Abstract
Mung bean protein was separated from wastewater of mung bean starch factory by utilizing an isoelectric precipitation method. The characteristics of freeze dried protein concentrate (having 88.93% protein content on dry basis) were oil absorption capacity, water absorption capacity, surface hydrophobicity and emulsifying activity index of 5.76 g/g, 2.41 g/g, 30, and 83, respectively. The lab scale procedure for production of microparticulated protein particle (MP3) included heating 5% w/w solution of mung bean protein concentrate in deionized water at 83°C for 15 min, coupled with homogenization at 17,000 rpm, homogenization at 23,000 rpm for 15 min, and centrifugation at 1000 × g for 10 min. The resulting supernatant produced 0.89 g of 0.1–3.0 μm MP3 per g of dry mung bean protein concentrate. Observations of MP3 using SEM showed a particle round shape indicating the potential for MP3 to provide creamy texture in oil-in-water emulsion foods to serve as a fat replacer.
INTRODUCTION
Microparticulated protein particles, MP3, are considered as mixtures of both soluble and insoluble protein aggregates to controlled size.[Citation1 Citation–2] They can be produced by thermal aggregation and/or chemical (acid or salt) precipitation under high shear conditions.[Citation2 Citation–3] MP3 are regarded as functional ingredients. The potential uses of MP3 include fat replacer, texturizer, and stabilizer in food products and controlled release microcapsules in cosmetic, pharmaceutical, and medical applications.[Citation3 Citation–4] The main application of MP3 is a fat replacer. As a food ingredient, fat contributes key sensory and physical benefits such as mouthfeel, taste, creaminess, and appearance.[Citation5] But high fat intake can increase risk of obesity, heart disease, and some types of cancer.[Citation6] Also consumers, researchers, and manufacturers are interested in fat replacers having fat-like physical properties that can maintain palatable texture of food.[Citation7–9] Fat replacers should provide less energy than fats and should provide mouthfeel similar to fats.[Citation4,Citation10–12] For fat replacer application, the size of particles has to be small enough that the tongue cannot feel the particles individually, but has the creamy rich texture of fats.[Citation11,Citation13] The critical characteristics of MP3 to provide creamy mouthfeel were reported to be spherical shape and diameter of 0.1–3.0 μm.[Citation10–12] Most of the protein-based fat replacers or MP3 were produced from whey protein by heating (at 80–130°C) and shearing (at 500,000 min−1) to obtain spherical MP3, having diameter of 0.1–2.0 μm. [Citation14]
Starch from mung bean [Vigna radiata (L.) Wilczek] is used as main ingredient for Asian-special texturize noodle (called glass noodle). In Thailand, the high protein content wastewater (1.5% protein) produced from mung bean starch factories was estimated to be about 600 tons/day. Treatment of this wastewater, which is mainly removal of protein, is required prior to disposal. To reduce cost of wastewater treatment and to utilize protein, some factories precipitate protein at pH 4.5 with HCl, centrifuge and (oven or drum) dry. The resulting denatured protein product is used for animal feed.
Proteins in mung bean are globular proteins consisting of globulins (≈ 70%), albumins (≈15–20%), and glutelins. The main globulin is vicilin, which has high content of acidic amino acid but low content of sulphur amino acid.[Citation15 Citation–16] Regardless of its low nutritional value, mung bean proteins might be utilized as functional ingredients. While there are numerous studies on utilization of whey protein and soy protein, research on mung bean protein is limited. Unlike soy protein, mung bean protein has bland taste and no smell. Therefore, it can be added into foods without giving undesirable flavor. This research aimed to develop MP3 from mung bean protein that is a by-product of mung bean starch production.
MATERIALS AND METHODS
Preparation of Mung Bean Protein Concentrate
Mung bean protein concentrate was prepared from precipitant wastewater of mung bean starch factory (Thaiwah Food Products Co. Ltd., Nakorn Pathom, Thailand). The process included precipitation at pH 4.5 with HCl, centrifugation at 20°C and 2000 × g for 20 min, washing of protein precipitate with distilled water (1:1 wt ratio) twice, centrifugation at 20°C and 2000 × g for 20 min after each washing step, adjusting to pH 7 with 1 N NaOH, centrifugation at 20°C and 1500 × g for 30 min, and freeze drying at chamber (or sample holder) pressure and condenser temperature lower than 0.5 Torr and −40°C, respectively. The contents of protein, moisture, fat and ash in this protein concentrate were determined using AOAC Methods 920.87, 925.10, 920.85, and 923.03, respectively.[Citation17]
Determination of Protein Functionality
pH-solubility profile
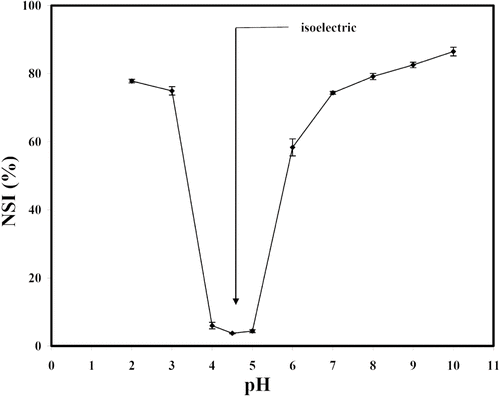
Protein concentrate was dissolved in deionized water to obtain 1% w/v solution. The pH of protein solution was adjusted with 1 N HCl or 1 N NaOH to cover the range 2 to 10 and then was centrifuged at 1500 × g for 30 min. The content of nitrogen in supernatant was determined by Kjeldahl method.[Citation18] Nitrogen solubility index was calculated as described by Vojdani.[Citation19] NSI versus pH was plotted and the isoelectric point of mung bean protein was determined.
Oil and water absorption capacity
To determine oil and water absorption capacity, the protein concentrate was mixed with oil (Soy bean oil, Thai Oil Co. Ltd., Nakorn Pathom, Thailand) or distilled water (at the weight ratio of 1 to 20) in a vortex mixer (MS1 Minishaker, IKA-Works, Inc., Wilmington, NC, USA) at 2500 rpm for 1 min. The mixture was allowed to stand at 27°C for 30 min and then was centrifuged at 3000 × g for 30 min. The supernatant was weighed and the absorption capacities were calculated. The results were expressed on a dry weight basis.
Surface hydrophobicity
Mung bean protein concentrate or bovine serum albumin (Sigma, St. Louis, MO, USA) was dissolved in 0.01 M phosphate buffer pH 7 to cover the concentration range 0.005 to 0.025 %w/v. To 4 ml protein solution, 20 μl of 8 mM solution of 1-anilinonapthalene-8-sulfonic acid (Sigma, St. Louis, MO, USA) in 0.1 M phosphate buffer (pH 7.4) was added. The fluorescent intensity of solution was measured by spectrofluorometer (Jasco FP-770, Japan Spectroscopic Co., Ltd., Tokyo, Japan) at excitation and emission wavelengths of 390 and 470 nm, respectively.[Citation20]
Emulsifying activity index
Mung bean protein solution of 0.5% w/v was prepared in 0.1 M phosphate buffer pH 7. It was then mixed with soy bean oil at a volume ratio of 3:1 using homogenizer (Ystral, Ballrechten-Dottingen, Germany) at speed No. 7 [which rpm was determined by digital tachometer (Digicon DT-240P, Digicon, Moorestown, NJ, USA) to be 25,000 rpm] for 2 min. The mixture was diluted 1000 times with 0.1% w/v solution of sodium dodecyl sulfate (Ajax, Auburn, N.S.W., Australia) in distilled water. Absorbency was measured at 500 nm and emulsifying activity index was calculated according to the method of Pearce and Kinsella.[Citation21]
Production of Microparticulated Particles from Mung Bean Protein
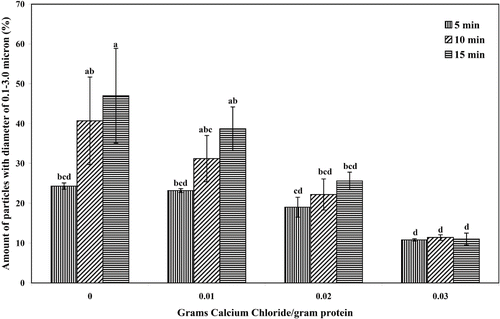
Mung bean protein concentrate was dissolved in deionized water to obtain concentration of 5% w/w.[Citation22] CaCl2 was added to protein solution to cover the range 0 to 0.03 g/g of protein (wet weight basis) in order to enhance aggregation of heat denaturated protein. The protein solution was heated at 83 ± 1°C for 5 to 15 min. The denaturation temperature of mung bean protein concentrate was determined by differential scanning calorimetry to be 83°C. During heating, protein solution was stirred with a homogenizer (Ystral, Ballrechten-Dottingen, Germany) at speed No. 1 (equivalent to 17,000 rpm as determined by digital tachometer). To maximize the process parameters, effects of CaCl2 concentration and heating time on size of protein aggregates were evaluated.
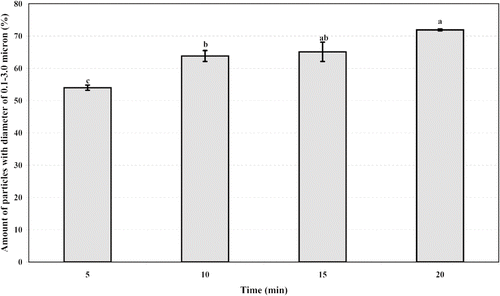
To reduce the size of protein aggregates, suspension of heat denaturated mung bean protein was homogenized at speeds No. 5, 6, 7, and 8 for 5 min. Rpms were determined by digital tachometer to be 23,000, 24,000, 25,000, and 27,000 rpm, respectively. The effect of homogenization time (5–20 min) on MP3 particle size was determined at 23,000 rpm. To precipitate protein aggregates of size higher than 3 μm, the homogenized suspension was centrifuged at 1000–4000 × g for 10 min. The effect of centrifugation force on particle size separation was evaluated by determination of size and size distribution of protein aggregates in precipitate and supernatant.
Characterization of Microparticulated Protein Particles (MP3)
Size and size distribution of MP3 were determined by laser particle size analyzer (Mastersizer, Malvern Instruments Ltd., Malvern, UK). The following process had to be done in order to determined the shape of MP3. After centrifugation, supernatant was diluted 50 times with distilled water, then filtered through 0.45 μm membrane. The membrane was dried and coated with gold. Two-dimensional micrograph of particles on the membrane was analyzed using Scanning Electron Microscope (JSM-5410LV, JEOL Ltd., Tokyo, Japan).
RESULTS AND DISCUSSION
Composition and Functionality of Mung Bean Protein Concentrate
Prepared mung bean concentrate had a moisture content of 6.38 ± 0.45% (wet weight basis). On dry weight basis, the contents of protein, fat, and ash were 88.93 ± 0.59%, 0.78 ± 0.78%, and 4.59 ± 0.04%, respectively. From a pH-solubility profile (), mung bean concentrate had maximum solubility in term of nitrogen solubility index of 86.5% at pH 10 and pH 4.5 was its isoelectric point. Oil absorption capacity, water absorption capacity, surface hydrophobicity and emulsifying activity index of this protein concentrate were 5.76 g/g, 2.41 g/g, 30 and 83, respectively.
Production of Microparticulated Particles from Mung Bean Protein
Protein denaturation and aggregation were induced by heating at 83°C. The purpose of homogenizing at 17,000 rpm was mainly to aid heat transfer within the sample. From particle size analysis, amount of aggregate having size smaller than 0.1 μm was about 1% in every treatment. Since CaCl2 enhanced protein aggregation, increase in CaCl2 concentration resulted in higher amount of particles having size larger than 3 μm.[Citation23]
The objective of MP3 production is to produce an ingredient that could be used as a fat replacer, therefore, the target size was in the range of 0.1 to 3.0 μm to develop creamy mouthfeel in oil-in-water emulsion.[Citation11] As shown in , amount of particles in this size range decreased as CaCl2 concentration increased. However, the number of particles in this size range increased with heating time while this effect was minimized as CaCl2 increased. Heating the protein solution without CaCl2 at 83°C for 15 min gave highest amount of 0.1–3.0 μm particles of 47.02 ± 16.76% (which that of > 3.0 μm particles was more than 50%). To increase amount of 0.1–3.0 μm particles, homogenization was required to break down particles having size larger than 3.0 μm.
Figure 2 Effect of CaCl2 concentration and heating time on amount of particles with diameter of 0.1–3.0 μm. Results are presented as mean ± standard error (SE). Statistical analysis of the data was done by using analysis of variance and Duncans multiple range test. Bars, not sharing even one common letter (a, b, c, and e), are significantly different (p ≤ 0.05).
The effect of further homogenizing at various rpm (0–27,000) for 5 min is shown in . Even though further homogenation of the protein aggregate suspension at 23,000–27,000 rpm did not increase amount of 0.1–3.0 μm particles significantly (p ≤ 0.05). But standard error (S.E.) of amount of 0.1–3.0 μm particles was smaller when homogenized at 23,000 and 24,000 rpm. An increase in homogenizing time required to break more particles of size larger than 3.0 μm was studied at 23,000 rpm. The amount of 0.1–3.0 μm particles increased with homogenizing time (). Sample containing about 65.14 ± 4.24% of this target size particles could be obtained by homogenizing for 15 min while increasing homogenizing time to 20 min did not increase the amount significantly (p ≤ 0.05).
Figure 3 Effect of speed of hand-operating homogenizer at constant time (5 min) on amount of particles with diameter of 0.1–3.0 μm. Results are presented as mean ± SE. Statistical analysis of the data was done by using analysis of variance and Duncans multiple range test. Bars bearing the letter ns (non-significant) indicate that means are not significantly different (p ≤ 0.05).
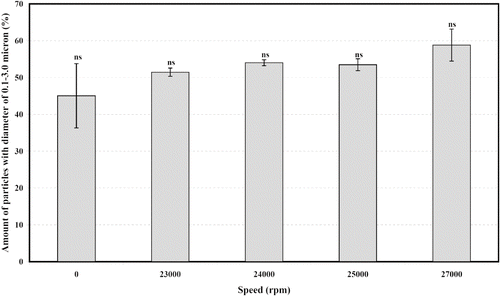
Figure 4 Effect of homogenizing time at constant speed (23,000 rpm) on amount of particles with diameter of 0.1–3.0 μm. Results are presented as mean ± SE. Statistical analysis of the data was done by using analysis of variance and Duncans multiple range test. Bars, not sharing even one common letter (a, b, c and e), are significantly different (p ≤ 0.05).
To precipitate particles larger than 3.0 μm, centrifugation was applied. By centrifugation at ≥ 2000 × g for 10 min, all of the particles larger than 3.0 μm were in the precipitate, however the amount of 0.1–3.0 μm particles in the supernatant decreased while that in the precipitate increased as centrifugation force increased (). Centrifugation at 1000 × g for 10 min was sufficient for removal of particles larger than 3.0 μm (only about 0.01% of them left in supernatant). Under these conditions, the amount of the target particles was 92.61 ± 0.40%.
Table 1 Effect of centrifugation force on the amount of particle of supernatant and precipitate (centrifugation time = 10 min)
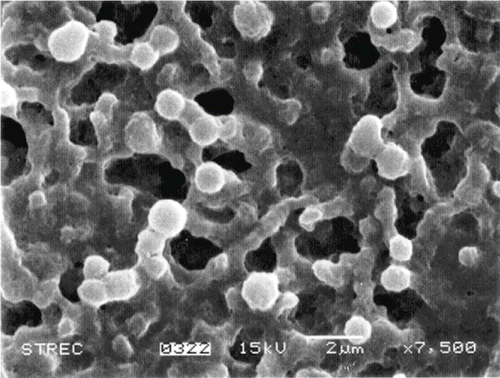
The yield of 0.1–3.0 μm particles (in term of gram of 0.1–3.0 μm particles per gram dry mung bean protein concentrate) was determined and illustrated in . The results showed that the yield in supernatant was 0.89 g/g dry protein when sample was centrifuged at 1000 × g for 10 min. Increasing centrifugation force showed a tendency to decrease this yield. The round shape particles, shown in SEM image (), indicated that the MP3 produced from mung bean protein had spherical shape.
Table 2 Effect of centrifugation force on the yield of particle in the range of 0.1–3.0 μm of supernatant and precipitate (centrifugation time = 10 min)
Figure 5 Scanning Electron Micrograph at 7500 times enlargement of 0.1–3 μm MP3 from mung bean protein concentrate.
Mung bean protein microparticulation was done using thermomechanical process. Heat and high shearing force unfolded/denatured protein.[Citation3,Citation24 Citation–25] Microparticles were formed by protein-protein interactions. These interactions were hydrogen bonds, van der Waals forces, hydrophobic interactions and disulfide linkages. The pH used in this process was 7, which was higher than the pI of the protein; therefore, the protein had a net negative charge. It was reported that formation of large particles was promoted by sulfhydryl-disulfide interchange reactions at pH > pI.[Citation3,Citation26] However, this interchange reaction might be lower in mung bean protein comparing with whey protein and egg albumin. This might be because of the content of sulfur-containing amino acids in mung bean protein which is lower than those in whey protein and egg albumin.[Citation16] In the presence of CaCl2, salt-bridges formed between calcium and the negatively charge amino acids, which exposed on the surface at pH 7. These interactions caused large protein aggregates.
The microparticulated particles can be produced from proteins (milk, egg and soy) and polysaccharides (starch, dextran, cellulose and gellan gum).[Citation1–3,Citation6,Citation9–13,Citation26–29] They are used as fat replacers.[Citation3,Citation28,Citation30] The most popular one in the market is Simplesse®, produced from whey protein. The main function of microparticulated particles is to provide the creamy mouthfeel similar to fats.[Citation1–3,Citation6,Citation10–14,Citation26–30] To provide this sensory property, the required physical characteristics were reported to be spheroidal shape and a mean particle size distribution ranging between 0.1–0.5 μm, with less than 30% of the total number of particles exceeding 5.0 μm in diameter.[Citation10,Citation13,Citation22,Citation29] The characters of MP3 produced from mung bean protein met the above criteria. Therefore, they had the potential to be used as fat replacers, which provided creamy mouthfeel. They might be used in oil-in-water emulsion, such as low fat salad dressing and sandwich spread.[Citation3,Citation28,Citation30 Citation–31]
CONCLUSION
Spherical shape microparticulated protein particles of 0.1–3.0 μm could be produced from mung bean concentrate. The lab scale procedure included heating of 5% w/w solution of protein concentrate in deionized water at 83°C for 15 min while homogenized at 17,000 rpm, homogenizing at 23,000 rpm for 15 min, and centrifugation at 1000 × g for 10 min. The resulting products contained 92% of 0.1–3.0 μm MP3 with 89% yield. The characteristics of this microparticulated particles produced from mung bean protein indicated that they could be used as fat replacer for oil-in-water emulsion.
ACKNOWLEDGMENTS
We would like to thank the Ministry of University Affairs—Chulalongkorn University Thesis Grant, Chulalongkorn University Graduate School Thesis Grant, TJTTP-JBIG, Chulalongkorn University Budget Funded for Research on Development of Agro-Industry/Food-Industry Project for giving us financial support, the Faculty of Pharmacy for providing the freeze dryer, and Thaiwah Food Products Co., Ltd., for supplying raw materials.
REFERENCES
- Renard , D. , Robert , P. , Faucheron , S. and Sanchez , C. 1999 . Rheological Properties of Mixed Gels Made of Microparticulated Whey Proteins and β-lactoglobulin . Colloids and Surfaces B: Biointerfaces , 12 : 113 – 121 .
- Renard , D. , Lavenant , L. , Sanchez , C. , Hemar , Y. and Horne , D. 2002 . Heat-induced Flocculation of Microparticulated Whey Proteins (MWP); Consequences for Mixed Gels Made of MWP and β-lactoglobulin . Colloids and Surfaces B: Biointerfaces , 24 : 73 – 85 .
- Sanchez , C. and Paquin , P. 1997 . “ Protein and Protein-Polysaccharide Microparticles ” . In Food Protein and Their Applications Edited by: Damodaran , S. and Paraf , A. 503 – 528 . Marcel Dekker, NY
- Alonso , M.J. 1996 . “ Nanoparticulate Drug Carrier Technology ” . In Microparticulate Systems for the Delivery of Proteins and Vaccines. Drugs and the Pharmaceutical Sciences , Edited by: Cohen , S. and Bernstein , H. Vol. 77 , 203 – 242 . Boca Raton, FL : CRC Press .
- Jones , S.A. 1996 . “ Issues in Fat Replacement ” . In Handbook of Fat Replacer , Edited by: Roller , S. and Jones , S. A. 3 – 26 . Boca Raton, FL : CRC Press .
- Akoh , C.C. 1999 . Fat Substitute . Food Ingredients and Analysis International , 21 ( 2 ) : 13 – 22 .
- Clark , J.P. 2005 . Processing . Food Technology , 59 ( 3 ) : 59 – 60 .
- Domagala , J. , Sady , M. , Grega , T. and Bonczar , G. 2005 . The Influence of Storage Time on Rheological Properties and Texture of Yoghurts with the Addition of Oat-maltodextrin as the Fat Substitute . International Journal of Food Properties , 8 : 395 – 404 .
- Domagala , J. , Sady , M. , Grega , T. and Bonczar , G. 2006 . Rheological Properties and Texture of Yoghurts When Oat-maltodextrin is Used as a Fat Substitute . International Journal of Food Properties , 9 : 1 – 11 .
- Singer , N.S. , Chang , H.-H. , Tang , P. and Dunn , J.M. Carbohydrate Cream Substitute . U.S. Patent 4,911,946 . Filed June 24, 1988 and issued March 27, 1990
- Singer , N.S. and Dunn , J.M. 1990 . Protein Microparticulation: The Principle and the Process . J. Am. Coll. Nutr. , 9 ( 4 ) : 388 – 397 .
- Singer , N.S. 1996 . “ Microparticulated Proteins as Fat Mimetics ” . In Handbook of Fat Replacer , Edited by: Roller , S. and Jones , S.A. 175 – 189 . Boca Raton, FL : CRC Press .
- Ziegler , G.R. Process for Producing Microparticulated Protein and the Product thereof . U.S. Patent 5,147,677 . Filed August 21, 1990 and issued September 15, 1992
- Singer , N.S. , Yamamoto , S. and Latella , J. Protein Product Base . U.S. Patent 4,734,287 . Filed May 4, 1984 and issued March 29, 1988
- Evans , R.J. and Bandemer , S.L. 1967 . Nutritive Value of Legume Seed Proteins . J. Agric. Food Chem. , 15 : 439 – 443 .
- Poehlman , J.M. 1991 . The Mungbean , Boulder, CO : Westview Press .
- Association of Official Analytical Chemists . 1995 . Official Method of Analysis , 16th , Washington, DC : AOAC .
- American Oil Chemists' Society . 1987 . Official Methods and Recommended Practices of the American Oil Chemists' Society , 3rd , Champaign, IL : AOCS .
- Solubility , Vojdani, F. 1996 . Method of Testing Protein Functionality , Edited by: Hall , G.H . 11 – 60 . London : Blackie Academic & Professional .
- Alizadeh-Pasdar , N. and Li-Chan , C.Y. 2000 . Comparison of Protein Surface Hydrophobicity Measured at Various pH Values Using Three Different Fluorescent Probes . J. Agric. Food Chem. , 48 : 328 – 334 .
- Pearce , K.N. and Kinsella , J.E. 1978 . Emulsifying Properties of Proteins: Evaluation of a Turbidimetric Technique . J. Agric. Food Chem. , 26 : 716 – 723 .
- McCarthy , A.J. and Maegli , J.W. Protein Fat Replacer and Method of Manufacture Thereof . U.S. Patent 5,350,590 . Filed December 15, 1992 and issued September 27, 1994
- Tay , S.L. , Tan , H.Y. and Perera , C. 2006 . The Coagulating Effects of Cations and Anions on Soy Protein . International Journal of Food Properties , 9 : 317 – 323 .
- Pradipasena , P. and Rha , C.K. 1977 . Pseudoplastic and Rheopectic properties of a Globular Protein Solution . J. Texture Stud. , 8 : 311 – 325 .
- Ker , Y.C. and Toledo , R.T. 1992 . Influence of Shear Treatments on Consistency and Gelling Properties of Whey Protein Isolate Suspensions . J. Food Sci. , 57 ( 1 ) : 82 – 85 .
- Cheftel , J.C. and Dumay , E. 1993 . Microcoagulation of Proteins for Development of “Creaminess.” . Food Rev. Int. , 9 ( 4 ) : 473 – 502 .
- Villagran , F.V. and Baughman , J.M. Low Fat Creamer Compositions . U.S. Patent 7,018,668 . Filed February 6, 2003 and issued March 28, 2006
- Akoh , C.C. 1998 . Fat Replacers . Food Technology , 52 ( 3 ) : 47 – 53 .
- Amankonah , O.J. , Valli , R.C. and Zdanis , D.A. Oil-coated Microparticulated Gellan Gum . U.S. Patent 5,516,543 . Filed November 30, 1994 and issued May 14, 1996
- American Dietetic Association . 2005 . Position of the American Dietetic Association: Fat Replacers . J. Amer. Dietetic Assoc. , 105 ( 2 ) : 266 – 275 .
- Cheung , I. , Gomes , F. , Ramsden , R. and Roberts , D.G. 2002 . Evaluation of Fat Replacers Avicel, N Lite S, and Simplesse in Mayonnaise . Int. J. Consumer Studies. , 26 : 27 – 33 .