Abstract
Commercial soy protein isolate (SPI) dispersions (10, 15, and 20% concentrations) were subjected to high pressure treatment at selected pressure levels (350, 450, 550, and 650 MPa) for 15 minutes at 23 ± 1.5°C. Calorimetric studies confirmed denaturation of SPI dispersions at 350 MPa irrespective of concentration. Frequency sweep data (0.1 to 10 Hz) of SPI dispersions during oscillation rheological measurement demonstrated that elastic modulus (G′) predominate over viscous component (G″) for all concentrations. Gel rigidity of pressurized samples, estimated by mechanical spectra analysis, showed no systematic pattern with applied pressure however concentration significantly increased mechanical strength. Contrary to thermal effect, high pressure treated samples exhibited predominant viscous property and overall there was no significant change on viscoelastic properties of treated samples to control. Electrophoresis results (both Native and SDS) confirmed rheological data with insignificant conformational change in protein subunits of post-process samples. As expected, thermal induced gel was firmer than that of pressure treated samples at similar concentration.
INTRODUCTION
Recently, high pressure processing of biological macromolecules has received tremendous research interest for the potential benefits in functionality leading to newer product development with desirable texture.[Citation1] Biopolymers like proteins exhibit reversible or irreversible changes of native structure during pressurization leading to denaturation, aggregation, and gelation.[Citation2] Thermal denaturation of proteins occurs irreversibly with protein unfolding by breaking of covalent bonds. In contrast to thermal denaturation, pressure induced denaturation mechanisms are substantially different.[Citation3] It has been suggested that pressure causes changes/breakage in ionic bonds of protein structure,[Citation4] and the pressure has a disruptive effect on the tertiary and quaternary structure of most globular proteins.[Citation5]
Numerous reports are available on the effects of high pressure processing on protein foods.[Citation6–10] These studies revealed high pressure affected protein foods differently: few cases high pressure assisted changes are insignificant (α-lactoalbumin), while others exhibited significant extent of modifications (β-lactoglobulin). Pressure treated gel of protein foods has industrial applications to produce newer product or analogue products with minimal changes in quality and sensory properties. Apichartsrangkoon et al.[Citation11] reported that pressure-time-temperature combination can be manipulated to manufacture desirable texture of soybean products.
Soybean protein isolates (SPI) have been utilized commercially as functional ingredients in food formulations for its high nutritional value and minimum cost. The protein isolates are also of special interest to processors and consumers due to low fat content and as a substitute of meat, fish and dairy products. The major functional properties of soy proteins are: hydration, gelation, emulsification, foaming, and adhesion.[Citation12]. In addition, several potential health benefits have been attributed to the consumptions of soy foods.[Citation13] Application areas for SPI in the food processing industry include baked goods, extruded high-protein foods along with cereals, nutritional bars, cured meats, and meat analogues. Presently, commercial manufacturers produce numerous types of isolates according to demand of industry and, therefore, functional properties vary significantly from one isolate from another.
An important property in SPI is gelation during pressurization with desirable water holding capacity. Protein gelation is an association or crosslinking of protein molecules to form a three-dimensional continuous network that traps and immobilizes water to form a rigid structure which is resistant to flow under pressure.[Citation14] Effect of HP treatment on various functional properties including texture and rheology of soy protein has been reported.[Citation11, Citation15–16] Pressure-temperature-time combination can manipulated to yield desirable soy texture. Soy protein forms gel at and above 300 MPa by rupturing non-covalent interactions within protein molecule which is relatively softer than thermally treated gel.[Citation17] Molina et al.[Citation16] found during differential scanning calorimetry scanning that pressure level of 400 MPa sufficient to denature 7S fraction while 11S remains intact. The degree of denaturation increased with pressure levels and denatured above 400 MPa. The firmness/texture of soy product is a function of pressure intensity. At very high pressure levels (> 700 MPa), secondary structure are altered and produce irreversible denaturation.
Small amplitude oscillatory shear (SAOS) measurements afford the measurement of dynamic rheological functions, without altering the internal network structure of materials tested.[Citation18] The gelation behaviour and characteristics of protein foods during pressurization could be well represented by SAOS measurement where the strain is restricted to less than 5%. The increase in elastic modulus (G′, a measurement of stored energy inside a material) values during gelation has been considered as the gel rigidity or stiffness for various protein foods.[Citation19] Viscoeleactic characteristics of gel can be well represented by either phase angle or complex viscosity. Though numerous research was carried out on the extent of protein denaturation by differential scanning calorimetry, there is limited information available on oscillation rheometry on pressurized gel.
The objective of this article was to study viscoelastic and calorimetric properties of pressure induced gels formed from commercial soy protein isolates (SPI) and changes in protein subunits during pressurization by electrophoresis. Furthermore, gel and viscoelastic characteristic of thermal and high pressure treatments of 20% SPI dispersions were compared using mechanical spectra.
MATERIALS AND METHODS
Materials
The readily dispersible isolated soy protein was a commercially available product (PRO-FAM® 066–932) from ADM, Decatur, IL. The composition was as follows: protein (N × 6.25) > 90%, moisture < 6.0%, fat < 5%, and ash < 5%, and 90% of the particles went through #100 U.S. Standard Screen (150 μ).
Preparation of Sample
Dispersions of different solids contents (10, 15, and 20 g per 100 mL water) were prepared on a weight percent basis in deionized water (conductance: 18 Ω, Milli-Q, Millipore, Bedford, USA) and kept for 8 hours at low temperature (10°C) for complete hydration. All measurements were conducted with the same lot of product from the same container. The pH values were found to be in the neutral range (6.5 to 6.7). The concentration was selected to evaluate changes of the rheological properties and to select critical gel formation concentration. Each sample was prepared in duplicate.
High-pressure Treatment
SPI dispersions were sealed (approx. 20–25 mL) in low-density polyethylene bags (Whirl-Pak®, USA) and pressure treated at 350, 450, 550, and 650 MPa for 15 minutes using a 5 l reactor unit (ACIP 6500/5/12VB; ACB Pressure Systems, Nantes, France) equipped with temperature and pressure regulator device. The pressurization rate was about 4.4 MPa/s and released at 26 MPa/s. The initial temperature of pressure medium was 18°C, which was sharply increased to 24.8 and 28.5°C due to the adiabatic effect during pressurization of 350 (minimum pressure used) and 650 MPa (maximum pressure used) however, temperature became steady at 22 and 25°C during holding period (15 minutes).
Differential Scanning Calorimetry (DSC) Analysis
The protein denaturation in soy protein isolate were analyzed by differential scanning calorimeter (DSC) (TA Q100, TA Instruments, Newcastle, DE, USA) calibrated with indium for heat flow and temperature. The DSC was equipped with a refrigerated cooling system that monitored temperature efficiently. Nitrogen was used as purge gas at a flow rate of 50 mL/min. Samples (13–15 mg) were accurately weighed into polymer coated aluminium pans, hermitically sealed, and allowed to equilibrate at the initial temperature for10 min. The heating rate of the DSC scans was 5°C/min over a range of 30 to 110°C. A four-axis robotic device automatically loads sample and reference pan of DSC. An empty aluminium pan was used as a reference. The DSC measurement was done in duplicate. The DSC data were analyzed with the Universal Analysis Software (version 3.6C) for thermal analysis, which was provided with the instrument (TA Instruments, Newcastle, NJ).
Rheological Measurements
Small amplitude dynamic rheological measurements were made using a controlled-stress rheometer (AR 2000, TA Instruments, New Castle, DE, USA) with parallel plate (60 mm dia) geometry. The width of the gap between two plates was 1000 μ. The AR 2000 System is based on efficient peltier temperature control which efficiently monitored the temperature during the experiments. For each test, a measured volume (approximately 2 mL) of well-mixed sample was transferred to the rheometer plate. The rheological measurement of pressure treated sample was carried out at 20°C where as another set of experiment, 20% SPI dispersions were isothermally heated at 90°C for 30 minutes, followed by immediate cooling back to 20°C in situ of the rheometer plate. Frequency sweep tests were carried out between 0.1 and 10 Hz at linear viscoelastic ranges. In order to ensure that all the measurements are carried out within the linear viscoelastic ranges, initially oscillation stress amplitude sweeps were tested for selected concentrations of SPI dispersion.
All the rheological measurements were carried out in triplicate and the experimental dynamic rheological data were obtained directly from the TA Rheology Advantage Data Analysis software V 5.1.42 (AR 2000, TA Instruments, New Castle, DE, USA). The deviation did not exceed 5% between duplicate runs, as the experiment was repeated. The average of the three runs was reported as the measured value.
Electrophoresis
Native and Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) were performed as described by Laemmli[Citation20] to demonstrate the effects of high pressure on the soy protein. Stacking gel and resolving gel of 4 and 12% acrylamide were used for SDS-PAGE. Slab gels (0.75-mm thickness) were run at a constant current of 15mA/gel; the electrophoresis was performed with a Bio-Rad Mini Trans blotting system following the method described by Ahmed et al.[Citation21] earlier.
RESULTS AND DISCUSSION
Differential Scanning Calorimetry
represents the differential scanning calorimetry thermograms of soybean protein isolates. From the thermogram (), the transition temperature of protein component was determined as the temperature at which the heat flow was maximal. The sample showed only one endothermal peak with a T m of 90.4°C. It indicates that the studied SPI contains 11S only with sharp peak. DSC scans of sample processed above 350 MPa for 15 min showed no endothermal peak which indicated that a pressure level of 350 MPa was sufficient to denature soy proteins by unfolding of the protein ().This indicated that after high pressure processing, the native structure of glycinin had been lost and the glycinin had been denatured completely.
Figure 1 DSC thermograms of SPI dispersions: a control 10%; b pressuruized 15% at 350 and 550 MPa for 15 minutes.
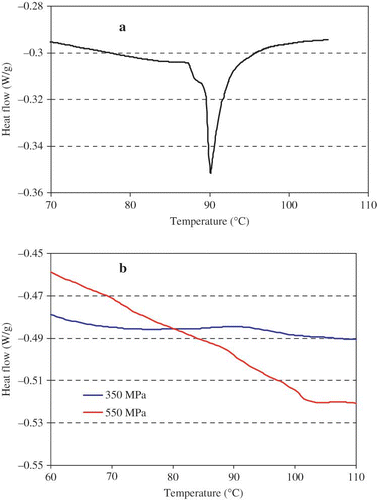
Our observation differed from earlier one reported by Molina et al.,[Citation16] where dispersions showed endothermic peaks at 78 and 96°C corresponding to the thermal denaturation of the β-conglycinin and glycinin fractions, respectively. In addition, those authors reported that a minimum pressure level of 400 MPa was required to denature 11S fraction. They suggested that 11S globulin contains 12 subunits which are linked by disulfide bonds and at higher pressure level sulfide bonds were reduced with resulting denaturation. Puppo et al.[Citation22] studied pressure effect of SPI at modified pH values at 3 and 8 respectively. Application of high pressure at 200 MPa did not affect denaturation but reduced denaturation enthalpy. Treatment at 400 and 600 MPa resulted denaturation and destabilization of both 7 and 11S fractions, with a new peak at 71°C. However, no such peak was observed in our study. These results indicated that high pressure resulted in unfolding as well as dissociation of glycinin. The difference could be attributed to differences in samples, protein content, pH, and isolation techniques.
Gel Rigidity and Viscoelastic Properties
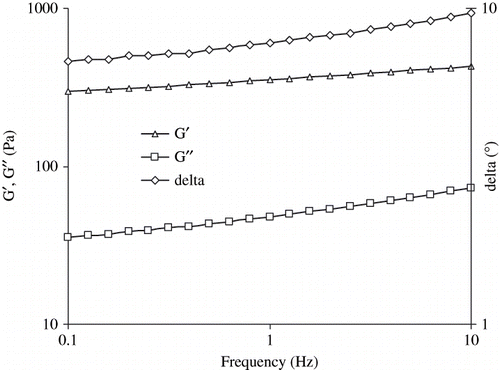
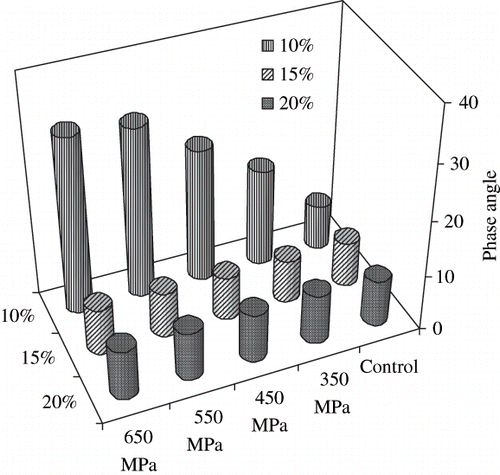
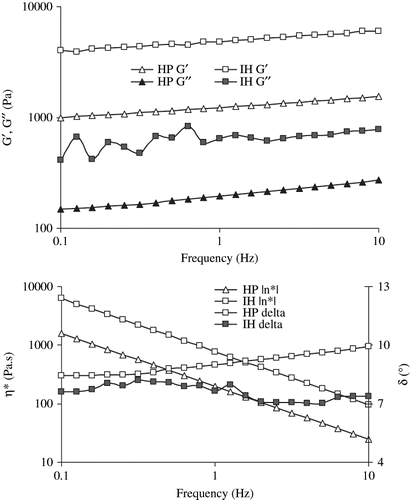
A typical mechanical spectra of pressure induced SPI gel is shown in . The elastic modulus predominates significantly over viscous component at frequency range of 0.1 to 10 Hz and slow change of G′ (undergoes little dispersion) indicating perfect elastic nature of pressurized gel.[Citation23] Phase angle (measures of the ratio of energy lost to energy stored in a cyclic deformation) data indicated visco-elastic nature of gel.
Effect of Pressure on Elastic and Viscous Modulii
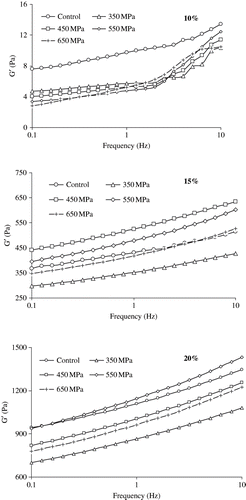
Effect of pressure on elastic modulus of soy gel is illustrated in . A control was the sample without pressurization. The elasticity of these gels increased linearly with concentration and, therefore, 20% SPI concentration resulting firmer gel compared to lower concentrations. Elasticity increased few folds with holding time (data not shown). However, no systematic pattern was observed with applied pressure levels at studied concentrations. For 10% concentration, as the pressure increased, G′ decreased systematically between 0.1 and 1 Hz with respect to control where as the trend was reversed at higher frequency ranges (1–10 Hz). At moderate concentration level (15%), a sharp increase in G′ was noticed at 450 MPa. Above 450 MPa, further G′ started to decrease, suggesting material started to undergo stress on structure. Interestingly, control and pressure induced SPI sample at 650 MPa for holding time of 15 minutes exhibited almost similar magnitudes of G′ especially at higher frequency. It indicated that highest pressure levels did not change elasticity of 15% SPI irrespective of nature of sample. Elasticity of pressurized gel at higher concentration (20%) decreased significantly compared to control sample except at 550 MPa where an increasing trend was observed. Earlier we have observed similar behaviour[Citation24] during thermorheological study of same lot of soy proteins at same concentration levels. Therefore, this study indicated that pressure has insignificant effect on mechanical strength of gel whether protein concentration played a major role in gel strength. Pressure resistance of soy proteins containing the major 11S protein has been reported,[Citation24–25] and it is believed stronger mechanical strength of pressurized soy gel was contributed by 7S fraction.
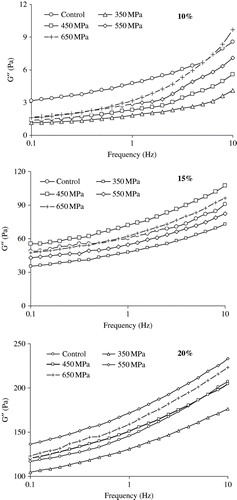
Pressure-induced gels at various concentrations (10–20%) exhibited non-systematic viscoelastic behavior in terms of G″ as function of pressure (). There was sudden drop of G″ value at 350 MPa for all concentration ranges indicated protein denaturation. At 10% concentration, G″ increased as function of pressure though pressure induced G″ values were lower than control sample except at 650 MPa at higher frequency. A complicated G″-P profile was noticed for 15 and 20% SPI concentrations.
Functional properties of soy proteins have been influenced by sulphydryl (SH) groups and disulfide (S–S) bonds and, it is expected that both SH groups and SS bonds undergo changes during high pressure application. During denaturation of glycinin, it might involve by cleavage of S-S bonds.[Citation16, Citation25] Zhang et al.[Citation25] observed that SH content increased with applied pressure (100–500 MPa) however decreased above 500 MPa. After pressurization, the glycinin was dissociated into subunits and creating new free SH residues, some of which recombined to give S–S exchange reactions or new S–S bonding by oxidation. Kajiyama et al.[Citation26] also reported increasing in free SH residues forming from the reduction of S-S bonds. These breaking and formation of bonds could be reason for unsystematic variations of dynamic modulii after pressurization. A decrease in texture parameters of 11S component of soybean protein gel above 600 MPa was reported by Molina et al.[Citation16] During steady shear measurement of pressurized (100–400 MPa for 30 minutes) 12.5% glycomacropeptides and 10% α-lactoalbumin non-systematic changes in consistency coefficients and yield stress values were observed during post-process samples.[Citation7–8] Recently, Patel et al.[Citation10] studied 12% whey protein concentrate solutions at 800 MPa for longer time periods (90 minutes) resulted weak gel with G′ less than 100 Pa.
Mathematically, the effects of pressure on gel characteristics can be represented by power type equation relating elastic (G′) and viscous (G″) modulus and frequency:
Linear regressions of ln ω vs ln G′ and ln G″ were performed at 20°C, and the magnitudes of slopes and intercepts (power parameters) were computed. The regression parameters obtained are reported in . It is evident from the table that both elastic and viscous modulus-frequency data fitted the power model adequately (R2 > 0.84). The elastic modulus values at higher concentration (15–20%) were found to be relatively independent of frequency as the slope varied between 0.070 and 0.099. It is reported earlier that a true gel is characterized by a zero slope of the power law model while viscous materials exhibit positive slopes.[Citation27] Pressure treatment reduced elastic component of gel compared to control sample where as elasticity of gel is almost independent of pressure. Similarly, slopes of ln ω vs ln G″ increased with pressure application for 10–15% SPI concentration and a decrease in magnitude was noticed for 20% concentration except at 650 MPa. In the present work, the slope of the power equation indicated the viscoelastic nature of heat treated SPI gels whereas the regression parameters increased with concentration and simultaneously solid-like character increased. It supports the earlier .
Table 1 Regression parameters of pressurized SPI dispersions
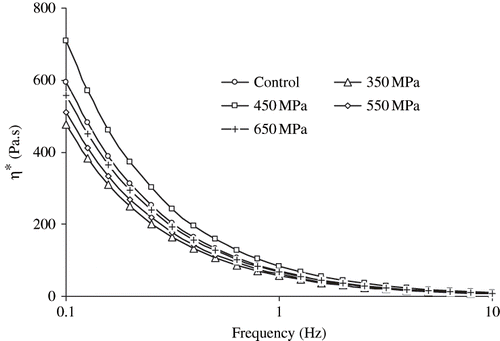
Complex viscosity (η∗) represents the true visco-elastic characteristics of gels. Complex viscosity versus angular frequency of SPI dispersions as function of concentration is plotted in . Following earlier trends of both G′ and G″ there was no systematic variation of η∗ with pressure levels. The values of η∗ significantly increased with concentration where as decreased as function of angular frequency. Changes of complex viscosity (η∗) with concentration (C) at constant frequency (1 Hz) was followed power type relationship (R2 > 0.92).
The regression coefficients A and n of Equationequation (3)(3) were varied between 5 × 10−8 – 1 × 10−6 and 6.99–8.13, respectively.
Changes of phase angle of post-process samples are shown in . Values of phase angle provide the nature of gel. Values of δ were almost alike for control sample which indicates irrespective of concentration viscoelasticity of SPI dispersions were same. During pressurization, no significant differences were observed on elasticity (δ ranged between 7.79 and 8.99) between 15 and 20% SPI samples where as 10% exhibited more viscous characteristics. This difference might be attributed by protein-water and protein-protein interaction.
Comparison Between Pressure and Heat-induced Gels
A dynamic rheological comparison was carried out between thermal and pressure-induced 20% SPI gels (). An isothermally heated (90°C for 30 minutes) gel exhibited more mechanical strength (about 4–6 times higher), viscous component (3–4 times higher) and viscoelasticity (4 times higher complex viscosity) compared to pressure-induced gel (650 MPa for 15 minutes). An increase in hardness of thermally treated gel over pressure-induced gel was reported by Molina et al.[Citation16] High protein content at higher temperature (90°C) resulted stronger gel which required four times rupture force than those formed at lower temperature.[Citation28] Pressure treatment induced changes in secondary structure of SPI samples: α-helix decreased by 15% while β-sheet remained unaffected at higher pressure.[Citation22] It is believed that the high-temperature heating generated more reaction sites for gel networks on the surface of protein molecule (mainly hydrophobic sites) and formed the disordered gel.[Citation29]
Electrophoresis
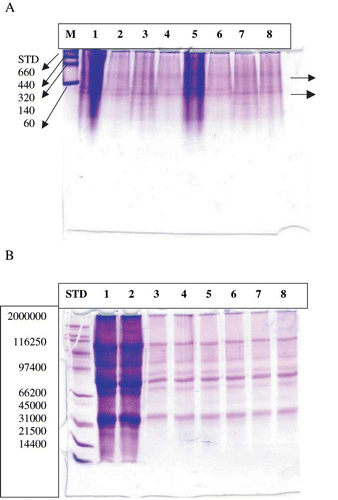
To study the effect of pressure treatment on protein fractions and subunits of SPI samples, native and SDS electrophoresis were carried out. The native analysis () exhibited that one band disappeared at 550 MPa (lanes 3 and 7) and again reappeared at 650 MPa (lanes 4 and 8). Similar observations of band appearance and disappearance were reported by Zhang et al.[Citation25] These results support our earlier rheological data. SDS-PAGE profile of the soybean protein isolates of the control and pressurized samples demonstrated that SPI has a heterogeneous protein structure. There was a gradual decrease in the mobility and intensity of protein subunits as function of pressure resulting shifting of bands (lanes 5–8). The results supported earlier reports on high pressure effect on globular proteins where it was reported that SPI (especially 11S component) involves SH/SS interchange during pressure treatment.[Citation16]
CONCLUSION
SPI was denatured completely and formed gel above 350 MPa. Application of various pressure levels (350–650 MPa) did not show any significant differences in mechanical strength of SPI dispersions and the changes were not systematic with pressure. However, 15–20% SPI concentrations exhibited excellent viscoelastic characteristics as supported by phase angle and complex viscosity data. Thermally induced gel showed more rigidity and viscoelasticity compared to pressure induced gel. Pressure treatment altered conformations of soy proteins duly supported by DSC and electrophoresis results. Pressure treated soft gel could be used to develop new food products in combination with other ingredients with known gel characteristics. The rheological properties of protein gels must be explained on the molecular level. Detailed investigation related to pressure induced protein gel structure is required.
REFERENCES
- Ledward , D.A. , Johnston , D.A. , Earnshaw , R.G. and Hasting , A.P.M. , eds. 1995 . High Pressure Processing of Foods , 208 Nottingham : Nottingham University Press .
- Cheftel , J.C. 1992 . “ Effects of High Hydrostatic Pressure on Food Constituents: An Overview ” . In High Pressure and Biotechnology , Edited by: Balny , C. , Hayashi , R. , Heremans , K. and Masson , P. 195 – 209 . Montrouge : John Libbey Eurotext . 887
- Knorr , D. , Hninz , V. and Buckow , R. 2006 . High Pressure Application for Food Biopolymers . Biochimica et Biophysica Acta , 1764 : 619 – 631 .
- Hayakawa , I. , Linko , Y.-Y. and Linko , P. 1996 . Mechanism of High-pressure Denaturation of Proteins . Lebensm.-Wiss. Technol. , 29 : 756 – 762 .
- Silva , J.L. and Weber , G. 1993 . Pressure Stability of Proteins . Annu. ReV.Phys. Chem. , 44 : 89 – 113 .
- Needs , E.C. , Stenning , R.A. , Gill , A.L. , Ferragut , V. and Rich , G.T. 2000 . High-pressure Treatment of Milk: Effects on Casein Micelle Structure and on Enzymic Coagulation . Journal of Dairy Research , 67 : 31 – 42 .
- Ahmed , J. and Ramaswamy , H.S. 2003 . Effect of High-hydrostatic Pressure and Temperature on Rheological Characteristics of Alpha-lactoalbumin . Australian Journal of Dairy Science , 58 : 233 – 237 .
- Ahmed , J. and Ramaswamy , H.S. 2003 . Effect of High-hydrostatic Pressure and Temperature on Rheological Characteristics of Alpha-lactoalbumin . Australian Journal of Dairy Technology , 58 : 233 – 237 .
- Anema , S.G. , Lowe , E.K. and Stockmann , R. 2005 . Particle Size Changes and Casein Solubilisation in High-pressure-treated Skim Milk . Food Hydrocolloids , 19 : 257 – 267 .
- Patel , H.A. , Singh , H. , Havea , P. , Considine , T. and Creamer , L.K. 2005 . Pressure-Induced Unfolding and Aggregation of the Proteins in Whey Protein Concentrate Solutions . J. Agric. Food Chem. , 53 : 9590 – 9601 .
- Apichartsrangkoon , A. , Ledward , D.A. , Bell , A.E. and Brennan , J.G. 1998 . Physicochemical Properties of High Pressure Treated Wheat Gluten . Food Chem. , 63 : 215 – 220 .
- Morr , C.V. 1990 . Current Status of Soy Protein Functionality in Food Systems . Journal of the American Oil Chemists Society , 67 ( 5 ) : 265 – 271 .
- Potter , S.M. , Baum , J.A. , Teng , H. , Stillman , R.J. , Shay , N.F. and Erdman , J.W. 1998 . Soy Protein and Isoflavones: Their Effects on Blood Lipids and Bone Density in Postmenopausal Women . Am. J. Clin. Nutr. , 68 ( suppl ) : 1375S – 1379S .
- Glicksman , M. 1982 . Food Hydrocolloids, Vol. I, II, III , Boca Raton, FL : CRC Press .
- Molina , E. , Papadopoulou , A. and Ledward , D.A. 2001 . Emulsifying Properties of High-pressure Treated Soy Protein Isolate and 7S and 11S Globulins . Food Hydrocolloids , 15 ( 3 ) : 49 – 55 .
- Molina , E. , Defaye , A.B. and Ledward , D.A. 2002 . Soy Protein Pressure-induced Gels . Food Hydrocolloids , 16 : 625 – 632 .
- Dumoulin , M. , Ozawa , S. and Hayashi , R. 1998 . Textural Properties of Pressure-induced Gels of Food Proteins Obtained under Different Temperatures Including Subzero . Journal of Food Science , 63 : 92 – 95 .
- Gunasekaran , S. and Ak , M.M. 2000 . Dynamic Oscillatory Shear Testing of Foods—Selected Applications . Trends Food Sci. Technol. , 11 ( 3 ) : 115 – 27 .
- Clark , A.H. , Lee-Tuffnell , C.D. , Mitchell and Ledward , D.A. 1986 . “ Gelation of Globular Proteins ” . In Functional Properties of Food Macromolecules , Edited by: Mitchell , J.R. and Ledward , D. A. 203 – 272 . London : Elsevier Applied Science Publishers .
- Laemmli , U.K. 1970 . Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4 . Nature , 227 : 680 – 685 .
- Ahmed , J. , Ramaswamy , H.S. , Alli , I. and Ngadi , M. 2003 . Effect of High-Pressure on Rheological Characteristics of Liquid Egg . Lebensmittel-wissenschaft und-technologie , 36 : 517 – 524 .
- Puppo , C. , Chapleau , N. , Speroni , F. , De Lamballerie-Anton , M. , Michel , F. , Anoan , C. and Anton , M. 2004 . Physicochemical Modifications of High-Pressure-Treated Soybean Protein Isolates . J. Agric. Food Chem. , 52 : 1564 – 1571 .
- Ferry , J.D. 1980 . Viscoelastic Properties of Polymer, , 3rd , 641 New York : John Wiley & Sons .
- Ahmed , J. , Ramaswamy , H.S. and Alli , I. 2006 . Thermorheological Characteristics of Soybean Protein Isolate . Journal of Food Science , 71 : E:158 – 163 .
- Zhang , H. , Li , L. , Tatsumi , E. and Kotwal , S. 2003 . Influence of High Pressure on Conformational Changes of Soybean Glycinin . Innovative Food Sci. & Emerging Technol. , 4 : 269 – 275 .
- Kajiyama , N. , Isobe , S. , Uemura , K. and Noguchi , A. 1995 . Changes of soy protein Under Ultra-high Hydraulic Pressure . InternationalJournal of Food Science and Technology , 30 : 147 – 158 .
- Ross-Murphy , S.B. 1984 . “ Rheological Methods ” . In Biophysical Methods in Food Research , Edited by: Chan , H.W.S. 138 – 199 . London : Blackwell Scientific .
- Kang , I.J. , Matsumura , Y. and Mori , T. 1991 . Characterization of Texture and Mechanical Properties of Heat-induced Soy Protein Gels . Journal of the American Oil Chemists'society , 68 ( 5 ) : 338 – 345 .
- Nagano , T. , Fukuda , Y. and Akasaka , T. 1996 . Dynamic Viscoelastic Study on the Gelation Properties of β-conglycinin-rich and Glycinin-rich Soybean Protein Isolates . Journal of Agricultural and Food Chemistry , 44 ( 11 ) : 3484 – 3488 .