Abstract
Lycopene is a functional component of great dietary importance obtained from many plant sources. In this article, we address the extraction of lycopene from various sources by efficient analytical support. Extraction, storage, and handling are described in detail. We also describe the effect of heat and time on amount of lycopene during extraction. Purification and applications are considered to understand lycopene's nutraceutical, epidemiological, and pharmaceutical importance, as well.
INTRODUCTION
For many decades, scientific studies and research have focused on functional foods and extracting the components from them that are responsible for their nutraceutical activity. According to Gibson and Roberfroid, a useful definition of functional food is dietary ingredients that affect its host in a targeted manner so as to exert positive effects that may, in due course, justify certain health claims.[Citation1] However, no universally accepted definitions of functional foods are available. The International Food Information Council (IFIC) defines functional foods as foods that provide health benefits beyond basic nutrition. This definition is similar to that of the International Life Sciences Institute of North America (ILSI), which has defined functional foods as foods that, by virtue of physiologically active food components, provide health benefits beyond basic nutrition. Health Canada defines functional foods as “similar in appearance to a conventional food, consumed as part of the usual diet, with demonstrated physiological benefits, and/or to reduce the risk of chronic disease beyond basic nutritional functions.” The Institute of Medicine of the National Academy of Sciences limits functional foods to those in which the concentrations of one or more ingredients have been manipulated or modified to enhance their contribution to a healthful diet.[Citation2] One branch of science is busy determining the number of natural sources, which have the anticancer activity. The cancer lowering effect of these foods was originally attributed to vitamin A active carotenoids. In 1831, Wackenroder[Citation3] isolated an orange pigment from the carrot (Daucus Carota) and coined the term “Carotene” from the Latin word carotacarota. The main carotenoids are −β-carotene, leutein and lycopene. Among these, lycopene, the biomolecular of interest, has been shown to have strong antioxidant activity and exhibits the highest physical quenching rate constant with singlet oxygen[Citation4]—approximately double than that of β-carotene (). There are also rising number of clinical evidences and studies supporting the role of lycopene to provide protection against different types of cancer.[Citation5]
Color is the first characteristic the consumer perceives of a food, and it confers expectations of quality and flavor. Moreover, the carotenoid pigments, either in isolation or jointly with other natural pigments such as chlorophylls and anthocyanins, are responsible for food color. The rich source of lycopene is “Tomatoes.” The scientific name of tomato is “Lycopersicon esculentum,” so the major antioxidant found in it is named “lycopene.” Other common sources are watermelon, grapefruit, and guava. These sources are easily available to all classes of people. Among these sources, tomatoes are the predominant source of lycopene in the world's diet because of their year round availability and high utility. Lycopene is a carotenoid present in human blood (0.5 mmol/liter plasma), and the tissue levels vary from 1 nmol/g wet wt in adipose tissue to up to 20 nmol/ g wet wt in adrenals and testes.[Citation6] In humans, lycopene achieves high level in testes, adrenal gland, prostate, and in human plasma.
In this article, we focus on the extraction, purification, stability, and applications of lycopene from various tomatoes. Further, we focus on the extraction of the functional components and measurement methods. For lycopene extraction, we mainly describe supercritical fluid extraction (SCFE) and solvent extraction in detail, followed by purification and detection of it. Effect of heat and time on amount of lycopene during extraction is also described. To understand the extraction of lycopene from different sources, we need to know the natural sources of lycopene and its intake in human body. Lycopene is obtained from many plant sources and almost all animals and microbes absorb it. The plant sources of lycopene, their common name, their species, and the part of the plant from where Lycopene is extracted[Citation7] are shown in . As we mentioned, among these the richest source of lycopene is tomatoes. The amount of Lycopene obtained from different varieties of tomatoes and its amount in pulp and peenl are shown in . The content of Lycopene in tomatoes differs with tomato varieties. Some of the red varieties—such as Flavourtop or Moneymaker—contain up to 50 mg/kg, whereas the lycopene content of yellow varieties is about 5 mg/kg. The content of several carotenoids in the tomato, including lycopene, increases with advancing maturity of the fruit.
Table 1 Common name, types, and species of lycopene
Table 2 Lycopene content (mg/100g−1)
Akanbi and Oludemi[Citation8] studied lycopene content and its stability in 2 commonly available tomato cultivars (Lycopersicon esculentum var. Roma VF and L. esculentum var. Ibadan Local) during processing and in-package storage. More lycopene was retained in the dried slices than in dried pulp and better in the presence of antioxidant (sodium metabisulphite). Daily lycopene intake is usually evaluated on the basis of food taken by general society. In a British study, the daily consumption of lycopene-rich food was equivalent to a lycopene intake of about 1.1 mg/day.[Citation9] During summer and fall, the intake was significantly higher than in winter and spring, but there was no significant seasonal variation in lycopene serum levels. In a study from the United States, a daily intake of lycopene of about 3.7 mg/day was observed. A lower intake was determined in the Finnish Mobile Clinic Health Examination Survey with a mean intake of 0.7 mg/day for men and 0.9 mg/day for women.[Citation10] The mean plasma levels of lycopene range from 0.22 to 1.06 nmol/ml. Lycopene contributes to between 21 and 43% of total carotenoids.
EXTRACTION AND PURIFICATION OF LYCOPENE
Extraction of Lycopene
The extraction, storage, handling, and analysis of Lycopene have to be carried out under controlled environmental factors to minimize losses of Lycopene through oxidation or isomerization. Actually, it has been suggested that the bioavailability of cis-lycopene in foods is higher than that of all trans-lycopene, but apparently, in vivo mechanisms, which are still unclear, promote isomerization of Lycopene from trans- to cis-form. In any case, considering that lycopene typically occurs in the all trans configuration, which is precisely the most thermodynamically stable form, there is clearly interest in avoiding trans-cis isomerization when lycopene into functional foods or nutraceuticals.
Super Critical Fluid Extraction (SCFE)
Gomez-Prieto[Citation5] et al. shows SCF Extraction was efficiently isolate the Lycopene from tomatoes (pear type). According to them, the first tomatoes were kept at 7°C for not more than 48 hours. Then, the tomatoes (skin and pulp without seeds) were dried in a freeze-dryer and subsequently ground, divided into different batches, and transferred to Teflon screw-cap tubes that were flushed with nitrogen, wrapped with aluminium foil, and stored at −18°C until the extraction was performed. Each batch was extracted at a different CO2 density, as explained below. At least two replicates of each extraction were performed. The extractions were carried out using a Hewlett-Packard 7680A extraction module fitted with a 7-ml thick walled stainless steel thimble as the extraction cell. The instant depressurization of the supercritical fluid and the independent control of both the pressure and the supercritical fluid flow rate are achieved through a nozzle-trap assembly, which acts as a controllable variable restrictor. The extraction system is fully automated and includes an internal trap, where the extracted analysts are retained once the supercritical fluid evaporates and leaves the system. Throughout the experimentation, different values for the CO2 density (namely 0.25, 0.35, 0.45, 0.55, 0.60, 0.70, 0.75, 0.80, 0.85, and 0.90 g/ml) were tested with 30 minutes being the extraction time. Other experimental conditions were as follows:
-
supercritical CO2 flow 4 ml/min;
-
sample weight in the extraction cell 0.5 g;
-
extraction cell temperature 40°C;
-
trap temperature 35°C;
-
restrictor temperature 45°C; and
-
the trap was packed with Hypersil-octadecylsilica (30–40 mm).
After the extraction, it was rinsed five times with 1 ml of dichloromethane at a rate of 2 ml/min.
Solvent Extraction
Solvent Extraction with Filter Medium
Generally, solvent extraction is done with the liquid extraction and saponification. The solvents used are acetone, petroleum ether, chloroform, hexane, and so on. A six to eight ounce can of tomato paste, having a bright red color, is added to 250 ml of a solution of 30% potassium hydroxide in methyl alcohol. The mixture is shaken at intervals until well mixed and placed at 5°C overnight. Either the whole or a portion of the saponified paste is mixed with distilled water, and a sufficient filter aid is added to disperse the paste conveniently, which is then spread on a thinly filter-aid-precoated filter paper (24 cm, no. 595 S&S) in a large suction funnel. The cake is washed with distilled water until it is approximately free of alkali, as shown by the almost colorless filtrate. Care should be taken to keep the cake covered with liquid. Washing may be completed in 10 to 15 minutes. If the cake separates from the funnel, the crack formed can be filled and sealed by a thin stream of a thick suspension of filter aid poured at the edge of the funnel. A tight seal is necessary for successful washing and extracting. The lycopene is extracted from the cake on the filter, which is held tightly at maximum suction, by acetone in charges of 50 to 75 ml. Approximately 300 ml of acetone per ounce of paste are required. To counteract the effect of decreasing temperature resulting from rapid evaporation of acetone under reduced pressure, the acetone is heated to 35°C before extraction. The washing and extracting can be completed in 25 to 30 minutes, and the first one or two charges may be discarded because they contain little lycopene. The glittering red crystals of lycopene start forming at once in the filtrate and can be filtered off immediately after extraction. However, a better yield with less effort is obtained by letting the extract stand at low temperature (as low as 0°F) until the crystals settle to the bottom of a tall cylinder or bottle. Most of the solution containing carotenes other than lycopene can be decanted. Lycopene in acetone deteriorates slowly at low temperatures. In Useful and simple methods are routinely adopted in many laboratories, for example, the extraction of lycopene with acetone in a blender, and the extract placed in a reparatory funnel and petroleum ether (40–60°C) is added giving two layers—the aqueous bottom layer and the petroleum layer. Distilled water is then added to dilute the aqueous layer, thereby enriching the petroleum ether layer with the carotene pigment. This layer is separated and sponified with alcoholic alkali. Again phase separation is carried out, and the upper layer is dried in a reduced pressure vacuums rotatory evaporator. The carotene pigment is dissolved in n-hexane. Separation of the resultant pigments carried out either by column chromatography or thin layer giving two major bands: lycopene and β-carotene.
Alternative method
An alternative method of extraction and saponification is the paste stirred in a blender with 1% metaphosphoric acid for 5 minutes, mixed with filter aid and distilled water. The use of metaphosphoric acid greatly hastens the filtration procedure. Acetone is poured on the cake in small charges, and 100 ml of acetone are sufficient to extract the pigments completely from 5 g of paste. After saponification, a reduction in the volume of the solvent, hexane, or petroleum ether is necessary to start crystallization. In either method, the cake of filter aid seems to act somewhat as a chromatographic column but gives a much more rapid extraction than the column. When crystals of lycopene are needed, they are rapidly filtered off on hard filter paper, washed with a little acetone, and dissolved in recently distilled chloroform. After being filtered again, they are recrystallized by addition of methyl alcohol. The crystals separate easily in a thick film on the hard paper and are then dried at low pressure (1 to 3 mm) in a desiccator over calcium chloride at room temperature for 1 hour. No precautions against deterioration, such as the use of inert gas, were used, except to work rapidly and avoid strong light. Twenty mg of lycopene are dissolved in 3 ml of chloroform and hexane is added to volume 200 ml.[Citation11]
Solvent extraction: (Liquid-liquid extraction)
The solvent system used by Emenhiser[Citation12] et.al. are hexane, ethanol, and acetone (50:25:25). One hundred ml of solvent is added to 125 ml beaker containing 12 ± 0.5 g sample of homogenized grapefruit. Fifteen ml water was added to it and agitation was provided for 5 minutes. Then the solution separated into distinct polar and nonpolar layers. The lycopene bearing upper hexane layer was injected into HPLC. The extracted pulp from the tomato samples was collected, centrifuged for 5 minutes. and then extracted. Moreover, the lower polar phase was also examined in HPLC for traces of Lycopene.
PURIFICATION/DETECTION OF LYCOPENE
Generally for purification and detection of lycopene, HPLC method is used. Other random methods[Citation1] reported are chromatography, thin layer chromatography, high-performance thin layer chromatography, and so on.
HPLC of SCF Extract and Salient Features of SCFE
The photodiode detector is used of wavelength range: 200–600 nm. Before the SF extract was injected onto a HPLC system, the solvent (CO2) was evaporated under a stream of nitrogen and subsequently re-dissolved in a 1 ml of dichloromethane. As a mobile phase, a mixture of eluent A: Methanol-water 96:4 w/v and eluent B: Methyl tert-butyl ether was used with 1000 ml/min flow rate. A linear gradient was applied in 60 minutes. from initial conditions (A:B, 83:17 v/v) to those maintained until the end of analysis (A:B, 33:67 v/v). Before use, Methanol and Methyl tert-butyl ether were filtered and degassed with Helium. Mainly, they used the 250 mm X 4.6 mm Develosil UG C30 column (Nomura Chemical, Solo, Japan) operated at 20°C for the HPLC analysis.[Citation13] The chromatograms were monitored at 285, 347, 450, and 472 nm to determine different carotenoids in the same run. The percentages of carotenoid compounds were calculated from peak areas measured. Based on experiments done by different authors,[Citation5,Citation14,Citation15 Citation16] there is no effect of time of extraction on trans-cis lycopene isomerization, but as the pressure increases, density of supercritical fluid increases and in it solubility of all trans-lycopene increases, while that of cis-lycopene decreases exponentially at ρ = 0.55 g/ml trans-lycopene = 31% and at ρ = 0.90 g/ml trans-Lycopene = 88%. Specifically, it is shown by Gomez-Prieto et al.[Citation5] when authors worked at 40°C and pressure range from low 77 bar up to intermediate value 281 bar, the effect on extraction yield of an increase of temperature would be either negative or non significant due to the balance between CO2 density and solute vapor pressure changes. In case of supercritical fluid extraction, the addition of different solvent modifiers like acetone, methanol, hexane, and dichloromethane is eliminated because it gives negative effect on extraction. The chromatograms monitored at 285, 347, 450 nm, obtained from the corresponding extracts showed that experimentation at the lowest density, i.e., 0.25 g/ml did not allow us to extract significant amounts of carotenoids, while the increase of their solubilities achieved at higher densities enabled us to detect different compounds. The enhancement of the solvating power, which implies the increase of the pressure from 77 bar (ρ = 0.25 g/ml) to 281 bar (ρ = 0.90g/ml), while maintaining the temperature of the extraction cell at 40 °C, made possible the extraction of different carotenoids, that is, phytoene, phytofluene, β‐carotene and lycopene. In these cases in which cis-isomers are not well separated from the all-trans forms, the analysis will result in a significant overestimation of the latter because their peaks will co-elute partly or totally with the cis-isomer peaks. In this case, the use of polymerically synthesized C30 column allowed us to obtain adequate selectivity and selectivity for the determination of all-trans-isomers from the cis-counterparts, as well as acceptable separations among the individual cis-isomers themselves. A CO2 density higher than 0.98 can extract other carotenoids (i.e., phytoene, phytofluene, and β-carotene), so densities lower than it used e.g. 0.85, 0.80, 0.75, 0.70, 0.60, and 0.55 g/ml. The yield of total Lycopene was 23.6, 14.5, 8.6, 4.5, 0.6, and 0.2%, respectively.[Citation5]
HPLC for Solvent Extraction
The mobile phase for solvent extraction is Methanol: THF: Water (67:27:6), flow rate 2ml/min. The routine detection of sample injection volume 20 μl is done at 475 nm—the adsorption maximum for lycopene in the mobile phase.[Citation17]
EFFECT OF HEAT AND TIME ON AMOUNT AND STABILITY OF LYCOPENE
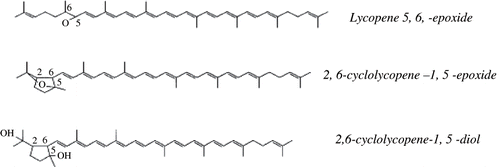
Regarding absorption of lycopene from dietary sources, surprisingly no increase in lycopene serum levels was observed after the single intake of large amounts of tomato juice. After ingestion of 180 g or even 700 g of tomato juice corresponding to a single dose of 12 or 80 mg of lycopene, respectively; no change in serum lycopene levels was observed. In contrast to unprocessed tomato juice, lycopene plasma levels increased significantly in human serum when processed juice was consumed. Boiling for 1 hour in the presence of 1% corn oil increased the bioavailability lycopene from tomato juice significantly.[Citation18] Lycopene absorption varies with individuals, and peak serum concentrations are observed at 24 to 48 hours after ingestion of processed tomato juice. Lycopene is eliminated from human plasma with a half-life of about 2 to 3 days, which is somewhat more rapid than β-carotene. Hydroxylated derivatives of lycopene have been detected in human serum. They contain lycopene 5,6-epoxide, which might be enzymatically or chemically modified the organism.
APPLICATIONS OF LYCOPENE
-
It has singlet oxygen quenching ability and so having antioxidant activity.[Citation19] Lycopene can oxidize in both human serum and plants. In human serum Lycopene-5,6-epoxide is the major oxidation product in the reaction with chloroperbenzoic acid. In 1996, Stahl[Citation6] isolated the final hydrolysis products following rearrangement are the epimers of 2,6-cyclolycopene-1, 5-diol, isolated by preparative HPLC from human serum.
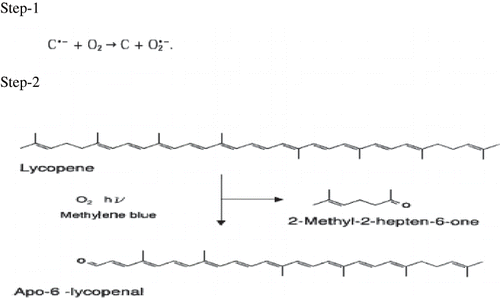
In plants, the rate for the singlet oxygen quenching by Lycopene is second-order and K = 7 × 10−9 M−1 s−1. shows the chemical pathway of the oxidation of lycopene in the plant where in step -1 active oxygen is generated which on reaction with lycopene in the presence of 2-methyl-2-hepten-6-one give Apo-6-lycopenal on oxidation.
-
Lycopene is natural coloring substance, so it avoids the harmful effects of artificial food colorants.[Citation20] The process of ripening of fruits or color development in some leafs (except green) coinciding with the transformation of chloroplasts into chromoplasts. During this Process, the esterification of carotenoids like Lycopene will take place with different fatty acids for removing and stabilizing free radicals of oxygen produced during the process.
-
Lycopene plays an important role in photosynthesis. As chloroplasts are also related to photosynthesis, many of the cell materials are co-related to it. Self-esterification of carotenoids like lycopene protects these cell materials from photo oxidative damage.[Citation20]
-
Lycopene is a vitamin A precursor. The general structure of lycopene is an aliphatic hydrocarbon with 11 conjugated carbon-carbon double bonds. Being acyclic, Lycopene possesses symmetrical planarity. When this structure under goes certain biochemical reactions, it forms provitamin A, and then it is converted to vitamins.[Citation21]
-
It is also helpful in certain critical in vivo biological processes like growth control, cell-to-cell communication, and modulating hormones.[Citation4]
-
It is useful for chemo-preventive treatment of cancer.[Citation22] Many of the experiments done on rats, rabbits, and human tumor cells show that the presence of lycopene inhibits the uncontrollable growth of the cells. In humans, Leukemia is a type of cancer, which is very hard to treat. Lycopene is one of the options, which can help treatment in early stages. Other cancers for which lycopene can be helpful are Prostate, Pancreas, and stomach cancers.
COMMERCIAL ASPECTS
More information is required to firmly establish lycopene's role in health protection and to identify the underlying biochemical mechanisms. Lycopene might interact with other food components providing additive or synergistic effects. One important aspect of lycopene research is the bioavailability of this compound and its metabolism to derivatives, which might also exhibit biological activities contributing to its potential cancer-preventing properties. With the increasing demand for nutritious food, many tablets available in the market contain vitamins and other compounds. Lycopene is also one of the important content of human dietary foods. Thus, more and more focus should be given on the manufacture of lycopene production on a commercial scale.
CONCLUSION
The effect of lycopene on tumor cell shows that lycopene restricts different cancer cells, e.g., prostate cancer cells. Furthermore, from different reported analyses, we conclude that tomatoes contain the maximum amount of lycopene compared to other sources, i.e., about 11.21 mg/100 g wet weight. Moreover, different methods are available for lycopene extraction, but the supercritical fluid extraction of Lycopene with CO2 gives better results than other methods. The extracted lycopene can be inserted into the diet, where natural sources of lycopene are not available. In some industrial processes, where oxygen quenching is required, extracted lycopene can be used. The experiments showed that heat-processed food gives a larger amount of available Lycopene than that was previous, but the degradation of extracted lycopene is greater at high-temperature environments. These controversial characteristics are still unexplained. For human dietary, Purposes lycopene should be one of the critical compounds because of its nutraceutical, epidemiological, and pharmaceutical importance.
NOMENCLATURE
| DWB | = |
Dry Weight Basis | |||||
| FWB | = |
Fresh Weight Basis | |||||
| SCFE | = |
Supercritical Fluid Extraction
| |||||
REFERENCES
- Hurst , W.J. 2002 . Methods of Analysis for Functional Foods and Nutraceuticals , 101 – 147 . Boca Raton : CRC Press .
- Marcel , B.R. 1999 . Concepts in Functional Foods: The Case of Inulin and Oligo Fructose . Journal of Nutrition , 129 : 1398s – 1401s .
- Coultate , T.P. 1996 . Food—The Chemistry of Its Components, , 3rd , 54 London : Royal Society of Chemistry .
- George , B. , Kaur , C. , Khurdia , D.S. and Kapoor , H.C. 2004 . Antioxidants in Tomato (Lycopersium esculentum)—as a Function of Genotype . Food Chemistry , 84 : 45 – 51 .
- Gomez-Prieto , S.M. , Caja , M.M. , Marta , H and Guillermo Santa-Maria Salud , M. 2003 . Supercritical Fluid Extraction of All Trans-Lycopene from Tomato . J. Agric. Food Chem. , 51 : 3 – 7 .
- Stahl , H.S. 1996 . Lycopene: A Biologically Important Carotenoid for Humans . Arch. Of Biochemistry and Biophysics. , 336 ( 1 ) : 1 – 9 .
- Minythyl , L.N. and Steven , J.S.. 1999 . Lycopene: Chemical and Biological Properties . Food Technology , 53 ( 2 ) : 38 – 43 .
- Akanbi , C.T. and Oludemi , F.O. 2004 . Effect of Processing and Packing on the Lycopene Content of Tomato Products . International Journal of Food Properties , 7 : 139 – 152 .
- Scalfi , L. , Fogliano , V. , Pentagelo , A. , Graziani , G. , Giordano , I. and Ritieni , A. 2000 . Antioxidant Activity and General Fruit Characteristics in Different Ecotypes of Corbarini Small Tomatoes . J. Agric. Food Chem. , 48 : 1363 – 1366 .
- Davis , W.B. 1949 . Preparation of Lycopene from Tomato Paste for use as a Spectrophotometric Standard . Analytical Chemistry , 10 : 1226 – 1228 .
- Sadler , G. , Davis , J. and Dezman , D. 1990 . A Research Note: Rapid Extraction of Lycopene and α-Carotene from Reconstituted Tomato Paste and Pink Grapefruit Homogenates . J. Food Science , 55 ( 5 ) : 1460 – 1461 .
- Emenhiser , C. , Sander , L.C. and Schwartz , S.J. 1995 . Capability of a Polymeric C30Stationary phase to Resolve Cis-Trans Carotenoids in Reversed Phase Liquid Chromatography . J. Chromatogr. , 707 : 205 – 216 .
- Baysal , T. , Erus , S. and Starmans , D.A. 2000 . Supercritical Extraction of α-−Carotene and Lycopene from Tomato Paste Waste . J. Agric. Food Chem. , 48 : 5507 – 5511 .
- Ollanketo , M. , Hartonen , K. , Riekkola , M.L. , Holm , Y. and Hiltunen , R. 2001 . Supercritical Carbondioxide Extraction of Lycopene in Tomato Skins . Eur. Food Res. Technol. , 212 : 561 – 565 .
- Rozzi , N.L. , Singh , R.K. , Vierling , R.A. and Watkings , B.A. 2002 . Supercritical Fluid Extraction of Lycopene from Tomato Processing Byproducts . J. Agric. Food Chem. , 50 : 2638 – 2643 .
- Takeoka , G.R. , Lan , D. , Stephan , F. , Gillespie , D.M. , Jewell , W.T. , Britta , H. , Daniel , B. and Ebeler , S.E. 2001 . Processing Effects on Lycopene Content and Antioxidant Activity of Tomatoes . J. Agric. Food Chem. , 49 : 3713 – 3717 .
- Joseph , S. , Werner , B. , Ilme , B. , Nicole , F. , Denise , H. , Kurt , S. and Willy , S. 1997 . Content and Isomeric Ratio of Lycopene in Food and Human Blood Plasma . Food Chemistry , 59 ( 3 ) : 459 – 465 .
- Codgell , R.J. 1985 . Carotenoids in Photosynthesis . Pure Appl. Chem. , 57 : 723 – 728 .
- Heinonen , M.I. , Ollilainen , V. , Linkola , E.K. , Varo , P.T. and Koivistoinen , P.E. 1989 . Carotenoids in Finnish Foods: Vegetable, Fruits and Berries . J. Agric. Food Chem. , 37 : 655 – 659 .
- Agrawal , S. and Rao , A.V. 1998 . Tomato Lycopene and Low Density Lipoprotein Oxidation: A Human Dietary Interversion Study . Lipids , 33 : 981 – 984 .
- Truscott , T.G. 1999 . New Trends in Photophysics and Photochemistry of the Carotenoids . J. Photochem. photobiol. , 54 : 359 – 371 .
- Wilhelm , S. and Helmut , S. 1996 . Lycopene: A Biologically Important Carotenoid for Humans . Archives of Biochem. Biophy. , 336 ( 1 ) : 1 – 9 .