDF membrane. This was analyzed for N-terminal sequencing by automated Edman degradation on an Applied Biosystems sequencer (Model 492HT, CA).
Carbonic Anhydrase Activity
Carbonic anhydrase activity staining was performed by colour change of bromothymol blue (BTB) having carbonic anhydrase activity with yellow bands against a blue background of BTB.[Citation13] A sample solution was mixed with an equal volume of 0.125 M Tris-HCl buffer (pH 6.8) containing 20% glycerol, 4% SDS, and 0.002% bromophenol blue. After SDS-PAGE, the gel was washed with 25% isopropanol in 10 mM Tris-HCl buffer (pH 7.9) to remove SDS before activity staining.
Carbonic Anhydrase Activity Inhibition Test
A sample solution was mixed with equal volume of electrophoresis sample buffer and SDS-PAGE was performed by the method of Laemmli.[Citation12] After electrophoresis, the gel was washed with 25% isopropanol in 10 mM Tris-HCl buffer (pH 7.9) to remove SDS before inhibition test. The gel was equilibrated in 100 mM Tris-HCl buffer (pH 8.5) containing 5 mM 2,6-pyridinedicarboxylic acid with gentle shaking at room temperature for 1 day. After washing with 100 mM Tris-HCl buffer (pH 8.5), the gel was staining for carbonic anhydrase activity.
RESULTS AND DISCUSSION
The detail of -purification of viscous protein is summarized in . Finally, specific kinematic viscosity of 0.18 cSt/mg protein and purification of 3.6-fold with a recovery rate of 12.4 % compared with the crude extract was attained. The protein content of the purified viscous protein was about 0.90 mg/mL. When the purified protein was concentrated using Ultrafree-MC (10,000 NMWL Filter Unit; Millipore, USA), the viscosity of protein solution was extremely increased (data not shown).
Table 1 Purification of viscous protein from yam tuber mucilage tororo.
Molecular Weight and N-terminal Amino Acid Sequence of Purified Protein
The molecular weight of the purified viscous protein was about 200 kDa, estimated by Native-PAGE () and Toyopearl HW-55F gel filtration (). According to SDS-PAGE, purified viscous protein appeared as a single protein band of molecular weight about 32 kDa, either without or with 2-mercaptoethanol, respectively (). It suggests that this protein may be a hexamer of identical subunits of molecular weight about 32 kDa. Moreover, the sequence of the purified protein revealed that the first 20 amino acid residues were identified by showing that the sample belonged to a homogeneous protein preparation. The N-terminus was VEDEFSYIEGNPNGPENWGN, which matches published sequences for dioscorin found in the data bank system (FASTA WWW System). The results indicate that about 50% of the amino acid residues are acidic amino acid with the highest levels corresponding to valine, phenylalanine, glycine, and asparatic acid.
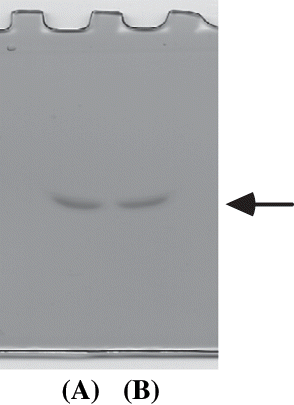
Figure 2 Nondenaturating polyacrylamide gel electrophoresis of the purified viscous protein from yam (D. opposita Thunb.) tuber mucilage tororo. (A) Molecular weight markers; (B) Purified viscous protein.
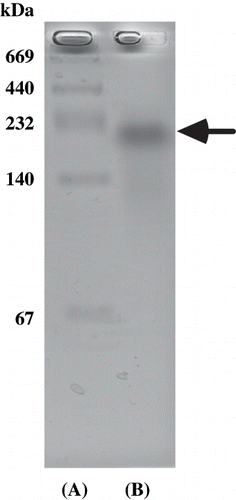
Figure 3 Estimation of molecular weight of the purified viscous protein from yam (D. opposita Thunb.) tuber mucilage tororo by gel filtration on Toyopearl HW-55F column (1.5 × 70 cm). The column was eluted with 10 mM sodium phosphate buffer (pH 6.0). The standard proteins used were as indicated.
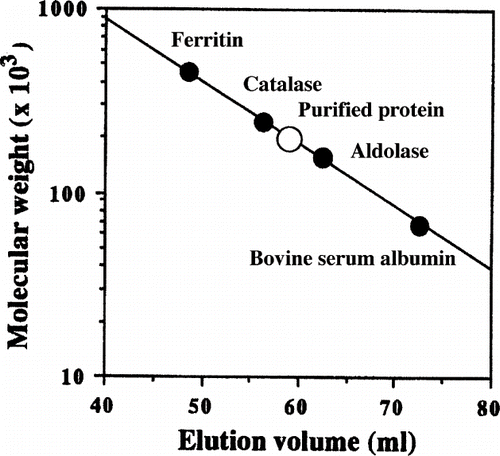
Figure 4 SDS-polyacrylamide gel electrophoresis of molecular weight markers and purified viscous protein from yam (D. opposita Thunb.) tuber mucilage tororo. (A) Low molecular weight markers; (B) purified protein without reducing reagent; (C) purified protein with reducing reagent; (D) High molecular weight markers.
Carbonic Anhydrase Activity of Purified Protein
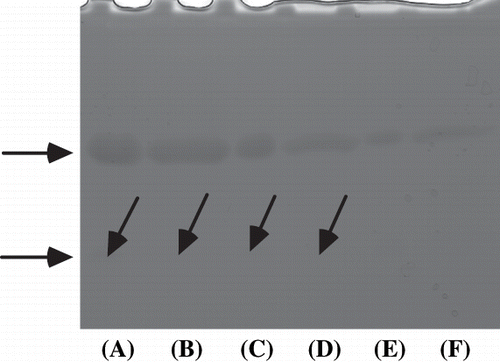
After SDS-PAGE, the gel was stained using bromothymol blue. As a result, at least two major bands (yellow bands on a blue background) were detected in crude extract and DEAE-Toyopearl 650M fraction matched the protein stained bands of molecular weight about 10 and 32 kDa, either without or with 2-mercaptoethanol (2‐ME) (). On the other hand, the purified protein also had a carbonic anhydrase activity band of molecular weight about 32 kDa, either without or with reducing reagent ().
Figure 5 Carbonic anhydrase activity staining of crude extracts, DEAE-Toyopearl 650M fraction, and the purified viscous protein from yam (D. opposita Thunb.) tuber mucilage tororo. (A) crude extracts without reducing reagent; (B) crude extracts with reducing reagent; (C) DEAE-Toyopearl 650M fraction without reducing reagent; (D) DEAE-Toyopearl 650M fraction with reducing reagent; (E) purified protein without reducing reagent; (F) purified protein with reducing reagent.
Carbonic Anhydrase Activity Inhibition Test
After SDS-PAGE, the influence of inhibitor on the carbonic anhydrase activity was analyzed. As shown in , carbonic anhydrase activity of the purified protein was not inhibited by 2,6-pyridinedicarboxylic acid as a zinc-chelating agent. The result indicated that zinc atoms were absent in the purified protein.
Effect of pH on Viscosity of Purified Protein
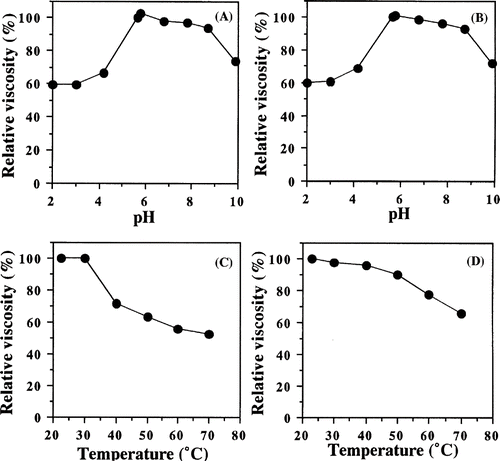
The viscosity of purified protein was measured at different pHs at 22.7°C. The optimum pH of the purified protein was about 5.8 (). The stability of the purified protein was examined at different pHs at 4°C for 1 day. As seen in , the protein retained more than 90 % of the original viscosity at pH between 5.7 and 8.7, but became extremely unstable when the pH was lower than 4.2 or higher than 9.9. It was suggested that the purified protein denatured at lower or higher pHs.
Effect of Temperature on Viscosity of Purified Protein
The effect of temperature on the viscosity of the purified protein was examined at 22.7°C in 10 mM sodium phosphate buffer (pH 6.0). The optimum temperature for the viscosity was around 20–30°C (). On the other hand, the purified protein was incubated at different temperatures at pH 6.0 for 60 min. After cooling, the residual viscosity was measured. The purified protein was stable until at 40°C, but the viscosity gradually decreased. At 70°C, the purified protein maintained about 66 % of the original viscosity ().
It is known that yam tuber contains approximately 67–84 % water, 2.0–4.5 % proteins, 0.1–0.5 % lipids, 13–27% carbohydrates, and 0.9–1.5% ash.[Citation14] On the other hand, it contains relatively high contents of vitamins B1 (0.08–0.15 mg/100 g edible portion) and C (4–17 mg/100 g edible portion), different micro and macro elements such as potassium (430–590 mg/100 g edible portion), iron (0.4–0.8 mg/100 g edible portion), zinc (0.3–0.7 mg/100 g edible portion), and enzymes as amylase, urease, oxidase. Moreover, yam tuber is rich in dietary fibers as water-soluble (0.2–0.7 g/100 g edible portion) and insoluble (0.8–1.8 g/100 g edible portion) ones. For these reasons the yam tuber is considered a more attractive food, as well as natural one. The Japanese have consumed yam tuber as yam rice boiled with barley, grated yam soup, fried food, food in sweetened vinegar, Japanese sweets, food made from fish paste, and a thickener for buckwheat flour. It is believed that sticky foods such as okra Abelmoschus esculentus (L.) Moench and taro Colocasis esculenta (L.) Schott, as well as yam are beneficial to the health.
In the present study we tried to isolate and partially characterize the viscous protein in representative Japanese yam (D. opposita Thunb.) tuber mucilage tororo. The viscosity of this protein solution was extremely high when it was concentrated. It suggests that this protein is a key substance that produced the viscosity in yam D. opposita -tuber mucilage. The purified protein appears as a molecular weight of 32 kDa without the presence of reducing reagent. It suggests that this viscous protein did not possess its intra-chain disulfide bonds. As per the result obtained from SDS-PAGE, there is a possibility that this protein may be dioscorin, the major storage proteins in yam species tubers. Moreover, the results from N-terminal amino acid sequence indicated that this protein is dioscorin. This amino acid sequence was highly homologous to deductive sequences of dioscorins from cDNA of D. cayenensis Lam (90% homology)[Citation7] and from D. batatas Decne (100 % homology).[Citation15] Harvey and Boulter[Citation8] reported that dioscorins usually contain a single disulfide bond, however Conlan et al.[Citation16] reported that the presence or absence of an intra‐chain disulfide bond could be used to discriminate between the major groups of components. Hou et al.[Citation15,Citation17] purified dioscorin from yam (D. batatas Decne) tuber by ammonium sulfate fractionation, DE-52 ion exchange chromatography, and Sephadex G-75 column. The purified dioscorin showed two protein bands of molecular weight of 28 and 82 kDa without 2-ME, but a single band of 32 kDa with 2-ME. On the other hand, Harvey and Boulter[Citation8] isolated dioscorin from yam tubers (D. rotundata) and its protein appeared as a molecular weight of 28 kDa.The -same results were reported in yam (D. cayenensis Lam.).[Citation7] The novel 82 kDa band reported by Hou et al.[Citation15,Citation17] might be due to the covalent linking of the intra- and intermolecular disulfide bridges of dioscorin from yam tuber. It is known that it exists the intra- and intermolecular disulfide bridges to form oligomers in yam tubers (D. rotundata) at different pH values, ionic strength, and protein concentrations.[Citation8] Moreover, it may be due to disruption of interactions between monomeric units of dioscorin oligomers, although 82 kDa protein band was disappeared by treatment of reducing agents such as 2-ME and dithiothreitol.
Our purified viscous protein in yam (D. opposita -Thunb.) tuber exhibited carbonic anhydrase activity in similar to those from yam species such as D. batatas, D. alata, and D. pseudojaponica.[Citation15,Citation18] Moreover, Hou et al.[Citation15,Citation18] reported that dioscorin obtained from these yam species showed not only trypsin inhibitor activity, but also dehydroascorbate reductase and monodehydroascorbate reductase activities.[Citation19] It was also reported that the mucilages contained in yam (Dioscorea alata Linn.) tubers is mamnan-protein complex.[Citation6] In recent years, the antioxidant activity of compounds from Taiwan yams D. alata tubers,[Citation20] a freeze-dried powder of Chinese yam D. alata cv. Tainung No. 2,[Citation21] mucilage,[Citation22] and dioscorin[Citation17] from yam (D. batatas Decna) have been reported.
The effects of pH and temperature in viscosity of the purified protein were investigated. It was more stable in weak acid to alkaline condition (pH 5.6–9.0), but was rapidly unstable in acid condition (under pH 5.6). Moreover, it was found that the purified protein retained more than 66% of the original viscosity at 70°C for 30 min; the viscous protein was more stable in high temperature.
CONCLUSION
The viscous protein was purified from Japanese yam (D. opposita Thunb.) tuber mucilage tororo and partially characterized. This report is, to our knowledge, the first on the protein with regard to viscosity in yam mucilage tororo. The understanding highlighted in this study will help to prevent the decrease of viscosity on yam mucilage tororo during storage and will be useful for application in food processing. Further research on the purified viscous protein of Japanese yam (D. opposita Thunb.) tuber mucilage tororo such as compositional and structural analysis and its functional properties such as antioxidant and free radical scavenging properties and angiotensin I-converting enzyme inhibitory activity will be considered in detail.
Notes
1. Satin, -M. Functional Properties of Starches. In Spotlight Tropical Starch Misses Market. AGSI repor, Agriculture21. FAO-Magazine, 1998; 11 pp.
REFERENCES
- 1. Satin, -M. Functional Properties of Starches. In Spotlight Tropical Starch Misses Market. AGSI repor, Agriculture21. FAO-Magazine, 1998; 11 pp.
- Myoda , T. , Nagai , T. and Nagashima , T. 2005 . “ Properties of Starches in Yam (Dioscorea spp.) Tuber ” . In Current Topics in Food Science and Technology , Edited by: Nagai , T. , Suzuki , N. and Nagashima , T. 105 – 114 . India : Research Signpost .
- Alexander , R.J. 1995 . Potato Starch: New Prospects for an Old Product . Cereal Food World , 40 : 763 – 764 .
- Sansavani , S. and Verzoni , D. . The Functional Properties of Starches as a Means to Expanding Their International Market. FAO Working document n3 . FAO-AGS as a part of policy-research Tasks in conjunction with the first world conference on research in horticulture (WCHR) . June 17–19 , Rome, Italy. pp. 28
- Misaki , A. , Ito , T. and Harada , T. 1972 . Constitutional Studies on the Mucilage of “Yamanoimo,” Dioscorea batatas Decne, forma Rsukune: Isolation and Structure of a Mannan . Agric. Biol. Chem. , 36 : 761 – 771 .
- Tsai , S.S. and Tai , F.J. 1984 . Studies on the Mucilage from Tuber of Yam (Dioscorea alata Linn.) I. Isolation and Purification of the Mucilage . J. Chinese Agric. Chem. Soc. , 22 : 88 – 94 .
- Conlan , R.S. , Griffiths , L.A. , Napier , J.A. , Shewry , P.R. , Mantel , S. and Ainsworth , C. 1995 . Isolation and Characterisation of cDNA Clones Representing the Genes Encoding the Major Tuber Storage Protein (Dioscorin) of Yam (Dioscorea cayenensis Lam.) . Plant Mol. Biol. , 28 : 369 – 380 .
- Harvey , P.J. and Boulter , D. 1983 . Isolation and Characterization of the Storage Protein of Yam Tubers (Dioscorea rotundata) . Phytochemistry , 22 : 1687 – 1693 .
- Sasikiran , K. , Padmaja , G. and Rekha , M.R. 2004 . Purification and Partial Characterization of Proteinase and α-amylase Inhibitors from Lesser Yam (Dioscorea esculenta) . Int. J. Food Properties , 7 : 185 – 199 .
- Lowry , O.H. , Rosebrough , N.J. , Farr , A.L. and Randall , R.J. 1951 . Protein Measurement with the Folin Phenol Reagent . J. Biol. Chem. , 193 : 265 – 275 .
- Davis , B.J. 1964 . Disk Electrophoresis-II. Method and Application to Human Serum Proteins . Ann. NY Acad. Sci. , 121 : 404 – 427 .
- Laemmli , U.K. 1970 . Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4 . Nature , 227 : 680 – 685 .
- Edwards , L.J. and Patton , R.L. 1966 . Visualization of Carbonic Anhydrase Activity in Polyacrylamide Gels . Stain Technol. , 41 : 333 – 334 .
- Kagawa Education Institute of Nutrition . 2003 . Standard Tables of Food Composition in Japan , Tokyo, , Japan : KEIN .
- Hou , W-C. , Liu , J-S. , Chen , H-J. , Chen , T-E. , Chang , C-F. and Lin , Y-H. 1999a . Dioscorin, the Major Tuber Storage Protein of Yam (Dioscorea batatas Decne), with Carbonic Anhydrase and Trypsin Inhibitor Activities . J. Agric. Food Chem. , 47 : 2168 – 2172 .
- Conlan , R.S. , Griffiths , L.A. , Turner , M. , Fido , R. , Tatham , A. , Ainsworth , C. and Shewry , P. 1998 . Characterisation of the Yam Tuber Storage Protein Dioscorin . J. Plant Physiol. , 153 : 25 – 31 .
- Hou , W-C. , Lee , M-H. , Chen , H-J. , Liang , W-L. , Han , C-H. , Liu , Y-W. and Lin , Y-H. 2001 . Antioxidant Activities of Dioscorin, the Storage Protein of Yam (Dioscorea batatas Decne) Tuber . J. Agric. Food Chem. , 49 : 4956 – 4960 .
- Hou , W-C. , Chen , H-J. and Lin , Y-H. 2000 . Dioscorin from Different Dioscorea Species all Exhibit Both Carbonic Anhydrase and Trypsin Inhibitor Activities . Bot. Bull. Acad. Sin. , 41 : 191 – 196 .
- Hou , W-C. , Chen , H-J. and Lin , Y-H. 1999 . Dioscorin, the Major Tuber Storage Proteins of Yam (Dioscorea batatas Decne), with Dehydroascorbate Reductase and Monodehydroascorbate Reductase Activities . Plant Sci. , 149 : 151 – 156 .
- Chen , P-Y. , Tu , Y-X. , Wu , C-T. , Jong , T-T. and Chang , C-M. J. 2004 . Continuous Hot Pressurized Solvent Extraction of 1,1-diphenyl-2-picrylhydrazyl Free Radical Scavenging Compounds from Taiwan Yams (Dioscorea alata) . J. Agric. Food Chem. , 52 : 1945 – 1949 .
- Chang , S-J. , Lee , Y-C. , Liu , S-Y. and Chang , T-W. 2004 . Chinese Yam (Dioscores alata cv. Tainung No. 2) Feeding Exhibited Antioxidative Effects in Hyperhomocysteinemia Rats . J. Agric. Food Chem. , 52 : 1720 – 1725 .
- Hou , W-C. , Hsu , F-L. and Lee , M-H. 2002 . Yam (Dioscorea batatas) Tuber Mucilage Exhibited Antioxidant Activities in vitro . Planta Med. , 68 : 1072 – 1076 .