Abstract
Nine different Lycopersicon esculentum varieties were analysed in order to determine differences in the antioxidant activity, both hydrophilic and lipophilic. To assess the nutritional value of all varieties, the total content of principal carotenoids, lycopene, and β-carotene was also analysed. On the basis of analyses performed on all lipophilic fractions, we can affirm that the total antioxidant activity of tomato is due to the integrated action of different compounds instead of that of any single compound such as lycopene and β-carotene. Anticytotoxic activities by brine shrimps assay were also tested on all lipophilic fractions to evaluate potential antitumoral activity.
INTRODUCTION
Fruits and vegetables play a significant role in human nutrition.[Citation1] Among vegetables, tomato is the most important both for its large consumption and for its richness in health-related food components. Several epidemiological studies indicated a beneficial effect of tomato consumption in the prevention of some major chronic diseases, such as some types of cancer and cardiovascular diseases.[Citation2,Citation3] It has been postulated that the protective role is due to tomato antioxidants that could contribute to the inhibition of abnormal oxidative processes.[Citation4] Tomatoes represent an effective way to supply several nutrients such as folate, vitamin C, and potassium, but the peculiar compounds of this vegetable are carotenoids, particularly β-carotene and lycopene,[Citation5] and due to its high consumption rates, it can provide significantly to the total intake of these components.[Citation6,Citation7,Citation8,Citation9] The antioxidant content of fresh tomatoes can be affected by many pre- and post-harvest factors. The influence of cultivar,[Citation10] cultural practices,[Citation11] ripening stage at harvest,[Citation12] and storage conditions[Citation13] on antioxidant accumulation has been studied during the past decade. Moreover, it is well known that the positive effect on health associated with the consumption is exerted by the pool of antioxidants, with noticeable synergistic effects. Therefore, to assess the nutritional quality of fresh tomatoes, it is important to study the main compounds having antioxidant activity.
Besides the compounds beneficial for human health, tomatoes could also contain tomatine and dehydrotomatine—glycoalkaloids having well-known toxic properties.[Citation14] The content of these glycoalkaloids decreases during ripening, whereas that of carotenoids increases.[Citation15,Citation16] Therefore, it can be concluded that the consumption of well-ripened tomatoes should ensure maximum health benefit, with a high level of carotenoids coupled with the absence of glycoalkaloids. However, great efforts are in progress to elucidate the physiological process, as well as the storage conditions that can control the phytonutrients content in foods.[Citation17,Citation18] Tomato is represented by several hundred cultivars and hybrids in response to the fresh consumption tomato market, which demands fruits which have very different characteristics.[Citation19] Therefore, tomato cultivars for fresh consumption show great differences in fruit characteristics in terms of fruit size (from a few to some hundreds of grams), shape (from flattened to elongated), and colour (from yellow to dark red). Besides, according to consumer and market requirements, tomato fruits are harvested at different stages of ripening: from breaking to red colour. There are several works describing the variation of the qualitative characteristics of tomatoes in relation to cultivars[Citation20–22] and growing conditions.[Citation23–25] Most of these works have taken into consideration only some qualitative characteristics (e.g., dry matter and soluble solids), whereas the antioxidative ability and the carotenoid composition, have not been considered. Our objective in this article was to establish the antioxidative ability and the level of β-carotene and lycopene in water-soluble and water-insoluble fractions of different cultivars of tomato commonly used for fresh consumption or by regional processing industries and differing in their pedoclimatic conditions. Moreover, anticytotoxic activities by brine shrimps assay were tested on all lipophilic extracts to evaluate potential antitumoral activity.
MATERIALS AND METHODS
Reagents and Spectrophotometry
We obtained analytical grade methanol, dichloromethane, diethyl ether, ethanol, and hexane from Carlo Erba (Italy). We used methanol and dichloromethane (HPLC grade) from Merck (Darmstadt, Germany). N,N-Dimethyl-p-phenylenediamine dihydrochloride (DMPD), 2,2′-Azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) as the crystallized diammonium salt and triethylamin (TEA) were from Fluka; 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) and butylated hydroxytoluene (BHT) were from Aldrich and β-carotene, lycopene, and potassium persulfate (K2S2O8) were purchased from Sigma Chemical Co. (Italy). We recorded spectrophotometric measurements at controlled room temperature (25°C) with a Varian DMS 90 UV-VIS spectrophotometer.
Plant Material
The samples of cultivars, San Marzano 823, Cirio 3, All Flash 900, Castiglione del Lago Perugia, and Roma (commonly used by Italian tomato processing industries or for fresh consumption) and the varieties Holland, Red Beefsteak, and Finest Tesco were cultivated in the area of Agro Nocerino-Sarnese (Salerno, Italy); the sample of variety Black Tomato, mainly used as fresh food, was cultivated in the area of Pachino (Siracusa, Italy). We purchased seeds of all varieties from MFM International S.r.l., Torre del Greco, Naples, and all varieties were cultivated outdoors with the same fertilizing and pedoclimatic conditions. The field showed good agronomic characteristics, such as good fertility, structure, and having a good presence of organic components. The seeding occurred in a greenhouse in the month of April; the transplantation into an open field happened in May. We harvested the berries at the end of July. All the fruits in our experiment appeared red in colour, both on the inside and the outside, with a good degree of ripening.
Each sample (5–10 fruits, 248.0–396.0 g) was homogenized in a blender, centrifuged at 9.500 rpm for 20 minutes, supernatant and pellet collected separately, and kept for analysis. Pellets were extracted with diethyl ether (w/v 1:2) under stirring at dark over night. Lipophilic extracts were filtered and concentrated in a rotary evaporator in vacuum (T < 35°C) and dried under N2.
Antioxidant Activity
The antioxidant activity was measured using the DMPD and ABTS decolouration methods,[Citation26–28] which is based on the capacity of different components to scavenge the DMPD and ABTS radical cations (DMPD• + and ABTS•+, respectively) compared to standard antioxidant (Trolox) in dose response curves. For the hydrophilic antioxidant activity, the reaction mixture contained 1 mM DMPD, 0.1 mM ferric chloride in acetate buffer 0.1 M (pH 5.25) in a total volume of 1 mL. The assay temperature was 25°C. The reaction was monitored at 505 nm until absorbance became stable. Then, 5 μL of aqueous phase was added to the reaction medium and the decrease in absorbance, which is proportional to the DMPD•+ quenched, was determined after 20 minutes. According to the method, the hydrophilic antioxidant activity was carried out in triplicate on supernatant and its diluted solutions 1:2, 1:3, 1:5, 1:7.5, 1:10. The antioxidant activity was expressed as T.E.A.C. (Trolox Equivalent Antioxidant Capacity — μM) per 1 mg of fresh weight of samples.
For the lipophilic antioxidant activity, the reaction mixture contained 56 μM ABTS and 24.5 μM K2S2O8 in ethanol (dilution 1: 100) in a total volume of 1 mL. In this case, 5 μL of the organic phase were added to the reaction medium and the decrease in absorbance at 734 nm was determined after 5 minutes. The total time needed to carry out each assay was approximately 6 minutes. The absorbance decrease was determined from the difference between the A734 values before and after the addition of the sample. According to the ABTS method, the lipophilic antioxidant activity was carried out in triplicate on crude diethyl ether extract of each sample, dissolved in dichloromethane analytical grade (10 mg/ml) and its four dilutions 1:2; 1:5; 1:10; 1:15. The antioxidant activity was expressed as T.E.A.C. (Trolox Equivalent Antioxidant Capacity — μM) per 1 mg of fresh weight of samples.
HPLC Analysis of Lycopene and β-Carotene in Lipophilic Extracts
Diethyl ether extract from each sample was analyzed in order to determine lycopene and β-carotene contents by reversed-phase High-Performance-Liquid-Chromatography (HPLC). The system was a Shimadzu LC 6A with a Kromasil 100A C18 column, 5 μm, 250 × 10 mm (Phenomenex) with UV-VIS detector SPD 10A VP, CR 3A recorder, system controller SCL 10A VP, and Chemstation integration software Class–VP 5.0. Immediately before injection, the diethyl ether extracts were dissolved in 2 mL of HPLC grade dichloromethane and filtered with 0,22 μm PTFE syringe filter. For every HPLC chromatographic run 30 μL was injected.
Lycopene and β-carotene were collected by using the following chromatographic conditions: gradient elution. 60:40 to 30:70, v/v, A/B (A was methanol/water 95/5 v/v (0,1% of butylated hydroxytoluene and 0,05% of triethylamin) and B was dichloromethane (0,1% of butylated hydroxytoluene and 0,05% of triethylamin), linear gradient changed over a period of 35 minutes and returned to the starting condition in 5 minutes before the next injection; flow rate, 1.5 mL min−1; wavelength of UV detector, 450 nm, sensitivity adjusted to 0.04 AUFS; room temperature. These chromatographic conditions were chosen as not to separate all the carotenoids present but to isolate lycopene and β-carotene as quickly and efficiently as possible. Three fractions were collected for each extract: fraction 1 with more polar components, fraction 2 containing the lycopene, and fraction 3 containing the β-carotene. The lycopene and β-carotene in each sample were identified involving the combined use of the retention times, and co-chromatography with authentic samples.
Brine Shrimps Assay
The Brine shrimps (Artemia salina) assay was performed in triplicate with appropriate amounts of lipophilic extracts of all cultivars of tomato dissolved in DMSO (1% final volume) to reach final concentrations of 1000, 100, and 10 ppm, using 10 freshly hatched larvae suspended in 5 ml of artificial sea water.[Citation29] Briefly, for each dose tested, surviving shrimp were counted after 24 hours, and the data statistically analysed by the Finney program,[Citation30] which affords LD50 values with 95% confidence intervals.
RESULTS AND DISCUSSION
The tomato typologies considered for our investigation were harvested at the stage at which they are usually consumed or processed by food industries. It is worth noting that the studied tomatoes presented relevant differences in their appearance, thus external fruit characteristics greatly varied. In this article, we have evaluated the total antioxidative activity of aqueous and lipophilic fractions of nine different cultivars of tomato used for fresh consumption and by local food processing industries, moreover because it is well known that the lipophilic antioxidants are related to the amount of carotenoids (in particular lycopene and β-carotene) present in each sample, we also have quantified the total amount of lycopene and β-carotene for each varieties. For each cultivar, 5 to 10 fruits (248.0–396.0g) were homogenated. After centrifugation, the aqueous fraction (crude supernatant, 140–380 ml) and its four dilutions (1:2; 1:5; 1:10; 1:15) were used for the DMPD assay to evaluate the antioxidative activity. We have chosen diethyl ether (solvent with low toxicity and high vapour pressure) to extract from pellets all carotenoids. The lipophilic fraction filtered, dried under nitrogen and dissolved in dichloromethane at concentration of 20 mg/mL and its four dilutions (1:2; 1:5; 1:10; 1:15) were used for the ABTS assay to test the antioxidative activity.
In , we report data showing the anti-oxidative activity of hydrophilic and lipophilic fractions of all nine cultivars of tomato. On the basis of the results obtained for hydrophilic fractions, the percentage of inhibition, calculated in terms of percent of inhibition per 1 mg of fresh material, ranged from 7.72% to 25.85%. The cultivar Holland and Finest Tesco were those with high activity (25.85% and 20.23%, respectively). The values obtained for lipophilic fractions, expressed in terms of percent of inhibition per 1 mg of fresh material, ranged from 0.25% to 2.12%. High activity was observed for the group of Italian cultivars (range 1.17% – 2.12%), where the variety San Marzano 823, commonly used for processing by industries, showed highest inhibition activity. The antioxidative ability expressed as T.E.A.C. present in 1 mg of fresh product is in accordance with that reported by Pellegrini et al.[Citation31]
Table 1 Anti-oxidative activities of hydrophilic and lipophilic fractions of nine cultivars of tomato
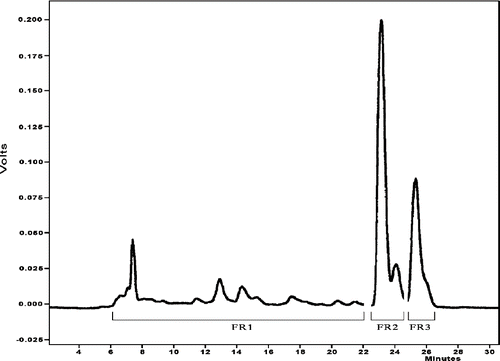
The diethyl ether extracts of nine cultivars were prepared for HPLC analyses to determine the concentration of lycopene and β-carotene. The analysis of HPLC chromatograms allowed us to collect three fractions named FR1, FR2 and FR3 (in we show, as an example, the HPLC chromatogram of lipophilic extracts of cultivar S.Marzano). FR1 contained more unidentified polar compounds in low concentration; FR2 and FR3 were identified as lycopene and β-carotene, respectively, by retention time and co-injection with purchased authentic standards.
Figure 1 Tomato lipophilic extracts HPLC chromatogram (cultivar S.Marzano). Detection was performed at 450 nm. Analytical conditions were described in the text.
In , we report the total concentrations of carotenoids recovered by HPLC analyses from crude lipophilic extracts. The amounts in terms of percentage of lycopene and β-carotene calculated per unit weight of fruits in all cultivars are comparable except for the concentration of lycopene of Holland variety (2.5 × 10−3%) and the content of β-carotene of San Marzano and Black tomato (0.047% and 0.06%, respectively). Previous extractions utilizing a mixture of hexane–acetone–ethanol, as suggested by Akanbi and Oludemi,[Citation6] allowed to recover lycopene (whose concentration is estimated by measuring the absorbance of its solutions in hexane at 472 nm on a UV-visible spectrophotometer) in average yields of 0.08% referred to 100 g of fresh material. In aim to collect, among carotenoids, β-carotene, we preferred to utilize diethyl ether, although the yield of lycopene was lower compared to that obtained in extracting with n-hexane.
Figure 2 Total concentrations of lycopene and β-carotene recovered by HPLC analyses from lipophilic extracts of nine cultivars of tomato. Results were expressed as % (× 10−3) of carotenoids per unit weight of fruits.
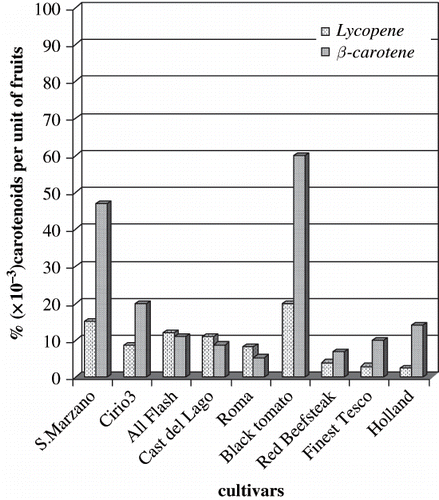
In analysing the data reported in , we can assess that there is no correlation between high contents of lycopene and β-carotene and anti-oxidative activity. Indeed, in the cultivar San Marzano 823, where the concentrations of lycopene and β-carotene are comparable to those contained in the Black tomato type, the total activity of its crude diethyl ether extract is more than double (0.55 μM and 0.26 μM, respectively, expressed as T.E.A.C. per 1 mg of fresh material).
In , we report the data concerning the citotoxic activity of the diethyl ether extracts of all cultivars expressed in terms of lethal dose (LD50). Among the crude extracts the Roma type exhibits the highest activity (lowest value of LD50). We also performed brine shrimp assay on fractions FR1, FR2 and FR3 recovered by HPLC analyses of all lipophilic extracts. As shown in , where we have reported only the data of activity of FR1, the high citotoxyc activity of Roma variety detected in diethyl ether extract was mainly concentrated in FR1 and due probably to minor unidentified compounds. In fact, FR2 (lycopene) and FR3 (β-carotene) citotoxic activity was, for all cultivar, on average 286,00 and 455,56, respectively. In conclusion, the lipophilic fraction of tomato, due to the high content of carotenoids, shows antioxidative activity and our results agree with those reported by several authors. The change in carotenoids and the variation in content of lycopene and β-carotene may be due to varietal, agricultural, technological, and environmental factors. On the basis of our results, lycopene and β-carotene content do not affect the antioxidative activity. When lycopene and β-carotene were tested in association with minor compounds (detected in FR1), an important effect on antioxidative activity was observed. Moreover, these minor compounds are also responsible for the citoxicity activity.
Table 2 Citotoxic activities in brine shrimps assay of total lipophilic fractions and fraction FR1 (unidentified carotenoids) of all cultivars
REFERENCES
- Goddard , M.S. and Matthews , R.H. 1979 . Contribution of Fruit and Vegetables to Human Nutrition . Hort. Science , 14 : 245 – 247 .
- Giovannucci , E. 1999 . Tomatoes, Tomato-based Products, Lycopene, and Cancer: Review of the Epidemiologic Literature . J. Natl Cancer Inst. , 91 : 317 – 331 .
- La Vecchia , C. 1998 . Mediterranean Epidemiological Evidence on Tomatoes and the Prevention of Digestive Tract Cancers . Proceeding of the Society for Experimental Biology and Medicine , 218 : 125 – 128 .
- Sies , H. and Stahl , W. 1998 . Lycopene: Antioxidant and Biological Effects and Its Bioavailability in the Human . Proceeding of the Society for Experimental Biology and Medicine , 218 : 121 – 124 .
- Akanbi , C.T. and Oludemi , F.O. 2004 . Effect of Processing and Packaging on the Lycopene Content of Tomato Products . International Journal of Food Properties , 1 : 139 – 152 .
- Beecher , G.R. 1997 . Nutrient Content of Tomatoes and Tomato Products . Proceeding of the Society for Experimental Biology and Medicine , 218 : 98 – 100 .
- Hart , D.J. and Scott , K.J. 1997 . Development and Evaluation of an HPLC Method for the Analysis of Carotenoids in Foods, and the Measurement of the Carotenoid Content of Vegetables and Fruits Commonly Consumed in the UK . Food Chem. , 54 : 101 – 111 .
- Abushita , A.A. , Hebshi , E.A. , Daood , H.G. and Biacs , P.A. 1997 . Determination of Antioxidant Vitamins in Tomatoes . Food Chem. , 60 : 207 – 212 .
- Crozier , A. , Lean , M.E. , McDonald , M.S. and Black , C. 1997 . Quantitative Analysis of the Flavonoid Content of Commercial Tomatoes, Onions, Lettuce and Celery . J. Agric. Food Chem. , 43 : 5590 – 5595 .
- Abushita , A.A. , Daood , H.G. and Biacs , P.A. 2000 . Change in Carotenoids and Antioxidant Vitamins in Tomato as a Function of Varietal and Technological Factors . J. Agric. Food Chem. , 48 : 2075 – 2081 .
- Audisio , M. , Dante , D. , De Cicco , A. and Suraci , C. 1993 . Il Contenuto di Vitamina C nei Pomodori in Relazione ai Metodi di Coltivazione . Rivista di Scienza dell'Alimentazione , 22 : 513 – 518 .
- Buta , J.G. and Spaulding , D.W. 1997 . Endogenous Levels of Phenolics in Tomato Fruit during Growth and Maturation . J. Plant Growth Regul. , 16 : 43 – 46 .
- Giovanelli , G. , Lavelli , V. , Peri , C. and Nobili , S. 1999 . Variation in Antioxidant Components of Tomato during Vine and Post-harvest Ripening . J. Sci. Food Agric. , 79 : 1583 – 1588 .
- Friedman , M. and McDonald , G.M. 1997 . Potato Glycoalkaloids: Chemistry, Analysis, Safety, and Plant Physiology . Critical Rev. Plant Sci. , 16 : 55 – 132 .
- Kozukue , N. , Kozukue , E. , Yamashita , H. and Fujii , S. 1994 . R-tomatine Purification and Quantification in Tomatoes by HPLC . J. Food Sci. , 59 : 1211 – 1212 .
- Rick , C.M. , Uhlig , J.W. and Jones , A.D. 1994 . High Alpha-tomatine Content in Ripe Fruit of Andean Lycopersicon Esculentumvar . Cerasiforme: Developmental and Genetic Aspects. Proceedings of National Academy of Science U.S.A. , 91 : 12,877 – 12,881 .
- Goldman , I.L. , Kader , A.A. and Heintz , C. 1999 . Influence of Production, Handling and Storage on Phytonutrients Contents in Foods . Nutrition Rev. , 57 : S46 – S52 .
- Grusak , M.A. , Della Penna , D. and Welch , M.R. 1999 . Physiologic Processes Affecting the Content and Distribution of Phytonutrients in Plants . Nutrition Rev. , 57 : S27 – S33 .
- Leonardi , C. Studi su Specie da Orto ai Fini Della Diversificazione Colturale . 1994 . Ph.D. Thesis, Università degli Studi di Parma, Parma-Italy.
- Davies , J.N. and Winsor , G.W. 1969 . Some Effects of Variety on the Composition and Quality of Tomato Fruit . J. Hort. Sci. , 44 : 331 – 342 .
- Gormley , T.R. , Mather , M.J. and Walshe , P.E. 1983 . Quality and Performance of Eight Tomato Cultivars in a Nutrient Film Technique System . Crop Res. , 23 : 83 – 93 .
- Stevens , M.A. , Kader , A.A. , Albright-Holton , M. and Algazi , M. 1977 . Genotypic Variation for Flavor and Composition in Fresh Market Tomatoes . J. Amer. Soc. Hort. Sci. , 102 : 680 – 689 .
- Blanc , D. 1989 . The Influence of Cultural Practices on the Quality of the Production in Protected Cultivation with Special References to Tomato Production . Acta Hort. , 191 : 85 – 98 .
- La Malfa , G. , Leonardi , C. and Romano , D. 1995 . Changes in Some Quality Parameters of Greenhouse Tomatoes in Relation to Thermal Levels and to Auxin Sprays . Agric. Med. , 125 : 404 – 412 .
- Mitchell , J.P. , Shennan , C. , Grattan , S.R. and May , D.M. 1991 . Tomato Fruit Yield and Quality under Water Deficit and Salinity . J. Am. Soc. Hort. Sci. , 116 : 215 – 221 .
- Fogliano , V. , Verde , V. , Randazzo , G. and Ritieni , A. 1999 . Method for Measuring Antioxidant Activity and Its Application to Monitoring the Antioxidant Capacity of Wines . J. Agric. Food Chem. , 47 : 1035 – 1040 .
- Miller , J.N. , Sampson , J. , Candeias , L.P. , Bramley , P.M. and Rice-Evans , C.A. 1996 . Antioxidant Activities of Carotenes and Xantophylls . FEBS Lett. , 384 : 240 – 242 .
- Miller , J.N. and Rice-Evans , C.A. 1997 . Factors Influencing the Antioxidant Activity Determined by the ABTS Radical Cation Assay . Free Rad. Res. , 26 : 195 – 199 .
- Meyer , B.N. , Ferrigni , N.R. , Putnam , J.E. , Jacobsen , L.B. , Nichols , D.E. and McLaughlin , J.L. 1982 . Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents . Planta Medica , 45 : 31 – 34 .
- Finney , D.J. 1971 . Statistical Logic in Monitoring of Reactions to Therapeutic Drugs . Meth. Inform. Med. , 10 ( 1 ) : 1 – 8 .
- Pellegrini , N. , Re , R. , Yang , M. and Rice-Evans , C. 1999 . Screening of Dietary Carotenoids and Carotenoid-rich Fruit Extracts for Antioxidant Activities Applying 2,2′-azinobis (3-ethylenebenzothiazoline-6-sulfonic acid) Radical Cation Decolourization Assay . Meth. Enzymol. , 299 : 379 – 389 .