Abstract
In this study, the cassava starch was micronized in a vacuum ball-mill to make tiny granules of different particle sizes (the d 50 value from 7.9 μm to 24.0 μm). The properties of the micronized cassava starch, such as granularity, gelatinization properties, and dispersibility in organic solvent, have been evaluated. Then the micronized cassava starch has also been modified using a dry-method to improve its hydrophobic property, with aluminate coupling agent (ACA). The results suggest that the granularity of the starch decreases sharply through micronization in the vacuum ball-mill. The d 50 value of micronized starch was reduced from 24.0 μm to 7.9 μm with the milling time from 0 h to 54 h. The active sites increase as the size of the cassava starch is reduced. The micronized starch has been found to be more easily gelatinized, dispersed in organic solvent and modified. The gelatinization temperature of starch was reduced from 59° C to 23° C with the milling time from 0 to 54 h.
Keywords:
INTRODUCTION
Starch is a kind of macromolecular compound composed of high sugars. It is widely used in paper, textile, adhesive, sweetener and food industries and these applications depend on starch properties such as viscosity, swelling volume, solubility, clarity, and so on. Among the different starches used by the industry, cassava starch is very important in view of its easy extractability, high viscosity, and paste clarity.[Citation1]
Cassava starch granules, with different size distribution characterizing each variety,[Citation2] are considerably irregular in shape with oval, round, and truncated granules.[Citation3] Cassava starch is also a semicrystalline compound consisting of amylose and amylopectin. Amylose is essentially a linear polymer in which the anhydroglucose units are linked through α-1, 4 indican bonds whereas amylopectin is a branched polymer with α-1, 4 indican bonds, having periodic branches at the O-6 position.[Citation4]
The use of starch has been challenged by some limitations, including the low moisture resistance, poor processability (high viscosity), high brittleness, and incompatibility with some hydrophobic polymers. Consequently, several strategies have been created to cope with these problems, involving treatment of starch granules by chemical or physical means.[Citation5] However, previous studies on the denatured starch were mainly focused on the chemical methods, and paid little attention to the physical methods and the applications of the physically denatured starch.[Citation6–18] The micronization of cassava starch has rarely been studied. Studies on the influence of granularity on the starch product properties, such as gelatinization properties and dispersibility in organic solvent, have not been studied in‐depth.[Citation9–13]
In the current work, we have micronized cassava starch granules and then investigated the effects of the treatment on the gelatinization properties, such as gelatinization properties and dispersibility in organic solvent. The cassava starch of different particle sizes was also modified to improve their dispersibility in organic solvent.
MATERIALS AND METHODS
Materials
Cassava starch was purchased from Beijing Quanfeng Starch Company. Its moisture content was 9.3% (w/w). Anhydrous alcohol (95%) was obtained from Beijing Beihua Chemical Company. It is an analytical pure compound. Liquid paraffin was chemical pure sourced from Beijing Yili Chemical Company. Aluminate coupling agent (ACA) was purchased from Beijing Dazheng Weiye Plastic Additives Ltd. Its variety is the “98–1”, and the industrial pure grade.
Micronization
A vacuum ball-mill was used (QM-1SP04, Instrument Company of Nanjing University, China). Capacity of each vacuum can was 50mL, and diameters of the balls were 10mm and 6mm, respectively. Thirty-six grams of dried cassava starch was dissolved in 80mL anhydrous alcohol. The suspension was then divided into 4 sub-samples and placed in 4 vacuum cans of the ball-grinding machine. Fourteen stainless steel balls (4×Φ10 mm and 10×Φ6 mm) were placed in each can. The cans were degassed to lower the inner pressure down to 0.05MPa. The grinding operation was done for 6 h, 18 h, 30 h, 42 h, and 54 h at 500 rpm, respectively. The suspensions were then dried in incubator at 105°C after the processing. Particle sizes after different milling time intervals were measured by Mastersizer 2000 Laser Particle Size Analyzer (Malvern Company, U.K.).
Gelatinization of Cassava Starch of Different Particle Sizes
Six grams of air-dried cassava starch at each particle size group was dissolved in 100mL de-ionized water. The suspension in 100 mL beaker was heated to 95°C at the rate of 0.67°C/min in heated water-bath; the viscosity was measured using the rotary viscometer (NDJ-8S, Shanghai Precise Scientific Instrument Company, China). The temperature of the gelatinized starch was then remained at 95°C for 1 h, and viscosity was measured once every 6 min. After the temperature was reduced to room temperature (about 23°C) at the speed of 2°C /min, the viscosity change in this process was again measured once every 6 min.
Dispersibility of Cassava Starch of Different Particle Sizes in Organic Solvent
Four grams of air-dried cassava starch of different particle size group was mixed with 10 g liquid paraffin in the colorimetric cylinder (25 mL). The cloud suspension was settled at room temperature, the settling volume was measured each hour. Dispersibility in liquid paraffin of cassava starch of different particle sizes was calculated as follows:
where Disp, V w and V x was the dispersibility of the cassava starch, volume of the suspension and the volume of solids settled to the bottom, respectively.
The bigger Disp is, the better performance of the starch dispersing in liquid paraffin and more stable dispersion system that one should expect.
Hydrophobic Modification of Cassava Starch of Different Particle Sizes
While being esterified by hydrophobic groups, the hydrophobicity of starch increases. Ten grams air-dried cassava starch of different particle sizes placed in round bottom flask were heated at 120°C for 10 min, respectively. Each preheated starch sample was mixed with 0.2 g ACA at 120°C for 20 min using motorized stirrer at 1000 rpm.[Citation14] The dispersibility in the organic solvent was thus measured as described above.
RESULTS AND DISCUSSION
Micronization
Particle size distributions of the cassava starch samples obtained with different milling time are shown in and the key size parameters are given in .
Figure 1 Particle size distribution of the cassava starch particles after different milling time (frequency distribution).
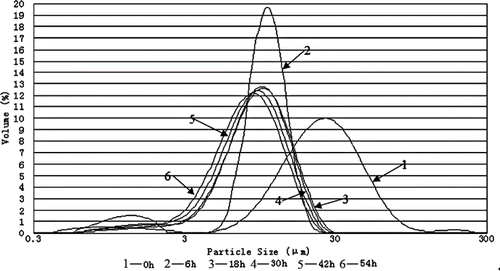
Table 1 Particle sizes of cassava starch of different milling time
The particle size distribution can be seen to shift towards the left with increasing milling time, which indicates the milling was effective. presents the precise sizes of cassava starch particles after different milling time. The d 50 value of micronized starch was reduced from 24.01 μm to 7.94 μm with the milling time from 0 h to 54 h. After a 54 h treatment, d 4.3 and d 50 of micronized starch were less than 1/3 of that of the native starch (non-pulverized, at 0 h treatment) granules. Corresponding, there was an increase in the specific surface area. Starch granules were impacted and rubbed by other granules or steel balls while being pulverized due to the sharply increased stress concentrated on any defect structures. The surface of starch granules lost flatness and smoothness while being pulverized.[Citation9,Citation12,Citation13] It also resulted in the increase in specific surface area.
also shows that there were remarkable changes in particle sizes at the very beginning of the pulverization, but after 6 h, changes were slight and asymptotic particle sizes were approached. When the particles become very small, specific surface areas and surface energies are so great that the particles can attract each other and agglomerate. Micronization process can in fact achieve a dynamic equilibrium: large particles are crushed and turn into smaller particles, while tiny particles agglomerate, forming larger particles. The breaking down of the agglomerates and the reagglomeration can attain a dynamic equilibrium state which shows an apparent final size distribution. However, Tamaki reported that granules of maize starch and potato starch retained whole figure and size after 320 h ball-mill treatment.[Citation12,Citation13] The difference between his results and ours might be caused by different methods to measure and calculate the particle sizes. The particle sizes that he obtained were measured by Scanning Electron Microscope (SEM). So they were just the sizes of some particles. However, our results obtained by laser particle size analyzer calculated all granules.
Gelatinization of Cassava Starch of Different Particle Sizes
Starch granules are known to be insoluble in cold water at room temperature.[Citation15] When heated, an aqueous suspension of starch granules undergoes an order-disorder transition known as gelatinization, resulting in viscosity increase. Starch gelatinization is the collapse of molecular orders within the starch granule manifested in irreversible changes in properties such as granular swelling, native crystallite melting, loss of birefringence, and starch solubilization.[Citation16] The process of gelatinization, which depends on the structure of starch, may be divided into the following three stages.[Citation17]
First stage
A reversible stage of absorbing/desorbing water. Water molecules just enter the interstitial spaces of the microcrystal clusters of the starch granules and associate with free hydroxyl of the amorphous part. Granules that have absorbed water slowly expand some what. At this stage, granule appearance does not change much. The crystal structure still remains. The viscosity of the suspension has no remarkable change.
Second stage
The irreversible stage of absorbing water. When gelatinization temperature is attained and beyond, water molecules would combine with some of the starch molecules, these starch granules absorb a large number of water molecules irreversibly and then expand rapidly. Association of the starch molecules is destroyed due to the fracture of the hydrogen bonds, double helix of the starch molecules are separated or stretched. The crystal structure of amylopectin is destroyed; smaller amylose can leak from the granule, so the suspension can turn into a thick yet transparent paste. The temperature where the suspension viscosity begins to rise significantly is referred as gelatinization temperature.
Third stage
The high-temperature stage. When subjecting the starch paste to more elevated temperatures, the major part of starch molecules would dissolve in water. The attraction forces between different molecules are very weak. All granules change in their forms, microcrystal structures now disintegrate into chips. The viscosity continuously rises to a maximum. Then the swollen granules rupture on continuous heating, resulting in a fall in viscosity.[Citation1]
Gelatinization of cassava starch of different milling time was monitored with the rotary viscometer and the results are shown in and listed in . indicates that gelatinization process of the micronized cassava starch can also be divided into three stages as described above, which is similar to that of the native cassava starch. Gelatinization curves of the micronized starch are less dramatic than that of the native starch. This may indicate that the thermal stability of the micronized starch samples is better.
Figure 2 Gelatinization curves (expressed using the viscosity records) of the cassava starch samples pulverized for different times.
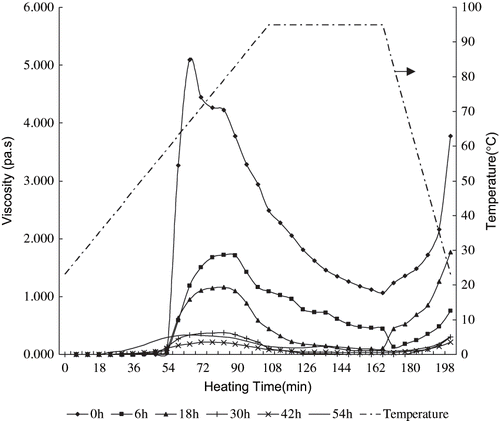
Table 2 Gelatinization characteristic of cassava starch of different milling time
shows that gelatinization of cassava is affected by micronization time directly. With the reduction in granularity, the gelatinization temperature decreases progressively. The gelatinization temperature of starch was reduced from 59°C to room temperature (23 ± 2°C). After 54 h milling, the starch particles were so small that the starch could dissolve in water at 23°C. This would be advantageous for such a product to be used in industry applications where energetic operation needs to be avoided. The viscosities of the starch paste, including V p , V 95, and V h , were found to decrease significantly with reducing granularity. It was in a good agreement with the study of Noda, which focused on the properties of potato starch.[Citation18] The paste would flow more easily when used. The crystal structure of the native starch has the crystal and non-crystal region staggering together inside the particles and the hydrogen bonds are strong. These prevent starch molecules from becoming associated with water molecules. The gelatinization temperature and paste viscosity of native starch is therefore higher.
The native structures are damaged through micronization which enable the starch molecules to become more easily associated with water molecules. This also reduces the gelatinization temperature and paste viscosity of cassava starch. After 54 h of pulverization, the sample's crystal structure would have been damaged severely, most hydrogen bonds would have been fractured, and the starch molecules become more active, making it more easily gelatinized.[Citation11]
Dispersibility of Cassava Starch of Different Particle Sizes in Organic Solvent
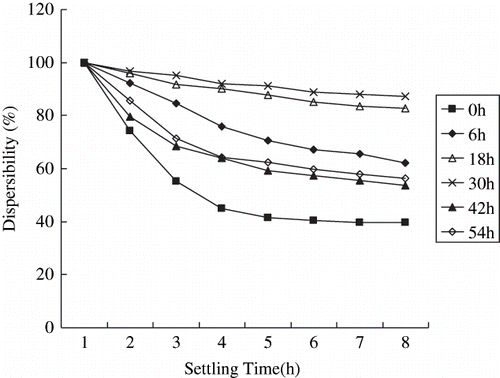
Dispersibility of starch in organic solvent is important for starch-based materials since it influences the producing processes, which in turn affects final properties of the products. A well-dispersed powder provides stable, uniform and high physicochemical properties of the final product. And dispersibility of starch granules in organic solvent is affected by a lot of factors, for example, particle sizes, moisture, ζ-potential, and so on. Dispersibility of cassava starch particles of different sizes in liquid paraffin has been investigated by the sedimentation test. Results of the test are shown in . Micronized cassava starch dispersed in liquid paraffin better than native cassava starch. It indicates that the dispersibility should been improved by reducing granularity appropriately.
Dispersibility of native cassava starch performed the worst because of its rather larger granules. The particles settled rapidly under their own gravity. After been pulverized, the granularity of cassava starch was reduced. Thus the dispersibility was improved gradually. However, the dispersibility of cassava starch pulverized for 42 h and 54 h was not improved any more, on the contrary, it was worse than that of the samples pulverized for less hours (6 h, 18 h, and 30 h). This might by explained by the agglomeration of very small particles due to their great surface energies.
Hydrophobic Modification of Cassava Starch of Different Particle Sizes
Molecules of natural starch are hydrophilic due to a large number of hydroxyls contained. Large amounts of emerged groups of hydroxyl can also reduce the compatibility between the starch and the ester based polymer. Hydrophobic modification of cassava starch in a dry-method can improve the compatibility to some degree. However, dry modification method can only take effect on the surface, so the extent of the modification used on the native starch is not large. Specific surface area and hydroxyls on the particle surface could be increased by reducing granularity, so the degree of modification could be improved by micronization of starch. General chemical formula of ACA is expressed as follows:
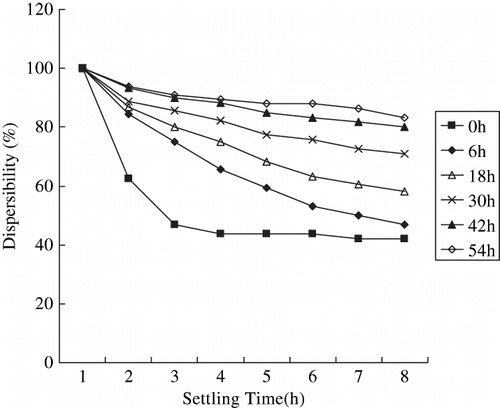
where RO, Al, R′ is the short alkoxy chain, which is easy to be hydrolyzed or exchanged, aluminum atom, and long non-polar alkoxy chain, respectively. The principle of hydrophobic modification of cassava starch with ACA as the coupling agent was described as follows: hydroxyls on the surface were covered by reaction with ACA while being modified. The starch structure changes into a hydrophobic one as a result. When modified starch is mixed with synthetic polymer, the long non-polar alkoxy chains-R′ join in and wrap around the chain of synthetic polymer molecules, so that the starch can be combined with the synthetic polymer well.[Citation14] Effect of ACA on the dispersibility of cassava starch in liquid paraffin was also investigated by the sedimentation test. Results are shown in .
Dispersibility of modified cassava starch was improved gradually in magnitude with decreasing granularity. In contrast with the unmodified samples, the cassava starch pulverized for 6 h, 18 h, and 30 h generated worse dispersions, while the cassava starch pulverized for 42 h and 54 h dispersed better in liquid paraffin. The difference among them might be resulted from their different particle sizes.
The ACA on particle surface produces steric stabilization when the surface-grafted layers overlaps as particles approach each other. It prevents particles from agglomeration. The short alkoxy chain of ACA attaches itself to the starch particle and the long non-polar alkoxy chain remains dissolved in liquid paraffin and provides steric stabilization. However, the success of steric stabilization depends on the surface coverage, the configuration of ACA and the thickness of the grafted layer.[Citation19] Hence, the amount of ACA must be proper and is a function of the hydrophilicity of starch. More ACA is needed for small particles due to their larger specific surface area and more hydroxyls on the surface.
It was reported that 2 wt% of ACA was sufficient for micronized corn starch sample which d 50 was 3.11 μm.[Citation14] So it was reasonable to presume that all of the cassava starch samples were well modified in our study. As we described above, the lipophilicity of the samples pulverized for 42 h and 54 h was improved dramatically for their increased specific surface area and hydroxyls on the particle surface. But the improvement of the samples pulverized for fewer hours was limited for lack of hydroxyls on the particle surface. Moreover, over-consumption of ACA increased the volume and weight of the particles sharply. That's why the dispersibility of the samples pulverized for 6 h, 18 h, and 30 h got worse. It can be concluded from this study that modification in a dry-method with ACA can improve the lipophilicity of cassava starch, but the effect is affected by particle sizes. The degree of modification can be improved by micronization of starch.
CONCLUSIONS
Based on these investigations, the following conclusions can be drawn: (i) ball-mill treatment can reduce the granularity of cassava starch evidently; (ii) gelatinization temperature and paste viscosity of cassava starch decrease while the granularity is reduced; (iii) dispersibility of cassava starch in liquid paraffin can be improved by reducing the granularity; and (iv) modification in a dry-method with ACA can improve the lipophilicity of cassava starch. Effect of the modification can be improved by reducing the granularity.
ACKNOWLEDGMENT
Research support was provided by the Funding System for Scientific Research Projects of Doctor Subject of Chinese Advanced University (No. 20020019044) and Funding System for Scientific Research Projects of China Agricultural University (No. 2004010).
REFERENCES
- Jyothi , A.N. , Sasikiran , K. , Sajeev , M.S. , Revamma , R. and Moorthy , S.N. 2005 . Gelatinisation Properties of Cassava Starch in the Presence of Salts, Acids and Oxidising Agents . Starch , 57 : 547 – 555 .
- Santisopasri , V. , Kurotjanawong , K. , Chotineeranat , S. , Piyachomkwan , K. , Sriroth , K. and Oates , C. G. 2001 . Impact of Water Stress on Yield and Quality of Cassava Starch . Industrial Crops and Products , 13 : 115 – 129 .
- Sriroth , K. , Santisopasri , V. , Petchalanuwat , C. , Kurotjanawong , K. , Piyachomkwan , K. and Oates , C.G. 1999 . Cassava Starch Granule Structure-function Properties: Infuence of Time and Conditions at Harvest on Four Cultivars of Cassava Starch . Carbohydate Polymers , 38 : 161 – 170 .
- Freitas , R.A. , Paula , R.C. , Feitosa , J.P.A. , Rocha , S. and Sierakowski , M.R. 2004 . Amylose Contents, Rheological Properties and Gelatinization Kinetics of Yam (Dioscorea alata) and Cassava (Manihot utilissima) Starches . Carbohydrate Polymers , 55 : 3 – 8 .
- Santayanon , R. and Wootthikanokkhan , J. 2003 . Modification of Cassava Starch by Using Propionic Anhydride and Properties of the Starch-blended Polyester Polyurethane . Carbohydrate Polymers , 51 : 17 – 24 .
- Mahmut , S. , Hasan , S. , Murat , O. and Milford , A.H. 2003 . Cross-linking of Starch with Reactive Extrusion and Expansion of Extrudates . International Journal of Food Properties , 6 ( 3 ) : 473 – 480 .
- Carvalho , A.J.F. , Curvelo , A.A.S. and Gandini , A. 2005 . Surface Chemical Modification of Thermoplastic Starch: Reactions with Isocyanates, Epoxy Functions and Stearoyl Chloride . Industrial Crops and Products , 21 : 331 – 336 .
- Aggarwal , P. and Dollimore , D. 1998 . The Effect of Chemical Modification on Starch Studied Using Thermal Analysis . Thermochimica Acta , 324 : 1 – 8 .
- Baldwin , P.M. , Adler , J. , Davies , M.C. and Melia , C.D. 1994 . Starch Damage Part 1: Characterisation of Granule Damage in Ball-milled Potato Starch Study by SEM . Starch , 46 : 247 – 251 .
- Adler , J. , Baldwin , P.M. and Melia , C.D. 1994 . Starch Damage Part 1: Types of Damage in Ball-milled Potato Starch, upon Hydration Observed by Confocal Microscope . Starch , 46 : 252 – 256 .
- Morrison , W.R. , Tester , R.F. and Gidley , M.J. 1994 . Properties of Damaged Starch Granules. II. Crystallinity, Molecular Order and Gelatinisation of Ball-milled Starches . Journal of Cereal Science , 19 : 209 – 217 .
- Tamaki , S. , Hisamatsu , M. , Teranishi , K. and Yamada , T. 1997 . Structural Change of Potato Starch Granules by Ball-mill Treatment . Starch , 49 : 431 – 438 .
- Tamaki , S. , Hisamatsu , M. , Teranishi , K. , Adachi , T. and Yamada , T . 1998 . Structural Change of Maize Starch Granules by Ball-mill Treatment . Starch , 50 : 342 – 348 .
- Wu , J. , Li , B. and Xie , B.J. 2004 . Studies on the Dry-method Hydrophobic Modification and Mechanism of Micronized Starch . Food Science , 25 ( 9 ) : 96 – 100 . (in Chinese).
- Nayouf , M. , Loisel , C. and Doublier , J.L. 2003 . Effect of Thermomechanical Treatment on the Rheological Properties of Crosslinked Waxy Corn Starch . Journal of Food Engineering , 59 : 209 – 219 .
- Maaruf , A.G. , Che Man , Y.B. , Asbi , B.A. , Junainah , A.H. and Kennedy , J.F. 2001 . Effect of Water Content on the Gelatinisation Temperature of Sago Starch . Carbohydrate Polymers , 46 : 331 – 337 .
- Pielichowski , K. , Tomasik , P. and Sikora , M. 1998 . Kinetics of Gelatinization of Potato Starch Studied by Non-isothermal DSC . Carbohydrate Polymers , 35 : 49 – 54 .
- Noda , T. , Takigawa , S. , Matsuura-Endo , C. , Hashimoto , N. , Yamauchi , H. , Hanashiro , I. and Takeda , Y. 2005 . Physicochemical Properties and Amylopectin Structures of Large, Small, and Extremely Small Potato Starch Granules . Carbohydrate Polymers , 60 : 245 – 251 .
- Shojai , F. , Petterson , A.B.A. , Mäntylä , T. and Rosenholm , J.B. 1997 . Dispersibility of Yttria-doped Zirconia Powders in Aqueous Media . Progress in Colloid and Polymer Science , 105 : 1 – 5 .