Abstract
Salt-fermented soybean foods are popular in Asia as an alternative food seasoning material. The α,α-Diphenyl-β-pricrylhydrazyl (DPPH) radical-scavenging activity, 2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) radical-scavenging activity and reducing power of the 50% ethanol extracts from one variety called in layman term as douchi were evaluated in this study. TEAC on DPPH radical and ABTS radical of extracts and the reducing powers of the extracts was found to increase significantly during the prefermentation (p < 0.05), through to the postfermentation. We investigated the effect of adding salt to the antioxidative activity during postfermentation. It was found that high salt content was not favorable for high TEAC on DPPH radical and the reducing powers. The effect of salt content on TEAC on ABTS radical of extracts was not significant (p > 0.05). The results showed that the antioxidative compounds released during the douchi fermentation were inhibited by the high salt addition.
INTRODUCTION
The traditional fermented soybean foods are divided in two types: salt-fermented and non-salt fermented. The non-salt fermented soybean foods include natto, tempeh and kinema, and so on; sufu, douchi (salt-fermented soybean), miso, and soy sauce are representative of the salt-fermented soybean foods. These fermented soybean foods play a very important role in people's diet in many centuries. Douchi is a traditional salt-fermented soybean product that originated in China, which has been consumed in large quantities since ancient times as the seasoning for dishes (see ). There are three types of douchi that are fermented by Mucor, Bacteria, and Aspergillus strains, respectively. Among them, Aspergillus-type douchi is the earliest, and has been the most popular type and is produced widely in China.
The traditional fermented soybean foods have been found to exhibit remarkably strong antioxidative activity, such as sufu,[Citation1–2] miso,[Citation3] natto,[Citation4–6] tempeh.[Citation7–9] Douchi is a similar fermented soybean food as natto, and tempeh, which were incubated with Baccillus natto, and Rhizopus oligosporus, respectively.[Citation10] One would expect douchi should also have reasonable antioxidative activity. Therefore, it is intended here to evaluate the antioxidative properties during the processing of douchi. Although there have been some reports relating to elicit dose-dependent antiglycemic effects in humans of douchi by Aspergillus cultured,[Citation11–12] it appears that no one has paid attention to the changes in the antioxidative activity of during douchi making.
Like in other salt-fermented soybean foods such as sufu,[Citation13] miso,[Citation14] salt has a multiple role in douchi. During the postfermentation of douchi, a salty taste to douchi by adding salt. The salt will control the microbial growth and enzyme activity in douchi as well. In this article, we analyzed the antioxiant activity of the 50% ethanol extracts from douchi, by using the DPPH (1,1-diphenyl-2-picrylhydrazyl) radical-scavenging assay, the ABTS (2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid)) radical-scavenging assay and the reducing power. We also investigated the the effect the salt supplementation during douchi postfermentation on the antioxiant activity of the extracts. By establishing the relationship between the function and process of douchi making, it would be possible to establish an improved procedure for producing highly functional douchi, and increasing its value as a healthy food product.
MATERIALS AND METHODS
Materials
Mold culture Aspergillus oryzae 3.951 was provided by the Institute of Microbiology, Chinese Academy of Sciences (Beijing, China). Soybeans named of Kefeng No. 6 (2003) were provided by the Agricultural Academy of Jilin Province (Jilin, China). ABTS, DPPH and Trolox ((+/−)-6-hydroxy-2, 5, 7, 8-tetramethyl-chroman-2-carboxylic acid, 97%) were purchased from Sigma Chemical Co (U.S.A.). All other chemicals were of the reagent grade.
Preparation of Douchi
Douchi preparation was performed as previous description,[Citation15] as illustrated in . The steps and parameters were as follows:
-
Pretreatment. Soybeans were washed, and soaked in tap water for 8 hours at room temperature (24°C ± 2°C). After draining, the soybeans were steamed for 30 minutes at 121°C in a retort (YMQ.L31.400, Beijing Jiangtai Medical Instrument CO., Ltd, Beijing, China).
-
Prefermentation. The cooked soybeans were cooled to 30°C–35°C, and then inoculated quickly with Aspergillus oryzae 3.951 incubated at 30°C for 60 hours, around 80% relative humidity in an incubator (LTI-601SD; Tokyo Rikakikai CO., Ltd, Tokyo, Japan). Semifinished products were called the douchi qu (koji).
-
Postfermentation. Aliquots of the douchi qu deposited into a series of glass jars were salted until the content of NaCl reached about 5, 7.5, 10, and 12.5% (w/w). Each sample was ripened for 1 month at 35°C in the same incubator.
Figure 2 Flow diagram for processing soybeans to make Douchi.[Citation15]
![Figure 2 Flow diagram for processing soybeans to make Douchi.[Citation15]](/cms/asset/1b378080-e711-4788-b6e6-255ad9379334/ljfp_a_205175_o_f0002g.gif)
Raw soybean, soaked soybean, and cooked soybean were sampled. Samples were taken at 12-hour intervals during the prefermentation, and at one-week intervals during the postfermentation. All the samples were lyophilized and ground using a coffee mill (TSK-U928S, CANKUN Co., China) to pass a 50-mesh size screen.
Preparation of the Douchi Extracts
One gram of freeze-dried sample powder was extracted with 10 mL 50% ethanol in a shaking incubator for 2 hours at room temperature. The suspension was centrifuged at 2000 × g for 15 minutes at room temperature. The supernatant was filtered and the serial dilutions were used for assaying. The antioxidative extracts were kept in the dark for further analysis.
DPPH Free Radical Scavenging Activity
The radical-scavenging activities of the extracts of douchi were determined according to the methods described by Suda[Citation16] with some modification. Diluted douchi extracts (100 μL) was mixed with 100 μL of a 200 μM DPPH solution in 0.1 M MES buffer (pH 6.0) containing 50% ethanol was then added. The mixture was kept at room temperature for 20 minutes, then the absorbance of the resulting solution was measured at 520 nm using a 96-well plate reader (model 550, Bio-Rad Laboratories, Japan). Trolox was used as an antioxidative standard to prepare the standard curve and calculate the trolox equivalent for each sample. The DPPH scavenging activity of the douchi extracts were expressed as Trolox Equivalent Antioxidative Capacity (TEAC).
ABTS Free Radical Scavenging Activity
ABTS free radical-scavenging assay was based on a method developed by Robert et al.[Citation17] with some modification. ABTS· + was generated by reacting ABTS (7.4 mM) with potassium persulphate (2.6 mM). The solution was diluted to obtain an absorbance of 1.4 units at 414 nm with ethanol. The ABTS· + antioxidative reaction mixture contained 200 μL of ABTS· + solution, and 50 μL of douchi extracts or 50 μL of 50% Ethanol for the control. The absorbance at 420 nm was measured at 6 min of the reaction by a 96-well plate reader (model 550, Bio-Rad Laboratories, Japan). Trolox was used as an antioxidative standard to prepare the standard curve and calculate the trolox equivalent for each sample. The radical scavenging activity of douchi extract was expressed as Trolox Equivalent Antioxidative Capacity (TEAC).
Reducing Power
The reducing power of douchi samples extracts was determined according to the method of Oyaizu[Citation18] with some modifications. Douchi extracts (5 mg/mL) 0.4 ml was mixed with phosphate buffer (1 mL, 0.2 mol/L, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (1 mL, 1%). The mixture was incubated at 50°C for 20 minutes. A portion (1 mL) of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 10000 × g for 10 minutes. The upper layer of solution (1 mL) was mixed with distilled water (2 mL) and FeCl3 (0.4 mL, 0.1%), and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power.
Statistical Analysis
All experiments were conducted in triplicates. The mean values of each experiment were calculated. The SAS system (SAS for Windows 6.12, SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. Duncan's mulitiple range test was used to estimate significant differences among the mean values at the 5% probability level.
RESULTS AND DISCUSSION
Changes in the Antioxidative Properties during Douchi Pretreatment Processing
The antioxidative properties of the extracts during douchi pre-treatment processing are shown in . TEAC on DPPH radical and ABTS radical were no significant changes (p > 0.05) after soaking and cooking comparing with raw soybean, but the reducing powers were significant increased (p < 0.05).
Table 1 Changes in the antioxidative properties for pretreatment of Douchi processing
Changes in the Antioxidative Properties during Douchi Prefermentation Processing
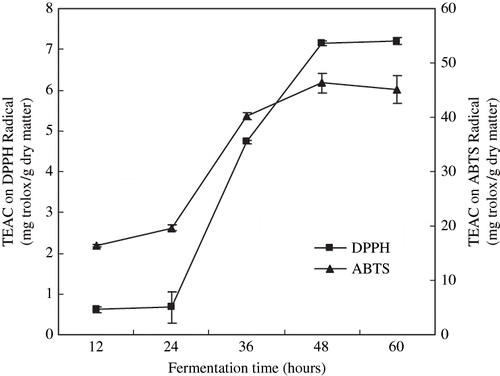
shows that the scavenging activity of extracts on DPPH radical and ABTS radical during the prefermentation. TEAC on DPPH radical and ABTS radical increased significantly (p < 0.05) from 0.61 to 7.21 and from 19.60 to 45.09 during the fermentation, respectively. Arnao[Citation19] reported the DPPH assay could significantly underestimate TEAC compared to ABTS assay. Our results were agreed with his.
Figure 3 Scavenging activity of Douchi extracts by DPPH method and ABTS method during the prefermentation. Results are the mean ± S. D. of three determinations.
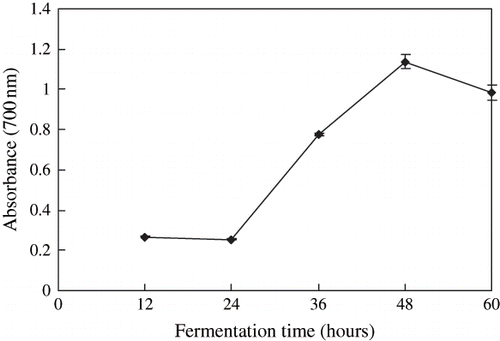
The reducing powers (absorbance at 700 nm) of the extracts during the pre-fermentation are shown in . The reducing power increased significantly from 0.27 to 0.96. The antioxidative properties during douchi prefermentation processing were increased significantly (p < 0.05). This might have been due to the fact that antioxidative compounds were released during the fermentation. The antioxidative effects of tempeh and natto were increased during fermentation, and were found to a significant influence by fermentation time.[Citation4, Citation8]
Changes in the Antioxidative Properties during Douchi Postfermentation Processing
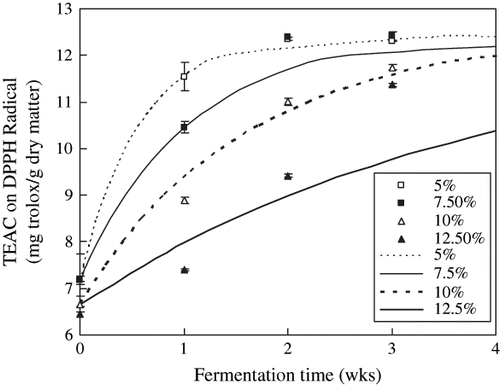
The scavenging activities the extracts on DPPH radical during the post-fermentation are shown in . TEAC increased significantly continually with the fermentation time of douchi (p < 0.05). TEAC were influenced significantly by the salt contents (p < 0.05). TEAC were increased with the decrease of the salt contents. TEAC were increased from 7.18 (5% salt content), 7.30 (7.5% salt content), 6.66 (10% salt content), and 6.45 (12.5% salt content) at 0 week (week) to 11.54, 10.46, 8.89, 7.40 at 1 week in postfermentation, respectively. The increasing rates of TEAC were evidently different with the different salt contents during fermentation.
Figure 5 Scavenging activity of Douchi extracts by DPPH method during the postfermentation. Symbols denoted the experimental data, the mean ± S. D. of three determinations; Lines denoted the predicted data according to the EquationEq. (1).
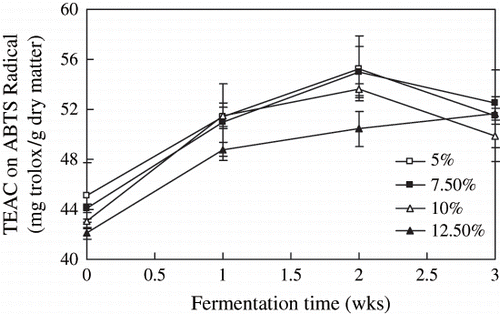
The scavenging activities of the extracts on ABTS radical during the postfermentation are shown in . The further increase of TEAC was significant, and reached the maximum point at 2 weeks in postfermentation. The effect of salt contents on TEAC were not significant (p > 0.05).
Figure 6 Scavenging activity of Douchi extracts by ABTS method during the postfermentation. Results are the mean ± S. D. of three determinations.
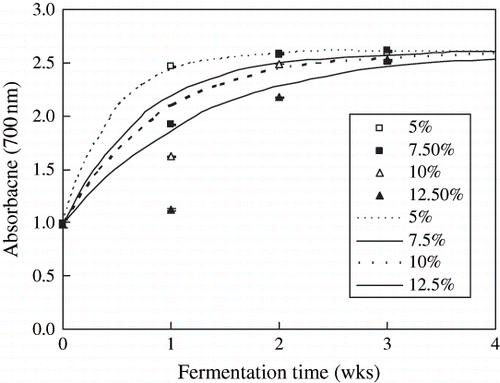
shows the reducing powers of the extracts during the post-fermentation, suggested that the reducing powers of the extracts were increased significantly during the first 2 weeks of postfermentation (p < 0.05), and the level of salt content did affect the reducing powers of the extracts, which decreased with increases in salt content, however, the effect of salt contents on the reducing powers were not significant at 3 weeks (p > 0.05). These results suggested that the antioxidative compounds on DPPH radical, on ABTS radical and reducing powers were released further during the postfermentation. The compounds on DPPH radical and reducing power were inhibited by higher salt addition. However, the compounds on ABTS radical were not affected by salt addition.
Figure 7 Reducing power of Douchi extracts during the postfermentation. Symbols denoted the experimental data, the mean ± S. D. of three determinations; Lines denoted the predicted data according to the EquationEq. (1).
Fermentation Time-Dependent Kinetics for Antioxidative Activity
As shown in and , the antioxidative activities of the extracts depended strongly on its fermentation time and the contents of salt. In general, the antioxidative activity increased with fermentation time to a certain extent, then leveled off with further increase in time. The antioxidative activities of the extracts have been found to be represented by the following equation:
where A is the antioxidative activity at any given fermentation time (weeks) in different the contents of salt; A 0 is the initial the antioxidative activity at 0 wk of postfermentation; A∗ is the equilibrium the antioxidative activity, which is as summed to remain constant for the processes; t is the time of postfermentation (wks); and k is a rate constant (1/wk). The proportional constant (k) can be obtained from the slope of a Ln(1 − (A − A 0) / (A − A∗)) against time (t) plot. The proportional constant (k) has therefore the unit of reciprocal time, and can be viewed as an indication for the antioxidative activity. A higher k value represents a higher rate of the growth of the antioxidative activity for the specific process.
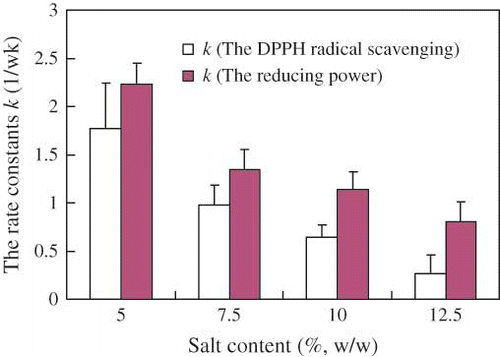
The rate constants (k) were plotted in . It has been found that the rate constant (k) for the DPPH radical scavenging activity and for the reducing power all decrease with the increase of the contents of salt. This indicated that the higher content of salt could result in lower reaction rates. The rate constant (k) for the reducing powder was higher than that of for the DPPH radical scavenging activity at the same salt content. According to simple regression analysis, the correlation coefficient of the TEAC on ABTS radical correlated with TEAC on DPPH radical was 0.96 and 0.64 in the prefermentation and the postfermentation, respectively. There were some reports about the correlation of ABTS assay and DPPH assay. A good correlation of TEAC between the ABTS model and DPPH model was observed in fruits.[Citation20] However, Significant differences by employing ABTS and DPPH scavenging assay were reported by Lissi et al.[Citation21] Wang et al.[Citation22] found that some compounds, which have ABTS radical scavenging activity may not show DPPH radical scavenging activity. The results obtained in this study showed a high correlation coefficient between the two assays in prefermentation, and a comparatively low correlation coefficient in postfermentation. These results indicated the compounds contributed the TEAC between the two methods were different at various stages of douchi fermentation.
Figure 8 The rate constants (k) for the DPPH radical scavenging activity and for the reducing power in different salt contents. Results are the mean + S. D. of at 1 week and 2 weeks in postfermentation.
The effect of antioxidative activity on DPPH radical scavenging and the reducing power of the douchi extracts were all thought to be due to its hydrogen donating ability.[Citation23] From our result, a good relationship (r = 0.99 in prefermentation, and r = 0.94 in postfermentation) was found between this two methods during fermentation. Hence, we suggest that the compounds contributed to the antioxidative properties and might be the same between the two methods.
Antioxidative peptides were found in the hydrolysates of soy proteins.[Citation24–25] Fan et al.[Citation26] reported the peptides by hydrolysate from soybean protein isolate for 4 hours showed the highest DPPH radical-scavenging activity, which the molecular weights were less than 33 kDa. Esaki et al.[Citation4] reported that the antioxidative activity of natto was dependent on the increase of water-soluble fractions of natto with fermentation time, rather than on the increase of isoflavone agalycons, such as daizen and genistein. The protein (albumin, globulin, and alkaline soluble) were rapidly degraded to amino acids and low-molecular-weight peptides during the tempeh fermentation.[Citation27] Markus et al.[Citation28] suggested the antioxidative activity of tempeh was a synergistic effect of tocopherols (present in the soybeans) and amino acids (liberated during the fermentation). Therefore, it was expected that peptides and amino acids were liberated during douchi fermentation process,[Citation29] contributing to the antioxidative activity of douchi extracts.
It was known that isoflavones participate in the antioxidative activity of soybean. Isoflavone aglycones showed higher physiological properties than their glucoside form. Daidzein and genistein were known for their antioxidative activity in biological systems with an emphasis on the scavenging activity on ABTS radical.[Citation30] These polyphenols can scavenge a peroxyl radical and protect against iron-induced free radical reaction.[Citation31] Daidzein and genistein were confirmed the main components responsible for the antioxidative activity of tempeh.[Citation7, Citation32] In our previous study,[Citation15] isoflavone glucosides were hydrolysed into their corresponding aglycones during Douchi fermentation. Hence, we presumed isoflavone transformation during douchi fermentation was one of the major reasons for the increase of antioxidative activity of douchi extracts.
Melanoidin, the end products of Maillard reaction process, was emphasized as one of the important factors on antioxidative activity of soy sauce.[Citation33] Maillard reaction was found during the douchi processing.[Citation34] This means that the melanoidin in douchi may play a more important role on its antioxidative activity effect. Although, peptide, amino acid, isoflavones, melanoidin, and tocopherols were all expected to be active ingredients in the Douchi extracts, isolation and identification of the antioxidative substances would have to be conducted to explore their contributions .This work is in progress.
A high NaCl addition may result in inhibiting the enzyme activity and preventing some compound contributing antioxidative active release during douchi fermentation. Some research about this phenomenon have been reported, such as that isoflavone transformation was inhibited during the fermentation of miso, sufu, and douchi;[Citation15, Citation35–36] protein degradation was reduced during the fermentation of Sufu.[Citation13] Furthermore, high NaCl content of food could increase the dietary sodium intake.[Citation37] Therefore, douchi with a low NaCl content may be preferable from the viewpoint of public nutritional health. Further investigation is needed to clarify the flavour, taste, texture, consumer acceptability, and shelf life of douchi with a lower NaCl content.
CONCLUSION
The antioxidative properties of the 50% ethanol extracts from douchi were effected during douchi processing. The antioxidative properties (TEAC on DPPH radical and ABTS radical) of the extracts during douchi pretreatment processing showed no significant changes (p > 0.05), but the reducing powers significantly increased (p < 0.05). The antioxidative properties of the extracts during douchi prefermentation processing were increased significantly (p < 0.05), through to the postfermentation. The content of NaCl did affect the antioxidative properties of the extracts. The higher the salt content, the lower the DPPH radical scavenging activity and the reducing power. These results indicate that the antioxidative compounds were released during the douchi fermentation and that they are inhibited by high salt addition. Furthermore, a simple model has been found to be useful for correlating the data of the effects of salt content on the DPPH radical scavenging activity and the reducing power.
ACKNOWLEDGMENTS
This study was conducted within the framework of the collaborative research project between Japan and China titled, “Development of sustainable production and utilization of major food resources in China,” supported by Japan International Research Center for Agricultural Sciences (JIRCAS). This work was supported using the fund (DIC2003-04) called, “Study on the antioxidative activities of the Chinese traditional fermented soybean foods,” from the Research/Communication Proposal of Danone Institute China.
REFERENCES
- Wang , L.J. , Saito , M. , Tatsumi , E. and Li , L.T. 2003 . Anti-oxidative and Angiotensin I-Converting Enzyme Inhibitory Activity of Sufu (Fermented Tofu) Extracts . Japan Agricutural Research Quarterly , 37 : 129 – 132 .
- Wang , L.J. , Li , L.T. , Fan , J.F. , Saito , M. and Tatsumi , E. 2004 . Radical-scavenging Activity and Isoflavone Content of Sufu (Fermented Tofu) Extracts from Various Regions in China . Food Science and Technology Research , 10 : 324 – 327 .
- Hirota , A. , Taki , S. , Kawaii , S. and Yano , M. 2000 . 1,1-diphenyl-2-picrylhydrazyl Radical-scavenging Compounds from Soybean Miso and Antiproliferative Activity of Isoflavones from Soybean Miso toward the Cancer Cell Lines . Bioscience Biotechnology and Biochemistry , 64 : 1038 – 1040 .
- Esaki , H. , Nohara , Y. , Onozaki , H. and Osawa , T. 1990 . Antioxidative Activity of Natto . Nippon Shokuhin Kogyo Gakkaishi , 37 : 474 – 477 .
- Iwai , K. , Nakaya , N. , Kawasaki , Y. and Matsue , H. 2002 . Inhibitory Effect of Natto, a Kind of Fermented Soybeans, on LDL Oxidation in vitro . Journal of Agricultural and Food Chemistry , 50 : 3592 – 3596 .
- Iwai , K. , Nakaya , N. , Kawasaki , Y. and Matsue , H. 2002 . Antioxidative Function of Natto, a Kind of Fermented Soybeans: Effect on LDL Oxidation and Lipid Metabolism in Cholesterol-fed Rats . Journal of Agricultural and Food Chemistry , 50 : 3597 – 3601 .
- György , P. , Murata , K. and Ikehata , H. 1964 . Antioxidatives Isolated from Fermented Soybeans (Tempeh) . Nature , l203 : 870 – 872 .
- Berghofer , E. , Grzeskowiak , B. , Mundigler , N. , Sentall , W.B. and Walcak , J. 1998 . Antioxidative Properties of Faba Bean-, Soybean- and Oat Tempeh . International Journal of Food Sciences and Nutrition , 49 : 45 – 54 .
- Sheih , I.C. , Wu , H.Y. , Lai , Y.J. and Lin , C.F. 2000 . Preparation of High Free Radical Scavenging Tempeh by a Newly Isolated Phizopus sp. R-69 from Indonesia . Food Science and Agricultural Chemistry , 2 : 35 – 40 .
- Li , L.T. , Zhang , J.H. , Li , Z.G. and Tatsumi , E. 2003 . The Comparison of Natto, Tempeh and Douchi . China Condiment , 5 : 3 – 7 . 10
- Fujita , H. , Yamagami , T. and Ohshima , K. 2001 . Fermented Soybean-derived Water-soluble Touchi Extract Inhibits α-glucosidase and is Antiglycemic in Rats and Humans after Single Oral Treatments . Journal of Nutrition , 131 : 1211 – 1213 .
- Fujita , H. , Yamagami , T. and Ohshima , K. 2001 . Long term Ingestion of a Fermented Soybean-derived Touchi-extract with α-glucosidase Inhibitory Activity is Safe and Effective in Humans with Borderline and Mild Type-2 Diabetes . Journal of Nutrition , 131 : 2105 – 2108 .
- Han , B.Z. , Wang , J.H , Rombouts , F.M. and Nout , M.J.R. 2003 . Efffect of NaCl on Textural Changes and Protein and Lipid Degradation during the Ripening Stage of Sufu, a Chinese Fermented Soybean Food . Journal of the Science of Food and Agriculture , 83 : 899 – 904 .
- Chiou , R.Y.Y. 1999 . Salt-free Miso Fermentation using Ethanol, Sugars, and Polyols . Journal of Food Science , 64 : 918 – 920 .
- Wang , L.J. , Yin , L.J. , Zou , L. , Saito , M. , Tatsumi , E. and Li , L.T. 2007 . Influences of Processing and NaCl Supplementation on Isoflavone Contents and Composition during Douchi Manufacturing . Food Chemistry , 101 : 1247 – 1253 .
- Suda , I. 2000 . “ Antioxidative Activity ” . In The Methods of Food Functions Analysis , Edited by: Shinohara , K. , Suzuki , T. and Kaminogaw , S. 218 – 220 . Japan : Korin .
- Robert , R. , Pellegrini , N. , Proteggente , A. , Pannala , A. , Yang , M. and Rice-Evans , C. 1999 . Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay . Free Radical Biology and Medicine , 26 : 1231 – 1237 .
- Oyaizu , M. 1986 . Studies on Product of Browning Reaction Prepared from Glucose Amine . Japanese Journal of Nutrition , 44 : 307 – 315 .
- Arnao , M.B. 2000 . Some Methodological Problems in the Determination of Antioxidant Activity using Chromogen Radicals: A Practical Case . Trends in Food Science and Technology , 11 : 419 – 421 .
- Leong , L.P. and Shui , G. 2002 . An Investigation of Antioxidant Activity of Fruits in Singapore Markets . Food Chemistry , 76 : 69 – 75 .
- Lissi , E.A. , Modak , B. , Torres , R. , Escobar , J. and Urzua , A. 1999 . Total Antioxidant Potential of Resinous Exudates from Heliotropium Species, and a Comparison of the ABTS and DPPH Methods . Free Radical Research , 30 : 471 – 477 .
- Wang , M. , Shao , Y. , Li , J.G. , Zhu , N.Q. , Rangarajan , M. , LaVoie , E.J. and Ho , C.T. 1998 . Antioxidative Phenolic Compounds from Saga (Salvia Officinalis) . Journal of Agricultural and Food Chemistry , 46 : 4869 – 4873 .
- Baumann , J. , Wurn , G. and Bruchlausen , F.V. 1979 . Prostaglandin Synthetase Inhibiting O2 − Radical Scavenging Properties of Some Flavonoids and Related Phenolic Compounds . Naunyn-Schmiedebergs Arch. Pharmacol. , 308 : R27
- Chen , H.M. , Muramoto , K. and Yamauchi , F. 1995 . Structural Analysis of Antioxidative Peptides from Soybean β-conglycinin . Journal of Agricultural and Food Chemistry , 43 : 574 – 578 .
- Chen , H.M. , Muramoto , K. , Yamauchi , F. , Fujimoto , K. and Nokihara , K. 1998 . Antioxidative Properties of Histidine-containing Peptides Designed from Peptide Fragments Found in the Digests of a Soybean Protein . Journal of Agricultural and Food Chemistry , 46 : 49 – 53 .
- Fan , J.F. , Saito , M. , Zhang , Y.Y. , Tan , S. , Wang , L.J. , Tatsumi , E. and Li , L.T. 2005 . Gel-forming Ability and Radical-scavenging Activity of Soy Protein Hydrolysate Treated with Transglutaminase . Journal of Food Science , 70 : 87 – 92 .
- Handoyo , T. and Morita , N. 2006 . Structural and Functional Properties of Fermented Soybean (tempeh) by Using Rhizopus Oligosporus . International Journal of Food Properties , 9 : 347 – 355 .
- Markus , B.H. , Hem , C.J. and Heinz , E. 1997 . Structure of an Antioxidant from Fermented Soybeans (Tempeh) . Journal of the American Oil Chemists' Society , 74 : 477 – 479 .
- Zhang , J.H. , Tatsumi , E. , Fan , J.F. and Li , L.T. 2006 . Chemical Components of Aspergillus- type Douchi, a Chinese Traditional Fermented Soybean Product, Change during the Fermentation Process . International Journal of Food Science and Technology , 41 : 1 – 6 .
- Ungar , Y. , Osundahunsi , O.F. and Shimoni , E. 2003 . Thermal Stability of Genistein and Daidzein and Its Effect on Their Antioxidant Activity . Journal of Agricultural and Food Chemistry , 51 : 4394 – 4399 .
- Rice-Evans , C.A. , Miller , N.J. , Bolwell , P.G. , Bramley , P.M. and Pridham , J.B. 1995 . The Relative Antioxidant Activities of Plan Derived Polyphenolic Flavonoids . Free Radical Research , 22 : 375 – 383 .
- Murakami , H. , Asakawa , T. , Terao , J. and Matsushita , S. 1984 . Antioxidative Stability of Tempeh and Liberation of Isoflavones by Fermentation . Agricultural and Biological Chemistry , 48 : 2971 – 2975 .
- Moon , G. , Lee , M. , Lee , Y. and Trakoontivakorn , G. 2002 . Main Component of Soy Sauce Representing Antioxidative Activity . International Congress Series , 1245 : 509 – 510 .
- Wang , L.J. , Zou , L. , Li , L.T. and Tatsumi , E. 2005 . Changes on Douchi Appearance, Temperature, Hardness and Color Tone during Fermentation . Food and Fermentation Industries , 31 : 53 – 56 .
- Chiou , R.Y.Y. and Cheng , S.L. 2001 . Isoflavone Transformation during Soybean Koji Preparation and Subsequent Miso Fermentation Supplemented with Ethanol and NaCl . Journal of Agricultural and Food Chemistry , 49 : 3656 – 3660 .
- Yin , L.J. , Li , L.T. , Li , Z.G. , Tatsumi , E. and Saito , M. 2004 . Changes in Isoflavone Contents and Composition of Sufu (Fermented Tofu) during Manufacturing . Food Chemistry , 87 : 587 – 592 .
- Baggott , J.E. , Ha , T. , Vaughn , W.H. , Juliana , M.M. , Hardin , J.M. and Grubbs , C.J. 1990 . Effect of Miso (Japanese Soybean Paste) and NaCl on DMBA-induced Rat Ammary Tumors . Nutrition and Cancer , 14 : 103 – 109 .