Abstract
Gel properties of radio frequency (RF) heated egg white dispersions at 27.12 MHz were studied as function of concentration (2.5–2.5 kg/100 kg sample), pH (3–11) and heating time (60–180 s). Egg white dispersions demonstrated a gradual liquid-solid transformation as they denatured and gelled during RF treatment. The critical concentration and heating period for egg white protein denaturation and gelation were found to be 7.5% (w/w) and 150 seconds. The elastic modulus (G′) of RF-heated samples increased with concentration and heating period (temperature), whereas complex viscosity (η*) increased exponentially with concentration. In an alkaline condition, the egg white dispersion did not produce a gel; however, in acidic condition it resulted in a strong gel with significantly (P < 0.05) higher G′. This could be attributed to the high dielectric constant (ε′) and loss factor (ε″) values of acidified samples as compared to the alkaline and control egg white dispersion. Effect of heating rate (1, 5, 10, and 20°C/min) in situ on rheometer plate significantly affected gel rigidity; the RF treated sample rigidity was comparable to samples heated at the rate of 5 and 10°C/min. Differential scanning calorimetry, dielectric measurement, and sodium dodecyl sulfate (SDS) PAGE electrophoresis results were used to confirm gelation behavior during both conventional and RF heating conditions.
INTRODUCTION
Radio frequency (RF) heating is an innovative technique among the several recent electro-technologies such as ohmic heating, microwave dielectric heating, inductive/ohmic combinations, inductive heating, and radiative magnetic heating. The RF generator produces an alternating electric field between two electrodes in radio frequency system. The material to be subjected to RF heating is conveyed between the electrodes, where the alternating current causes the polar molecules in the material to continuously try to align themselves with the oscillating electrical field much like the way bar magnets behave in an alternating magnetic field. The friction resulting from molecular movement causes the material to rapidly heat throughout its entire mass. The amount of heat generated in the product is a function of frequency, the square of the applied voltage, dimensions of the product, and the dielectric properties (mostly the loss factor) of the material. The dielectric properties of materials dictate, to a large extent, the behavior of the materials when subjected to RF or microwave (MW) heating of the materials.[Citation1]
RF heating (13.56, 27.12, and 40 MHz) differs from the higher frequency microwave heating (915 MHz and 2450 MHz) in which the wavelength is comparable or smaller than the dimensions of the sample and heating occurs in a metal chamber with resonant electromagnetic standing waves. Since RF waves have a longer wavelength than MW, the electromagnetic waves in the RF system can penetrate deeper into the product so there is less surface overheating, and hot or colds spots—a common problem with MW heating. The RF heating also offers simple uniform field patterns as opposed to the more complex and characteristically non-uniform standing wave patterns in a microwave oven.
One of the carliest applications of RF heating was in the baking of bread and thereafter the technology has been extensively exploited by the bakery industry for post-bake drying/finishing purposes.[Citation2–3] Several review articles are available on the RF-heating and its applications.[Citation4–7] Demeczky[Citation8] reported that RF pasteurised fruit juices had better microbiological and sensory qualities as compared to conventional thermal treatments. Some studies have been carried out on RF pasteurisation of meat products.[Citation6,Citation9] Since, most of the liquid foods are now processed aseptically, development and optimization of RF heating will be attractive for processing and preservation of liquid foods.[Citation10]
Zhong et al.[Citation5] reported that a 30 kW, 40.68 MHz RF system heated water uniformly, while heating of carboxymethylcellulose (CMC) solution was non-uniform in both batch and continuous modes of operation. The difference was attributed to the dielectric properties of water and CMC samples. Luechapattanaporn et al.[Citation11] studied the thermal resistance of Clostridium sporogenes (PA 3679) in phosphate buffer and mashed potatoes in aluminium thermal-death-time tubes using 27.12 MHz RF heating and reported that the RF heating system could be used to produce safe and shelf stable packaged foods. Although, the RF heating process has been experimented with pasteurization or sterilization, there has been no detailed study on the evaluation of protein denaturation or gelation behavior of RF heated foods.
Egg white protein (EWP) has the unique characteristics and is extensively used in many food applications. Gel formation, water holding capacity, foaming, and emulsifying properties are some of the important functional characteristics of egg white. Heat induced gelation of protein is considered to form a three-dimensional network assembled from protein molecules or aggregates, surrounded by water. The gel network is generally measured in terms of rheological properties, water holding capacity, and/or micro-structural changes all of which are affected by protein concentration, pH, temperature, heating period, and rate of heating.[Citation12–13] Recently, some studies have focused on subjecting whey and muscle proteins to dielectric heating and evaluating the denaturation behaviour by observing changes in their dielectric properties.[Citation14–15]
Food gels are aqueous dispersions or solutions of high molecular weight carbohydrates or proteins, cross-linked so as to form an interconnected molecular network that spans the volume of the liquid medium. Rheological techniques have been used to measure the rigidity and deformation of gels using various techniques (small and large-strain rheology).[Citation16–22] Gel network structure and interactions within the network strand control the rheological properties of protein gels. Most works conclude that gel rigidity is increased with an increase in protein concentration, temperature, and pH. However, for egg white, no information is available on denaturation and gel formation during radio frequency heating.
The objective of this work was to study application of radio frequency heating to liquid egg white and evaluate its effect on gelation and gel strength as function of protein concentration, pH, and heating time in comparison with conventionally heated product. Since, RF heating is directly dependent on dielectric properties; the second objective was to study the dielectric properties of egg white dispersions as function of temperature and pH in the RF range.
MATERIALS AND METHODS
Material
Sample of albumin from chicken egg white was obtained from Sigma Chemicals (Sigma-Aldrich Inc., St Louis, MO). The product was a crude preparation of dried egg white, light yellow in color. The moisture content of the powder sample was 9.2% and contained 62–88% ovalbumin with a minimum 12.5% nitrogen. The molecular weight of the sample was approximately 44.3 kDa (as per specifications).
Preparation of Sample
Samples of egg white dispersions were prepared (2.5, 5, 7.5, 10, and 12.5 kg/100 kg water) by mixing the required amount egg white powder and deionized water (conductance: 18 Ω, Milli-Q, Millipore, Bedford, CT) under continuous stirring after which it was held for 2 hours at room temperature (20°C) for hydration. The different concentrations were chosen to evaluate the critical level required for gel formation. The normal pH of the dispersion was 6.89. The pH of the samples were adjusted to various levels (3, 5, 9, and 11) by adding required volume of hydrochloric acid or sodium hydroxide solution while the control sample is considered to at pH 7 for comparison purposes. Each sample was prepared in triplicate.
Apparatus
A laboratory scale radio frequency dielectric heating system (Model IRF-01; iCorz Technologies, Guelph, Ontario, Canada) was used in this work operating at 27.12 MHz and a nominal output power of 2 kW. Apart from the generator, the RF system was equipped with a vertical applicator tube of 0.0254 m diameter and 0.762 m height.
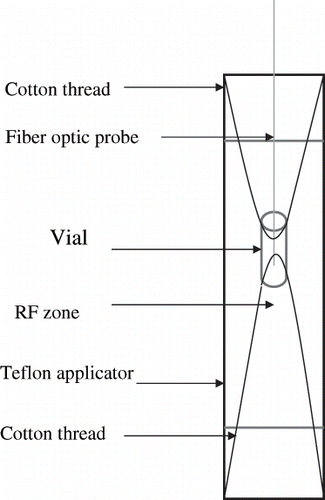
The RF load applicator was adjusted with load and tune to match 50 Ω impedance network. The unit was designed to work either in batch or continuous mode with the connection of the applicator tube to a pump. However, the present work was carried out in a batch mode by closing at the bottom of the applicator tube with a plug. Water was placed in the applicator, and the level was kept slightly above radio frequency zone (∼210 mL). A polypropylene vial (Nalge Nunc Int., Rochester, NY) of 2 mL capacity containing egg white sample was suspended in the applicator radio frequency zone and held in place using a cotton thread during the heating period A fiber optic probe (FISO Technologies Inc., Quebec, Canada) was inserted inside the vial (). The sample temperature was recorded using the data logger and software provided by the fiber optic probe supplier. The top of the applicator tube was covered with aluminium foil padded wad to prevent leakage of RF waves and minimize heat loss.
Rheological Measurements
The rheological measurements of RF heated egg white dispersions were carried out by using a controlled-stress rheometer (AR 2000, TA Instruments, New Castle, DE). A parallel plate geometry with a 60-mm diameter of upper plate was used with a gap of 1000 microns. The sample temperature was internally controlled by peltier system (−20 to 200°C with an accuracy of ±0.1°C) attached with a water circulation unit. A platinum resistance thermometer sensor positioned at the centre of the plate ensured temperature control and measurement. For each test, a measured volume of thoroughly mixed sample (approximately 2 mL) was placed at the center of the rheometer plate for 5 minutes for stress relaxation and temperature equilibration before the actual measurements.
Small-amplitude oscillation (SAOS) measurements were performed within linear viscoelastic region over a frequency range between 0.1 and 10 Hz, which enables the material to preserve the structure. To study the effect of heating rate, the sample was heated from 30 to 92°C (1Hz frequency) at selected rates (1,5,10, and 20°C/min) and followed by immediate cooling to 20°C with the internal peltier heating system. Measurements were made after temperature equilibration to 20°C and each time new sample was used for rheological measurement. All the rheological measurements were carried out in triplicate and rheological parameters (elastic modulus, G′; viscous modulus, G″; phase angle, δ; and complex viscosity, η*) were directly obtained using the manufacturer supplied computer software (Rheology Advantage Data Analysis Program, TA Instruments, New Castle, DE).
Dielectric Properties Measurement
A vector network analyzer (Model: Agilent 8722ES, Agilent Technology, Palo Alto, CA) with an open-ended coaxial probe was used to measure dielectric properties of egg white dispersions. The built-in S-parameter test set provided a full range of magnitude and phase measurements in both the forward and reverse directions. The sample (approximately 40 mL) was placed in a wide glass tube (50 mL), and the open co-axial probe (probe diameter ≈20 mm) was set into the tube. The sample holder was dipped into a temperature controlled water bath with agitation. A thermocouple temperature sensor (BK Precision 390A, Yorba Linda, CA) was used to monitor the temperature during study and the temperature variation was minimal, ± 0.5°C. The network analyzer probe and the cable were fixed together so that there could be no movement during sample measurement and data acquisition. The instrument was calibrated using air, short, and water before each experiment. The dielectric properties of the samples were measured from 200 to 2500 MHz within temperature range of 20 to 90°C. The dielectric spectra of the samples (dielectric constant ∈′, and dielectric loss factor ∈″) were automatically computed and recorded with the manufacturer supplied computer software (Version 85070 D1.00, Agilent Technologies, Palo Alto, CA). All the measurements were carried out in duplicate and were reproducible ± 5%.
Differential Scanning Calorimetry
A differential scanning calorimenter DSC Q 500 (TA Instruments Newcastle, NJ) was used for gathering the thermograms. Control and RF heated egg white samples were weighed (10–13 mg ± 0.12 mg) into an aluminum DSC sample pan (Part No 900793.901) and uniformly distributed across the bottom of the pan. The upper aluminium hermetic lid (Part No 900794.901) was placed and hermetically encapsulated samples for DSC measurements. All samples were analyzed within 3h of RF heating.
The DSC was calibrated with indium and sapphire for temperature and heat capacity. An empty pan was used as a reference to balance the heat capacity of the sample pan. In the experiment, sample pans were equilibrated to 20°C and held isothermal for another 2 minutes. The heating rate of the DSC scans was 5°C/min over a range of 30 to 120°C. The DSC measurements were done in triplicate. The DSC data were analyzed with the Universal Analysis Software (version 3.6C) for thermal analysis, which was provided with the instrument (TA Instruments, Newcastle, NJ).
Electrophoresis
Sodium dodecyl sulfate (SDS)-PAGE was performed using the method of Fritz et al.[Citation23] to demonstrate the effects of RF heating on the egg protein. Stacking gel and resolving gel of 4 and 12% acrylamide were used for SDS-PAGE. Slab gels (0.75-mm thickness) were run at a constant current of 15 mA/gel; the electrophoresis was performed with a Bio-Rad Mini Trans blotting system as detailed in earlier studies.[Citation24]
Data analysis
The Microsoft Excel software package (Microsoft Corporation, Redmond, WA) was used to carry out the statistical analysis and linear regressions of experimental data.
RESULTS AND DISCUSSION
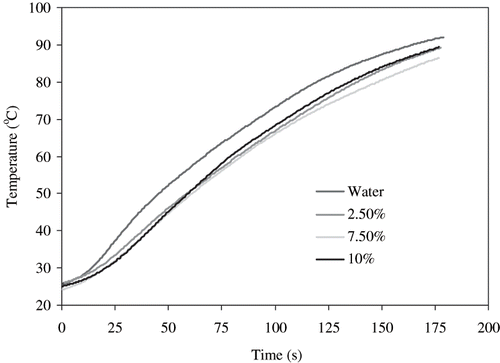
Temperature profiles of water (medium) and egg white dispersions at different concentrations are shown in . Protein concentrations did not affect the heating pattern. Normally, in conventional heating, the heating pattern gradually changes from convection to conduction with an increase in protein concentration in the dispersions thereby considerably slowing down the rate of heating. However, this was not the case with RF heating. This could be attributed to the dielectric heating in the RF system with a much deeper penetration power (uniform heating), which was independent of sample concentration. The temperature along wall side of the applicator was not significantly (P > 0.05) different from that at the center of the Teflon tube (difference less than 2–3°C, data are not shown). Similar observation has been reported by Zhong et al.[Citation5] during experiments with RF heating of water and CMC dispersion.
Gel Formation and Gel Rigidity Measurement by SAOS
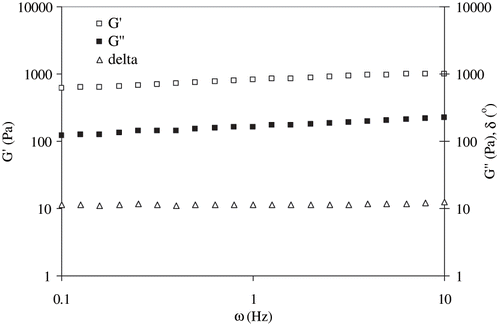
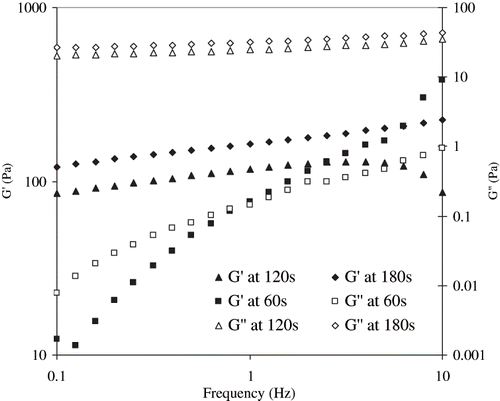
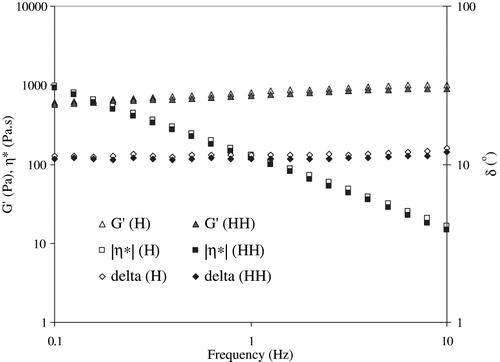
SAOS measurement indicated the possible gelation of egg white dispersions during RF-heating. The gel characteristics were represented by dynamic rheological parameters: elastic modulus (G′), viscous modulus (G″), phase angle (δ), and complex viscosity (η*). A typical rheogram of RF-treated egg white dispersion is shown in . The elastic modulus predominated significantly (P < 0.05) over viscous component in whole frequency range; however, with a slow change in G′ indicating a more elastic nature of the RF treated egg white dispersions.[Citation25] Lower values of phase angle (which is a measure of the ratio of energy lost to energy stored in a cyclic deformation) data supported the visco-clastic nature of heated egg white sample.
Effect of concentration
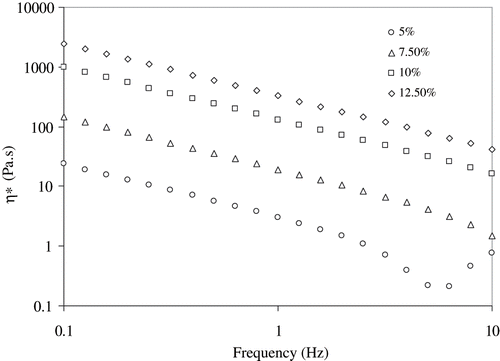
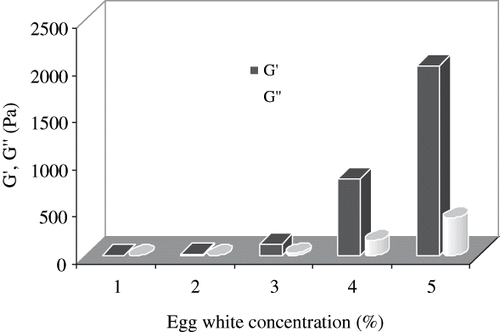
Both G′ and G″ of RF treated egg white dispersions are shown as function of concentration in . RF heating changed the physical state of egg white dispersions leading to gelation. Visually it was noticed that a concentration of 7.5% was required to form a gel. Both G′ and G″ for the 2.5% heat-treated sample were too low, and it was difficult to obtain data of significance within the range of oscillation stress. Therefore, this concentration was excluded from further study. Sample concentration of 5% produced precipitation. Rheological data supported the visual observation with significantly different magnitudes of G′ and G″. Both these parameters increased with concentration and the maximum value was found at the egg white concentration of 12.5% (). Higher G′ values indicate the ability of gel to maintain an intact network structure.
Figure 4 Effect of concentration on elastic and viscous modulus of RF-heated egg white dispersion at 1 Hz and 180 seconds heating (1: 2.5, 2: 5, 3: 7.5, 4: 10, 5: 12.5%).
Complex viscosity (η*) measures the true visco-elastic nature of gels. Complex viscosity versus angular frequency of egg white dispersions as a function of concentration is shown in . The values of η* significantly (P < 0.05) increased with concentration, however systematically decreased with angular frequency. The increase in complex viscosity (η*) followed an exponential relationship with concentration (C) at a constant frequency (1 Hz) (EquationEq. 1).
Effect of heating time
The RF heating time was varied between 60 and 180 seconds, and it was noted that heating period significantly (P < 0.05) affected protein denaturation process and gel formation of egg white dispersion (). Both G′ and G″ increased as a function of heating period, and the peak values were noted at 180 seconds. However, a minimum heating period of 150 seconds (critical gelling time, corresponding temperature 78–80°C) was required to denature egg white dispersion by RF heating and, to form a gel. The gel formation is an integrated effect of time, temperature, and reaction rates.[Citation26] Increase in heating period and even holding for an additional 180 seconds and cooling immediately did not enhance either elastic or viscous modulus of samples (). It is possible that gelation of egg white protein was complete with the 180 seconds RF heating treatment, and hence, no further increase in rheological parameters was observed during the extended holding time. All the rheological parameters (elastic modulus, phase angle, and complex viscosity) demonstrated superimposition with each another.
Effect of heating rate and comparison with conventional heating
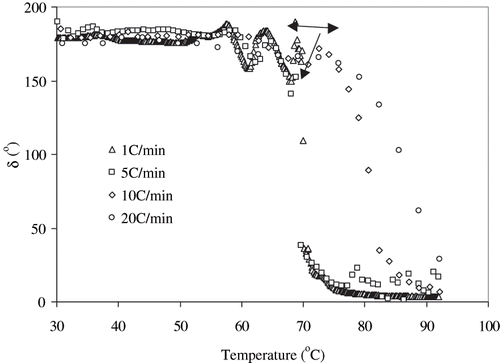
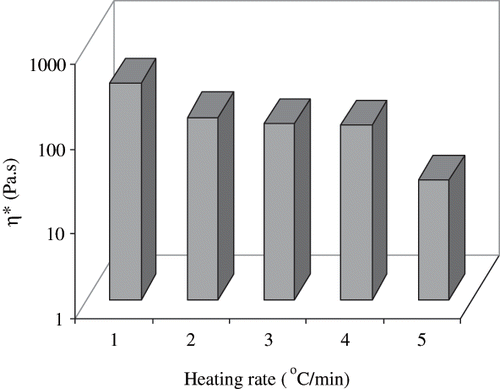
The heating rate during RF treatment was found to be 22°C/min. To compare gelation behavior between RF heating and conventional heating, a control sample of 10% was heated in rheometer plate from 30 to 92°C at different heating rates of 1, 5, 10, and 20°C/min (maximum heating rate of rheometer and a near comparison to the RF heating rate of 22°C/min), and the thermorheological behavior was measured in terms of phase angle which is an important parameter for visco-elastic fluid[Citation26] (). A sharp decrease in phase angle was observed at temperature of about 70°C which indicated initiation of protein denaturation. Heating rate at 1 and 5°C/min exhibited almost similar patterns of changes in phase angle, while a large scattering of data were noticed with a more rapid heating rate. The significant decrease in phase angle indicated protein denaturation with visco-elastic texture. However, at rapid heating rates, the denaturation got initiated at a slightly higher temperature due to short residence times. There are several hypotheses for protein gel formation and gel rigidity. Many researchers have reported that faster heating rates would allow less time at temperature above the denaturation temperature of the proteins for aggregation to occur and, therefore, result in a weak gel formation.[Citation27–28] This is also very much consistent with the concept of high temperature short time (HTST) processes in which better quality retention has been traditionally observed indicating smaller thermal effects on quality changes such as starch gelatinization, protein denaturation or enzyme inactivation (except for microbial kill which gets accelerated).
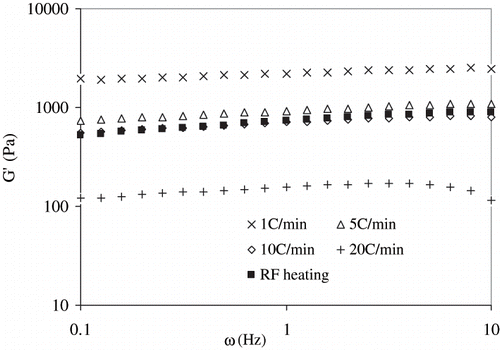
Gel rigidity of RF and conventional thermal treated samples (at different heating rate) were compared after heating at 92°C, followed by immediate cooling to 20°C.
Interestingly, the gel rigidity produced by heating at 1°C/min of egg white sample was found to be the highest (65 minutes duration of heating-cooling), while gels produced by heating rate of 5°C/min (15 minutes duration of heating-cooling) and 10°C/min (7.5 min) were comparable with radio frequency heated sample (). The gel rigidity of RF-heated sample was significantly higher than sample heated at rate of 20°C/min (4 minutes). We observed similar results for complex viscosity of heated egg white dispersions (). This observation indicated that in addition to temperature effect, RF heating could have some complementary effect on protein molecules to enhance denaturation and gel formation in the alternating current electric field. This could be attributed to the total thermal input (combination of time and temperature), rather than only the heating rate, is the determining factor in heat-induced gel formation by egg white proteins. Recently, Riemann et al.[Citation26] advocated that rapid heating followed by brief holding resulted similar gel texture of meat, which were cooked by slow heating and cooled immediately.
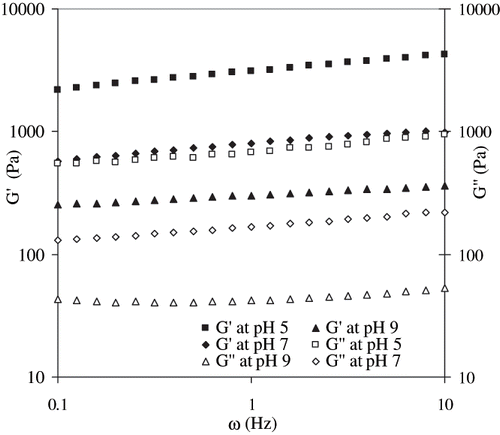
Effect of pH
Egg white dispersions at various pH levels showed significant differences in gel formation. Egg white dispersions adjusted to lower pH and subjected to RF heating formed a stronger gel as compared to control or higher pH sample (). This observation is contradictory to earlier work on water bath heating of egg white dispersions.[Citation17] The previous authors reported that alkaline gels exhibited a fine ordered network and the maximum hardness was found at pH 11. On the other hand, Renkema[Citation21] observed highest G′ values at lower pH (3.2) as compared to 5.2 and 7.6, respectively. In our study, the acidification resulted stronger gel, while alkaline dispersions (pH 9 and 11) did not form gel during RF heating. Elastic modulus of acidified samples was stronger than control pH sample (6.87). The reason is not clear. The possible reason for non-gel formation of egg white in the alkaline pH region could be ionic interference in the radio frequency zone.
Dielectric Properties
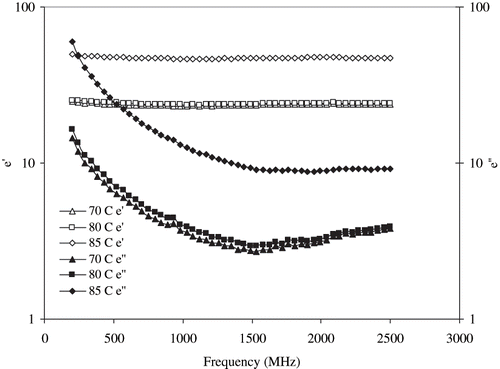
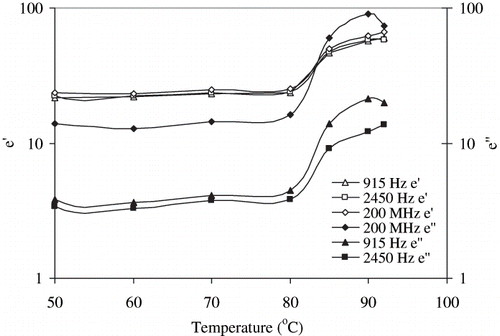
Effect of temperature on dielectric parameters of egg white dispersion is shown in . The dielectric constant (ε′) was independent of frequency; however, the dielectric loss factor (ε″) decreased up to 1200 MHz and remained constant at higher frequencies. Both the dielectric constant (ε′) and dielectric loss factor (ε″) increased with an increase in temperature. However, no significant differences (P > 0.05) were observed in dielectric parameters between 70 and 80°C, while the parameters became significant (P < 0.05) at 85°C. Effect of temperature at selected frequencies (200, 915, and 2450 MHz) is illustrated in . It further confirmed the frequency independency of dielectric constant, whereas loss factor decreased with an increase in frequency within the temperature range employed. Temperature did not significantly affect dielectric parameters in the temperature range between 50 and 80°C. A sharp increase in both ε′ and ε″ values observed between 85 and 90°C, which finally decreased at 92°C. These temperature ranges correspond to egg white denaturation. Similar observation was earlier reported by Bircan et al.[Citation14] for whey protein, and the authors reported that the temperature where an increase or decrease in dielectric parameters observed corresponded to whey protein denaturation temperature. Further, Bircan and Barringer[Citation15] found that both dielectric parameters increased with temperature for muscle proteins, and a sudden increase in dielectric parameters represented the muscle protein denaturation temperature.
Figure 11b Changes in the dielectric constants at 50–92°C of 10% egg white dispersion at 200, 915, and 2450 MHz.
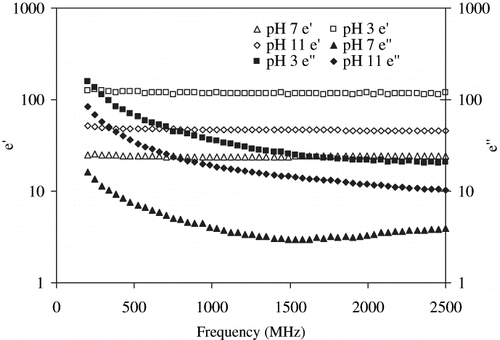
Effect of pH on dielectric parameters of egg white dispersion is shown in . Change in pH increased both dielectric parameters of egg white dispersions while the highly acidic pH (pH = 3) exhibited significantly (P < 0.05) higher values. It has been well established that salt concentration is an influencing factor on the dielectric properties of foods.[Citation29] The additional hydrogen ions affect the parameters to a large extent as compared to excess sodium ions in the alkaline zone (pH = 11) and, therefore result in a higher dielectric constant for acidified egg white dispersions. It infers that egg proteins in acidic condition denatures faster in electric field than alkaline or neutral conditions due to more rapid dielectric heating.
Protein Denaturation
Protein denaturation during RF-heating was monitored by DSC () and electrophoresis (), respectively. The protein denaturation temperature (Td) of egg white protein (mainly ovalbumin) was found to be 84.1°C. Some RF heating conditions (60, 90, and 120 seconds) did not denature ovalbumin (–f). Effects of pH (3 and 11) on egg protein denaturation are compared in Figures 11d and 11e, respectively. The aggregation of protein occurred at both pH after heating for 120 seconds at 110°C. The shift of protein thermogram could be the effect of RF heating. The sample heated for 180 seconds and longer irrespective of pH denatured and no ovalbumin peak was observed (Fig. 11f).
Figure 13 DSC thermograms of egg white dispersions: a. Control sample (12.5% at pH 7); b. 10%, pH 9 and 60 seconds; c. 5%, pH 5 and 90 seconds; d. 7.5%, pH 11 and 120 seconds; e. 7.5%, pH 3 and 120 seconds; f. 7.5%, pH 7 and 150 seconds.
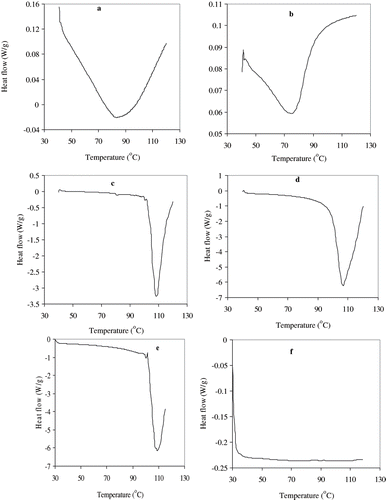
Figure 14 SDS-PAGE results of RF and convention heating of egg white dispersions (10%); 1- standard; 2-control sample; RF heating:3–60 seconds; 4–90 seconds; 5–120 seconds; 6–150 seconds; 7–180 seconds; Convention heating: 8-non-RF heating 180 seconds and 9-non-RF heating 60 seconds.
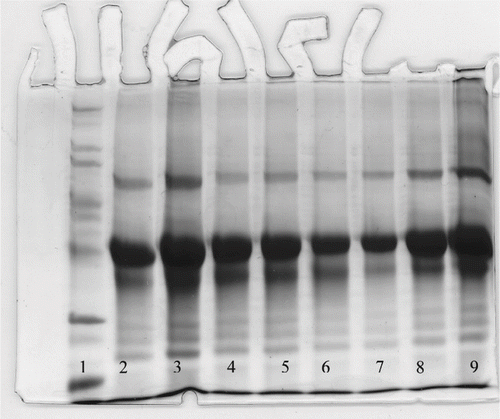
SDS PAGE () compares between RF heating and conventional heating of egg white dispersions at similar conditions. It shows that RF heating time affected the egg white protein subunits. The ovalbumin band intensity decreased with heating time period and major changes noticed in samples heated for 150 seconds and longer. The ovalbumin intensity was found to be higher for conventional heat treatments (column 8) as compared to RF treatment (column 7). RF heating indicated a systematic realignment of protein structure with strong gel rigidity. Alzagtat[Citation30] observed that SDS-PAGE detected molecular changes of ovalbumin as a result of interaction with various lipids. The SDS-PAGE results supported protein denaturation behavior from rheological and calorimetric observation of RF treated ovalbumin.
CONCLUSIONS
Radio frequency heating denatured egg protein dispersions and produced a stronger gel. Elastic modulus of RF-treated samples increased with concentration and heating period where as complex viscosity increased exponentially with sample concentration. The critical concentration and optimum time for the gelation was found to be 7.5% (w/w) and 150 seconds, respectively. Acidic dispersions produced stronger gel where as alkaline solution of egg white did not produce a gel. Higher dielectric properties of acidified egg white dispersion made faster RF heating and consequently produced a stronger gel compared to neutral and alkaline dispersions.
ACKNOWLEDGMENTS
The authors thank Muhammad Alu'datt, Department of Food Science & Agricultural Chemistry, and Yvan Gariepy, Department of Bioresource Engineering, McGill University for performing the electrophoresis experiment and helping to set up the network analyzer, respectively. The authors would also like to thank the Natural Sciences and Engineering Research Council of Canada for proving partial funding to carryout the research.
REFERENCES
- Nelson , S.O. and Datta , A.K. 2001 . “ Dielectric Properties of Food Materials and Electric Field Interactions ” . In Handbook of Microwave Technology for Food Applications , Edited by: Datta , A.K. and Anatheswaran , R.C. 69 – 107 . New York : Marcel Dekker, Inc .
- Decareau , R.V. 1985 . Microwaves in the Food Processing Industry , 1 – 23 . New York : Academic Press .
- Jones , P.L. 1997 . “ RF Heating, An Old Technology with a Future ” . In Microwaves: Theory and Applications in Materials Processing , Edited by: Clark , D.E. , Sutton , W.H. and Lewis , D.A. Vol. IV , 261 – 268 . Westerville, Ohio : The American Ceramic Society .
- Piyasena , P. , Dussault , C. , Koutchma , T. , Ramaswamy , H.S. and Awuah , G.B. 2003 . Radio Frequency Heating of Foods: Principles Applications and Related Properties—A Review . Crit Rev Food Sci Nutr. , 43 ( 6 ) : 587 – 606 .
- Zhong , Q. , Sandeep , K.P. and Swartzel , K.R. 2003 . Continuous Flow Radio Frequency Heating of Water and Carboxymethylcellulose Solutions . Journal of Food Science , 68 : 217 – 223 .
- Orsat , V. , Bai , L. , Raghavan , G.S.V. and Smith , J.P. 2005 . Radio-Frequency Heating of Ham to Enhance Shelf-life in Vacuum Packaging . Journal of Food Process Engineering , 27 : 267 – 283 .
- Brunton , N.P. , Lyng , J.G. , Li , W. , Cronin , D.A. , Morgan , D. and McKenna , B. 2005 . Effect of Radio Frequency (RF) Heating on the Texture, Colour and Sensory Properties of a Comminuted Pork Meat Product . Food Research International , 38 : 337 – 344 .
- Demeczky , M. Continuous Pasteurisation of Bottled Fruit Juices by High Frequency Energy . Proceedings of IV International Congress on Food Science and Technology . 1974 , Madrid. Vol. IV , pp. 11 – 20 . Madrid, Spain : Instituto Nacional de Ciencia y Tecnologia a de Alimentos .
- Houben , J. , Schoenmakers , L. , van Putten , E. , van Roon , P. and Krol , B. 1991 . Radio-Frequency Pasteurization of Sausage Emulsions as a Continuous Process . Journal of Microwave Power Electromagnetic Energy , 26 ( 4 ) : 202 – 205 .
- Piyasena , P. and Dussault , C. June 1999 . “ Evaluation of a 1.5 kW Radio-Frequency Heater for its Potential Use in a High Temperature Short Time (HTST) Process ” . In CIFST Annual Conference June , Kelowna, BC
- Luechapattanaporn , K. , Wang , Y. , Wang , J. , Al-Holy , M. , Kang , D.H. , Tang , J. and Hallberg , L.M. 2004 . Microbial Safety in Radio-Frequency Processing of Packaged Foods . Journal of Food Science , 69 : M201 – 206 .
- Boye , I.J. , Ma , C.Y. and Harwalkar , V.R. 1997 . “ Thermal Denaturation and Coagulation of Food Proteins ” . In Food Proteins and Their Applications , Edited by: Damodaran , S. and Paraf , A. 25 – 56 . New York : Marcel Dekker .
- Ahmed , J. , Ramaswamy , H.S. and Alli , I. 2006 . Thermorheological Characteristics of Soybean Protein Isolate . Journal of Food Science , 71 : E:158 – 163 .
- Bircan , C. , Barringer , S.A. and Mnagino , M.E. 2001 . Use of Dielectric Properties to Detect Whey Protein Denaturation . J. Microwave Power & Electromagnetic Energy , 36 : 179 – 186 .
- Bircan , C. and Barringer , S.A. 2002 . Determination of Protein Denaturation of Muscle Foods Using the Dielectric Properties . Journal of Food Science , 67 ( 1 ) : 202 – 205 .
- Clark , A.H. , Judge , F.J. , Richards , J.B. , Stubbs , J.M. and Suggett . 1981 . A. Electron Microscopy of Network Structures in Thermally-Induced Globular Protein Gels . Int. J. Peptide Protein Res. , 17 : 380 – 392 .
- Bowland , E.L. and Foegeding , E.A. 1995 . Effects of Anions on Thermally Induced Whey Protein Isolate Gels . Food Hydrocolloids , 9 : 47 – 56 .
- Handa , A. , Takahashi , K. , Kuroda , N. and Froning , G. 1998 . Heat-Induced Egg White Gels as Affected by pH . Journal of Food Science , 63 : 403 – 407 .
- Li , H. , Errington , AD and Foegeding , EA . 1999 . Isostrength Comparison of Large-Strain (Fracture) Rheological Properties of Egg White and Whey Protein Gels . Journal of Food Science , 69 : 893 – 898 .
- Ngarize , S. , Herman , H. , Adams , A. and Howell , N. 2004 . Comparison of Changes in the Secondary Structure of Unheated, Heated, and High-Pressure-Treated Beta-Lactoglobulin and Ovalbumin Proteins Using Fourier Transform Raman Spectroscopy and Self-Deconvolution . J Agric Food Chem. , 52 : 6470 – 6477 .
- Renkema , J.M.S. 2004 . Relations Between Rheological Properties and Network Structure of Soy Protein Gels . Food Hydrocolloids , 18 : 39 – 47 .
- Ahmed , J. , Ramaswamy , H.S. and Ngadi , M. 2005 . Rheological Characteristics of Arabic Gum in Combination with Guar and Xanthan Gum Using Response Surface Methodology: Effect of Temperature and Concentration . International Journal of Food Properties , 8 : 179 – 192 .
- Fritz , D.J. , Swartz , D.R. and Greazer , M.L. 1989 . Factors Affecting Polyacrylamide Gel Electrophoresis and Electroblotting of High Molecular Weight Myofibrillar Proteins . Anaytical Biochemistry , 180 : 205 – 210 .
- Ahmed , J. , Ramaswamy , H.S. , Alli , I. and Ngadi , M. 2003 . Effect of High-Pressure on Rheological Characteristics of Liquid Egg . Lebensmittel-wissenschaft und-Technologie , 36 : 517 – 524 .
- Ferry , J.D. 1970 . Viscoelastic Properties of Polymers , 2nd , 292 New York : John Wiley & Sons, Inc .
- Riemann , A.E. , Lanier , T.C. and Swartzel , K.R. 2004 . Rapid Heating Effects on Gelation of Muscle Proteins . Journal of Food Science , 69 : E308 – E314 .
- Foegeding , E.A. , Dayton , W.R. and Allen , C.E. 1986 . Interactions of Myosin-Albumin and Myosin-Fibrinogen to Form Protein Gels . Journal of Food Science , 51 : 109 – 112 .
- Hermansson , A.-M. 1978 . Physico-Chemical Aspects of Soy Proteins Structure Formation . Journal of Texture Studies , 9 : 33 – 58 .
- Kent , M. 1987 . “ Electrical and Dielectrical Properties of Food Amterails ” . In A COST 90bis production , London : Science & Technology Publishers .
- Alzagtat , A.A. 2002 . “ Effects of Protein-Lipid Interactions on Physicochemical and Functional Properties of Food Proteins ” . Canada : McGill University . PhD thesis