Abstract
Water sorption isotherms of fried yam chips were determined using a static gravimetric method with saturated salt solutions in the range of water activity between 0.22 and 0.85 at 293, 303 and 313K. Four sorption models namely GAB, Peleg, modified Mizrahi, and BET were fitted with the sorption data generated. The GAB model followed by Peleg and modified Mizrahi models were found to best represent the experimental data in the aw range of 0.22–0.85. However, the BET model was more applicable between aw range of 0.22–0.55. The adsorption isotherm of fried yam chips clearly showed the influence of temperature, decreasing the moisture content at a fixed water activity value with higher temperature. The net isosteric heats of sorption of water were estimated by applying the Clausius–clapeyron equation to the adsorption isotherms at different temperatures. The net isosteric heat of sorption was observed to be decreasing as moisture content increases. Samples stored in desiccators of 0.44 and 0.55 aw at 303 and 313K, respectively, were rated higher in terms of textural properties investigated.
INTRODUCTION
Frying, resulting in dehydration, is an established process of food preparation worldwide[Citation1] and has long been a means of food preparation for achieving desired texture and flavour attributes of a variety of products.[Citation2] Fried yam chips are prepared by frying yam slices at a high temperature,[Citation3] which combines heat treatment at high moisture with dehydration and results in gelatinization of starch, softening of tuber tissues and at least a partial inactivation of enzymes.[Citation4] Dehydration during frying leads to formation of a dry crust with its typical porous structure and crisp texture, and there is good evidence that this rapid drying is critical for ensuring favourable structural and textural properties of food products.[Citation5] Dehydration in hot oil is characterized by very high drying rates.[Citation4]
The graphical relationship between the amount of water adsorbed and the corresponding environmental humidity, expressed as water activity at constant temperature is called a sorption isotherm.[Citation6] The relationship between water activity and equilibrium moisture content is fundamental to the design of dehydration operations (optimal moisture content and energy requirements) and also, to determine suitable storage and packaging conditions (to improve several characteristics such as texture or stability related to the quality)[Citation7] of fried food material. Moisture sorption isotherms are usefull thermodynamic tools for determining the interactions between water and the products dry surface and provide information useful for assessing food processing operation such as frying (drying), mixing, and storage.[Citation8] Sorption isotherms can also be used to investigate structural features of a food product such as specific surface area and pore size distribution. Such data can be used for selecting appropriate storage conditions and packaging systems that optimize retention of aroma, flavour, colour, texture, nutrients and biological stability.[Citation9]
It is well known that microbial and physiochemical stability of foods strongly depends on water activity (aw) of the food system[Citation10] hence, it is important to determine the optimal storage conditions of fried yam chips in order to ensure its availability all year round while still retaining its quality attributes. A common procedure used to preserve the quality of a food material is reducing the water activity (aw) in order to inhibit microbial growth[Citation11] and maintain crispness. Several models are available in the literature for predicting sorption isotherms of food materials. These include GAB, BET, Peleg, Modified Mizrahi, Kuhn, Oswin, Smith among others used for some agricultural products and foods like intermediate moisture meat products,[Citation12] peanuts,[Citation13] sweet potatoes,[Citation14] lafun and soy flour,[Citation15] fufu and tapioca,[Citation16] cassava shrimp chips,[Citation17] and cassava-based products.[Citation18] However, applications of these models to predict sorption isotherms of fried yam chips have not been presented in the literature. Taking into account food materials with similar chemical composition but different physical characteristics may give different sorption isotherms; hence it is necessary to obtain experimental data for fried yam chips as well as fit these data to available mathematical models. Hence, the aims of the present study were to: (i) determine the adsorption isotherms at different temperatures; fit the data to mathematical models; correlate it with experimental data; (ii) evaluate the heats of sorption, which is a basic parameter for energy requirement determination; and (iii) examine the textural properties of fried yam chips during sorption study.
MATERIALS AND METHODS
Product Preparation Used for Adsorption Experiments
Fried yam chips were produced under laboratory condition as depicted in by modifying the method of.[Citation3] Fresh yam tubers (Dioscorea rotundata), purchased from a local market were sorted and then washed to remove sand, dirt, and other adhering materials. The cleaned tubers were peeled using sharp knives and then sliced using a kitchen slicer (ART No: SF 923-1) to obtain a slice thickness of about 1.2 ± 0.01mm. This method was modified by washing off the starch surface followed by partial drying at 32 ± 1°C for 20 min. Samples were then fried in a deep-fat fryer (Model DF5T, China) at 170 ± 1°C for 3 min.
Figure 1 Production of fried yam chips. Source: Modified from Enwere.[Citation3]
![Figure 1 Production of fried yam chips. Source: Modified from Enwere.[Citation3]](/cms/asset/e87f1dc1-4c0a-4543-a70a-8cdccc1c0274/ljfp_a_203459_o_f0001g.gif)
Experimental Procedure
The fried yam chips were immediately transferred into a glass dessicator containing phosphorus pentoxide (P2O5) for about 3 days. The equilibrium isotherms of the samples fried at 170 ± 1°C were determined by the static gravimetric method at 293, 303, and 313K in ventilated incubators (Model SG 93/06/369). Triplicates of the samples of known weight (about 2 g) were placed above saturated salt solutions (analytical grade, Merck), CH3COOK: 0.220; MgCl2: 0.330; Ca(NO3)2: 0.440; Mg(NO3)2: 0.550; NaBr: 0.591; CuCl2: 0.680; NaCl: 0.755 and KCl: 0.845[Citation19,Citation20] with aw in the range (0.220–0.845) in separate tightly closed glass jars of 12 cm diameter for 15–20 days until constant weight was reached. Samples weights were determined using a Mettler AJ150 balance. Crystalline thymol was placed inside the high relative humidity (> 65%) desiccators to protect samples from microbial spoilage.[Citation11,Citation21] The final moisture content at equilibration was determined by drying at 105°C for 8 h.[Citation22] All moisture content was expressed as a percentage of non-fat dry weight because fat is known to exhibit no sorption of water below RH 90%.[Citation23] The initial moisture content of the fried samples was found to be 2.6 ± 0.01% (wb). The equilibrium moisture content at each water activity is the mean value of three replications.
Mathematical Modeling
In order to solve a number of processing and /or storage problems using sorption isotherm data with computer techniques, there is a need to fit sorption data to mathematical models.[Citation10] Such problems include: prediction of drying times to reach a desired awlevel, prediction of shelf-life of processed products under specified storage conditions, prediction of minimum packaging requirements and equilibrium product characteristics. In addition to such practical consideration, certain theoretical isotherm equations are also needed for evaluation of important thermodynamic parameters associated with water adsorption in heats, i.e., heat of sorption and monolayer moisture content.
A research of the literature showed that moisture sorption isotherms of food materials can be described by more than one sorption model.[Citation24] The BET, GAB, Peleg and modified Mizrahi models are widely used for modeling of the sorption isotherms of food. Therefore, the four models, eqs. (Equation1–4) were chosen to fit the experimental sorption data and the parameters of the models were estimated from the experimental results using the non-linear regression analysis of data fit version 6.1,[Citation25] which minimizes the residual sum of squares.
GAB:[Citation26]
BET:[Citation27]
Peleg:[Citation28]
Modified Mizrahi:[Citation29]
where C, K, and a-d, are constants in sorption models, aw water activity, M the equilibrium moisture content (dry basis), and Mo is the monolayer value (dry basis). The goodness of fit was determined using the coefficient of determination (R2), the average residual (A), percent average relative deviation (P %), and standard deviation (S).[Citation30] Values of P below 10% are indications of a good fit.[Citation28]
Heat of Sorption
The location of the dynamic equilibrium between water vapour and the adsorb amount of water is affected by temperature. This influence was obtained at the investigated temperatures using the theoretical Clausius-Clapeyron EquationEq. (5) which relates temperature dependence to the water activity at constant water content.[Citation31]
where, Qs (kJ/mol) is the net isosteric heat of sorption; R is the gas constant (8.314 kJ/Kmol); T2 and T1 are absolute temperatures at aw2; and aw1, respectively.
An empirical function describing the relationship between Qs and the equilibrium moisture content was used:[Citation32]
where Mo is the inverse of the gradient produced by the linear fit of ln (Qs) vs. Mw. The slope indicates the change in binding energy with changes in water content, and Qo is the isosteric heat of sorption for the strongest bound water molecules.
Textural Analysis of Fried Yam Chips under Sorption Studies
The profiling method based on quantitative descriptive analysis was used. Ten trained testers were asked to rate by number the following descriptors of texture:[Citation33] crispness intensity (0 = no crispness, flexible; and 100 = very crispy, breaking easily when bitten); crispness acceptance (0 = unacceptable; 100 = highly acceptable); hardness (0 = delicate or soft for samples lacking crispness; 100 = very hard); and texture acceptance (0 = unacceptable texture; 100 = highly acceptable texture). An unstructured linear scale was used that covered the range 0 to 100 points, described at both ends as shown above.[Citation34] For each session, each tester judges eight samples with two each for samples kept at aw between 0.22–0.55 at a particular temperature. Testing results were converted to numbers assuming the total scale to be 5 points.
Breaking Force Determination
The breaking force of the chips was determined using a universal testing machine (Model–M500, Testometric AX, Rochdale, England), equipped with a 50 kN load cell. Fried yam chips of uniform sizes were selected and then placed on a metal support with jaws at a distance of about 25 mm. They were pressed in the middle with a cylindrical flat-end plunger (70 mm diameter) at a speed of 2.5 mm/min. The measurement was recorded by a computer connected directly to the equipment. The breaking force (N) interpreted as chips hardness was obtained as the peak force from the force-deformation curve.[Citation33]
RESULTS AND DISCUSSIONS
Effect of Temperature on Adsorption Isotherm
The moisture content of fresh yam slices was found to be 75 ± 2% (wb), while the moisture content of fried chips was determined to be 2.6 ± 0.01% (wb). The experimental adsorption isotherm of the samples at 293, 303, and 313K are shown in . Similar trend showing effects of temperature have been reported for high starchy food products. These include cassava and cassava products like lafun;[Citation15] fufu and tapioca;[Citation16] cassava products;[Citation17,Citation18] potato;[Citation35] and sweet potato slices.[Citation14] From , a significant temperature effect on the adsorption for the full range of aw was observed for the product similar to the observations of some researchers.[Citation36,Citation37,Citation11,Citation21] From , higher storage temperature resulted in progressively higher aw values for a particular EMC. The practical implications of this phenomenon are especially important in the higher aw range. For an EMC of 0.1 kg/kg dry solids; aw shifts between 0.70 and 0.78 as storage temperature increased between 293 and 313K. As a result, an increased storage temperature could be detrimental to the microbial stability and sensory attributes of the fried yam chips. On the other hand, lower storage temperature results in lower aw values for a given EMC thus improving stability of the products.
Figure 2 Experimental equilibrium data of sorption isotherms for fried yam chips at different temperature.
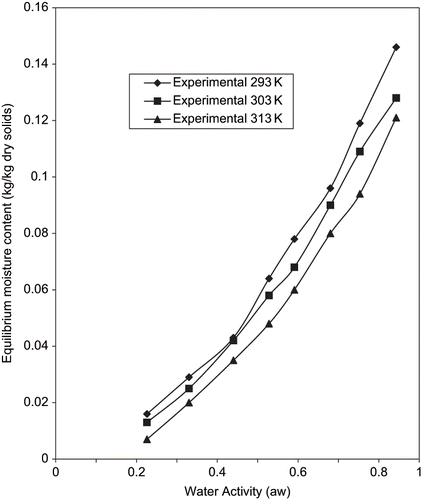
If aw is kept constant, an increase in temperature causes a decrease in the amount of sorbed water. As earlier reported by[Citation38] this is necessitated by the thermodynamic relationship, ΔF = ΔH–TΔS where ΔF, ΔH, and ΔS are the changes in free energy, enthalpy, and entropy (in J/kmol), respectively, and T the absolute temperature (K). Since ΔF < 0 (sorption is a spontaneous process) and ΔS < 0 (the sorbed water molecule has less freedom), hence ΔH < 0. Therefore, an increase in temperature represents a condition unfavorable to water adsorption. Temperature shift can have an important practical effect on chemical and microbiological reactivity related to quality deterioration of a food material.[Citation12] However, at lower temperature of storage, the product may become less dried to preserve it but most food materials are considered as dry foods when aw <0.6.[Citation36] The quantity of sugars present in food materials plays a role in whether or not crossing of isotherms with temperature at high water activities will take place.[Citation39] From , no crossing over was observed which may be attributed to very low sugar content of yam tubers (0.02–0.5%).[Citation3] A similar result was observed for low sugar apples.[Citation40]
Fitting of Sorption Models to Experimental Data
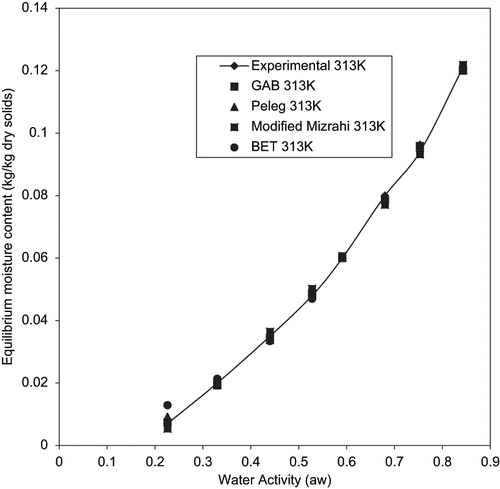
The parameters for the sorption models for fried yam chips with the statistical results using a non linear regression package data fit version 6.1[Citation25] are as shown on . A close examination of the results on indicates that the GAB, Peleg, and Modified Mizrahi models adequately describes the experimental adsorption data for fried yam chips throughout the entire water activity range due to higher R2. However, since R2 alone is not a reliable criterion for evaluation of EMC/ERH models, the P (%) values are a necessity.[Citation28] Based on this the GAB and Peleg models could be said to best describe the sorption data of fried yam chips with the former having P values ranging from 0.40723% to 2.99689% and an average value of 1.2797%; compared with the latter having P values between 0.06784% to 3.1767% with an average of 1.28%. The mean relative percent deviation (P %) of these two models was observed to be less than 10% within the temperature of study (293 to 313K). Hence, the GAB model could be said to satisfactorily predict the equilibrium moisture content of fried yam chips. Similar results have been reported for other starchy products like cassava—shrimp chips,[Citation17] sweet potato slices,[Citation14] fufu, tapioca, and lafun.[Citation15,Citation16]
Table 1 Model parameter and statistical data of water sorption isotherms of fried yam chips at different temperature
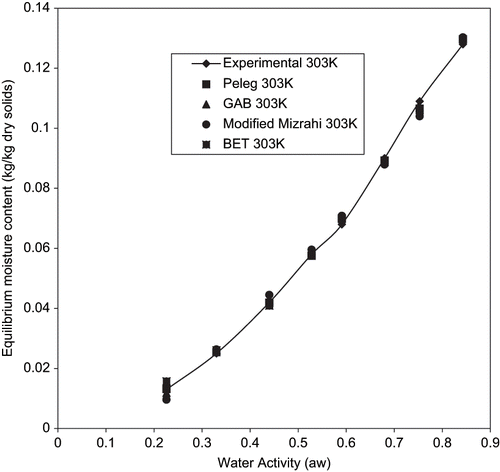
The table also shows that the BET model gave a good fit for temperatures between 293 to 303K for aw between 0.22 to 0.55. Since the P value within 0.22 to 0.55 aw at 313K is greater than 10% despite having high R2 (0.95773), it has a poor fit under this condition. However, the BET model is known to hold for aw up to about 0.5.[Citation38] Since the applicability of the BET model to fried yam chips is limited, it has to be admitted that prediction of data will be simple and reliable if a single equation fits the entire span of aw.[Citation28] to , shows the plots of experimental and predicted using the four models tested. The closeness of the experimental and predicted data obtained by GAB model confirms the suitability of this model in predicting the sorption isotherms of fried yam chips under the condition of investigation.
Figure 3 Experimental equilibrium data and predicted sorption isotherms for fried yam chips at 293K.
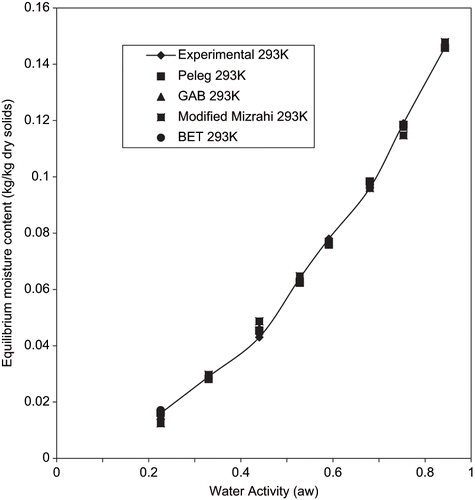
Figure 4 Experimental equilibrium data and predicted sorption isotherms for fried yam chips at 303K.
It can also be seen from the table that the monolayer value (Mo) which is a measure of sorption capability of the material obtained by GAB model increases as temperature increases from 293 to 303K but falls when temperature gets to 313K. However, that of BET decreases as temperature increases from 293 to 313K within the aw range of 0.22 to 0.55. These values (Mo) obtained for this study was observed to be less than 0.1kg/kg dry basis which was the maximum value earlier reported for food materials.[Citation26,Citation36]
Isosteric Heat of Sorption
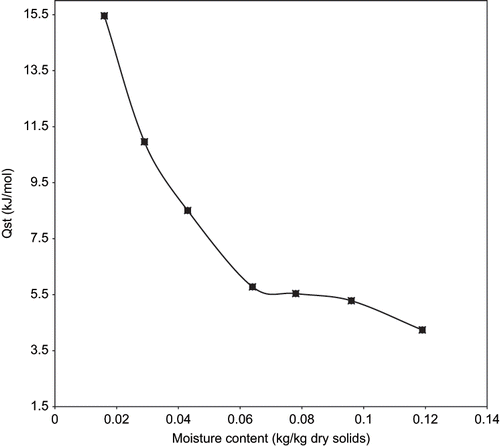
The net isosteric heats of sorption or differential enthalpy shows the energy requirement for removing moisture from food material (water-solid binding strength) and has a practical use in complete drying calculations and modeling of energy.[Citation41] shows the plots of log aw vs the reciprocal of the absolute temperature of fried yam chips at different moisture contents. The slope of the line (Qst – heat of sorption) decreases as moisture content increases indicating a decrease in the binding energy for water molecules. From , it was also observed that the calculated heats of sorption (slope of the graph), decreases with increasing temperature. This can be explained by the higher mobility and energy of water molecules with increasing temperature, with the energetic difference between sorption and no sorption being reduced.[Citation36]
Figure 6 Plots of Loge aw Vs the reciprocal of the absolute temperature of fried yam chips at various moisture contents (g H2O/100g solids).
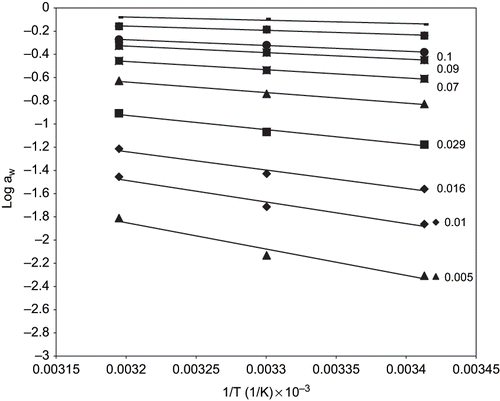
If Qst for a particular food were known, it would be possible to predict the aw of that food at any temperature. Since however, standard tables for Qst for different foods do not exist, prediction of aw requires the determination of moisture isotherms for at least two temperatures. In , the calculated heats of sorption were plotted as a function of water content. At low moisture content, the heat of sorption is high indicating the highest binding energy for removal of water. These results indicate the high interaction energy between the water molecules and food matrix at low moisture content. These interactions reduces, lowering the heat of sorption, as the moisture behaves as free water. As may be expected, when starting with an absolutely dry material like fried yam chips, water first adsorbs preferentially on the most active sites with great interaction energy, and as these sites become predominantly occupied, further adsorption will take place on less active site with lower heats of adsorption. With increasing amounts of adsorbed water, the specific adsorption enthalpy decreases and attains ultimately the level of condensation of pure water.[Citation23] A similar trend was observed by[Citation11,Citation16,Citation21,Citation41] in their work.
Changes of Textural Properties
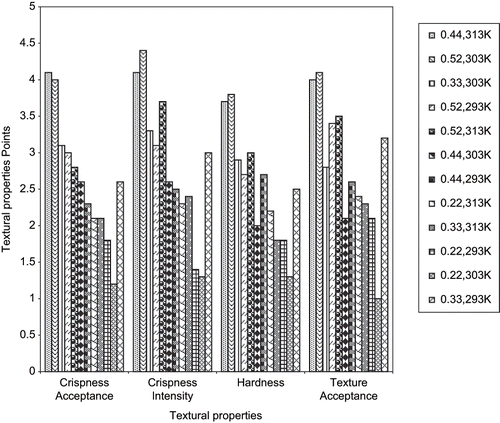
shows the results of sensory test of fried yam chips at various water activities and temperatures. Expectedly, the aw affected texture (ANOVA not shown). From the figure, it was observed that samples under aw 0.44 at 313K and aw 0.55 at 303K were assessed similarly and significant differences between these samples were not observed. These chips were highly acceptable in terms of the textural properties investigated. However, samples kept in the aw 0.22 at various temperatures were judged significantly lower by the panelists. Similar results have been reported for apple chips.[Citation34] From this study, we concluded that temperature seems to have a significant effect on textural properties. We expected that samples at lower aw range will be more acceptable but this was not the case. Hence, it seems that storing fried yam chips at aw range of 0.44–0.55 at temperatures 293K to 303K gave samples of high textural acceptance.
The breaking force which was interpreted as chips hardness also confirms sensory results as shown in . It was observed that the breaking force, i.e., peak force for rupture of chips in 0.44aw at 313K has the lowest value followed closely by samples kept in 0.52aw at 303K. Significant differences occurs between values for other conditions with samples kept in 0.22aw at temperatures between 293K and 313k having the highest breaking force hence low acceptance in terms of hardness. Similar results have been reported for carrot chips.[Citation33] From the table, it could be observed that the breaking force was significantly affected by temperature of storage and water activity.
Table 2 Mean values of breaking force of fried yam chips at different water activity and temperature of sorption study
CONCLUSION AND RECOMMENDATIONS
The equilibrium experimental data at 293, 303, and 313K and aw range of 0.22 to 0.85 for fried yam chips were successfully fitted to four sorption models available in the literature with the GAB and Peleg showing a good fit for the range of water activities studied. The adsorption isotherm indicates that temperature affects the sorption characteristics of the samples. The EMC decreases with increasing temperature. The net isosteric heat of sorption decreases with increasing moisture content and at lower moisture content, a strong heat of sorption was evaluated. The heat of sorption also decreases with increase in temperature. The lowest breaking force and subsequently better textural properties was observed for samples kept between 0.44–0.55aw range at 303 and 313K, while those kept in 0.22 aw at any temperature were rated poorly.
ACKNOWLEDGMENT
The authors wish to express our gratitude to the management of the National Centre for Agricultural Mechanization (NCAM) Ilorin, for the use of their Universal testing machine.
REFERENCES
- Lui-ping , F. , Min , Z. , Gong-Nian , X. , Jin-Cai , S. and Qian , T. 2005 . The Optimization of Vacuum Frying to Dehydrate Carrot Chips . International Journal of Food Science and Technology , 40 : 911 – 919 .
- Saguy , I.S. and Pinthus , E.J. 1995 . Oil Uptake during Deep-fat Frying: Factors and Mechanisms . Food Technology , 49 : 142 – 145 . 152.
- Enwere , N.J. 1998 . Food of Plant Origin: Root and Tuber Processing—A Technological Approach , 127 – 137 . Nsukka, , Nigeria : Afro-orbis Publishing Ltd. .
- Baumann , B. and Escher , F. 1995 . Mass and Heat Transfer during Deep-fat Frying of Potato Slices—1. Rate of Drying and Oil Uptake . Lebensm. Wiss. U-Technology , 28 : 395 – 403 .
- Singh , R.P. 2000 . Moving Boundaries in Food Engineering . Food Technology , 54 ( 2 ) : 45
- Caurie , M. 2005 . The Unimolecular Character of the Classified Brunauer, Emmett and Teller Adsorption Equation and Moisture Adsorption . International Journal of Food Science and Technology , 40 : 283 – 293 .
- Al-muhtaseb , A.H. , McMinn , W.A.M. and Magee , T.R.A. 2004 . Water Sorption Isotherms of Starch Powders. Part 1: Mathematical Description of Experimental Data . Journal of Food Engineering , 61 : 297 – 307 .
- Perez-Alonso , C. , Beristain , C.I. , Labato-Calleros , C. , Rodriguez-Huezo , M.E. and Vernon-Carter , E.J. In press . Thermodynamic Analysis of the Sorption Isotherms of Pure and Blended Carbohydrate Polymer . Journal of Food Engineering ,
- Beristan , C.I. , Azuara , E. and Vernon-Carter , E.J. 2002 . Effect of Water Activity on the Stability to Oxidation of Spray-dried Encapsulated Orange Peel Oil Using Mesquite Gum (Prosopis juliflora) as Wall Material . Journal of Food Science , 55 : 475 – 477 .
- Labuza , T.P. 1968 . Sorption Phenomena in Foods . Food Technology , 22 : 263 – 262 .
- Moreira , R. , Chenlo , F. , Vazquez , M.J. and Camean , P. 2005 . Sorption Isotherms of Turnip Top Leaves and Stems in the Temperature Range from 298 to 328K . Journal of Food Engineering , 71 : 193 – 199 .
- Lazarides , H.N. 1990 . Sorption Isotherm Characteristics of an Intermediate Moisture Meat Product . Lebensm. Wiss. U-Technology , 23 ( 5 ) : 418 – 421 .
- Chen , C. 2000 . A Rapid Method to Determine the Sorption Isotherms of Peanuts . Journal of Agricultural Engineering Research , 75 ( 2 ) : 401 – 408 .
- Chen , C. 2002 . Sorption Isotherms of Sweet Potato Slices . Biosystems Engineering , 83 ( 1 ) : 85 – 95 .
- Kuye , A. and Sanni , L.O. 2002 . Analysis of the Equilibrium Moisture Sorption Data for Lafun and Soyflour . Journal of Modelling, Design and Management of Engineering Systems , 1 ( 1 ) : 63 – 71 .
- Sanni , L.O. , Atere , C. and Kuye , A. 1997 . Moisture Sorption Isotherms of Fufu and Tapioca at Different Temperatures . Journal of Food Engineering , 34 ( 2 ) : 203 – 212 .
- Tungsangprateep , S. and Jinalal , V.K. 2004 . Sorption Isotherms and Moisture Diffusivity in Fried Cassava—Shrimp Chips . International Journal of Food Properties , 7 ( 2 ) : 215 – 227 .
- Kuye , A. and Ariri , I. 2005 . Modeling the Equilibrium Moisture Sorption Data for Some Nigerian Foods . International Journal of Food Properties , 8 ( 1 ) : 1 – 13 .
- Greenspan , L. 1977 . Humidity Fixed Points of Binary Saturated Aqueous Solutions . Journal of Research National Bureau of Standard , 81 : 89 – 102 .
- Bizot , H. , Buleon , A. , Mouhous-Riou , N and Multon , J.L. 1985 . Some Facts Concerning Water Vapour Sorption Hysteresis on Potato Starch , Edited by: Simatos , D. and Multon , J.L. Dordrecht : Martinus Nijhoft . Properties of water in foods
- Jamali , A. , Kouhila , M. , Ait Mohammed , L. , Jaouhari , J.T , Idlimam , A. and Abdenouri , N. 2006 . Sorption Isotherms of Chenopodium AmbrosioidesLeaves at Three Temperatures . Journal of Food Engineering , 72 : 77 – 84 .
- Association of Official Analytical Chemists . 1990 . Official methods of analysis of the AOAC , Edited by: Helrich , K. Arlington : Helrich, K. AOAC .
- De Jong , G.I.W. , Vander Berg , C. and Kokelaar , A.J. 1996 . Water Vapour Sorption Behaviour of Original and Defatted Wheat Gluten . International Journal of Food Science and Technology , 31 : 519 – 526 .
- Sablani , S.S. , Kasapsis , S. , Rahman , M.S. , Al-Jabri , A. and Al-Habsi , N. 2004 . Sorption Isotherms and the State Diagram for Evaluating Stability Criteria of Abalone . Food Research International , 37 : 915 – 924 .
- Oakdale Engineering . 1999 . Data fit version 6.1. Oakdale, PA
- Van den Berg , C. 1985 . “ Development of B.E.T. Like Models for Sorption of Water of Foods; Theory and Relevance ” . In Properties of water in foods , Edited by: Simatos , D. and Multon , J.L. Dordrecht : Martinus Nijhoft .
- Brunauer , S. , Emmett , P.H. and Teller , H. 1938 . Adsorption of Gases in Multimolecular Layer . Journal of American Chemists Society , 60 : 309 – 319 .
- Moreira , R. , Chenlo , F. , Vanquez , M.J. and Camean , P. 2005 . Sorption Isotherms of Turnip Top Leaves and Stems in the Temperature Range from 298 to 328K . Journal of Food Engineering , 71 : 193 – 199 .
- Rao , K.J. 1993 . Application of Hurdle Technology in the Development of Long Life Paneer Based Convenience , Karnal, , India : National Dairy Research Institute (ICAR) . Ph.D. Thesis,
- Hen , C. C. and Morey , R.V. 1989 . Comparison of Four EMC/ERH Equations . Transactions of the America Society of Agricultural Engineers , 32 : 983 – 989 .
- Bell , L.N. and Labuza , T.P. 2000 . Moisture Sorption Isotherm: Practical Aspects of Isotherm Measurement and Use , 2nd , St. Paul, MN : American Association of Cereal Chemists, Inc .
- Tsami , E. , Marinos Kouris , D. and Maroulis , Z.B. 1990 . Heat of Sorption and Water in Dried Fruits . International Journal of Food Science and Technology , 25 : 350 – 362 .
- Fan , L. , Zhang , M. , Xiao , G. , Suri , J. and Tao , Q. 2005 . The Optimization of Vacuum Frying to Dehydrate Carrot Chips . International Journal of Food Science and Technology , 40 : 911 – 919 .
- Konopacka , D. , Plocharski , W. and Beveridge , T. 2002 . Water Sorption and Crispness of Fat-free Apple Chips . Journal of Food Science , 67 ( 1 ) : 87 – 92 .
- McMinn , W.A.M. and Magee , T.R.A. 2003 . Thermodynamic Properties of Moisture Sorption of Potato . Journal of Food Engineering , 60 : 155 – 237 .
- Labuza , T.P. , Kaanane , A. and Chen , J.Y. 1985 . Effect of Temperature on the Moisture Isotherms and Water Activity Shift of Two Dehydrated Foods . Journal of Food Science , 50 : 385 – 391 .
- Pagano , A.M. and Mascheroni , R.H. 2003 . Water Sorption of Amaranthus Cruentus L. Seeds Modeled by GAB Equation . International Journal of Food Properties , 6 ( 3 ) : 369 – 391 .
- Iglesias , H.A. and Chirife , J. 1982 . Handbook of Food Isotherms: Water Sorption Parameters for Food and Food Components , London : Academic Press .
- Bandyopadhyay , S. , Weisser , H. and Loncin , M. 1980 . Water Adsorption Isotherms of Foods at High Temperature . Lebensm. Wiss. U.–Technology , 13 : 182
- Roman , G.N. , Urbicain , M.J and Rotstein , E. 1982 . Moisture Equilibrium in Apples at Several Temperatures. Experimental Data and Theoretical Consideration . Journal of Food Science , 47 : 1484
- Kaya , S. and Kahyaoglu , T. 2005 . Thermodynamic Properties and Sorption Equilibrium of Pestil (Grape Leather) . Journal of Food Engineering , 71 : 2000 – 2007 .