Abstract
Spray drying behavior of orange juice concentrate with various levels of maltodextrin (DE 6) was studied. Five combinations of orange juice concentrate and maltodextrin (25:75, 30:70, 35:65, 40:60, and 50:50) were spray dried at 160 and 65°C inlet and outlet temperatures, respectively. The product recovered with 50% maltodextrin concentration was sticky and only 20% powder was recovered. The recovery of orange juice powder increased as the amount of maltodextrin in powders increased. The particle size and bulk density remained almost the same in all except in 50% maltodextrin powder which was slightly larger and more dense. The moisture content of spray dried powders was high and desiccated before measuring glass transition temperature. The anhydrous spray dried powders showed increased Tg values with increasing maltodextrin concentration, from 66°C in 50% maltodextrin to 97°C in 75% maltodextrin containing powders. The glass rubber transition (Tg-r) values of all the products measured using novel Thermal Mechanical Compression Test (TMCT) were higher than Tg values measured by DSC; the difference in values increased with increase in maltodextrin concentration.
INTRODUCTION
Fruit juice powders have many benefits and economic potentials over their liquid counterparts such as reduced volume or weight, reduced packaging, easier handling and transportation, and much longer shelf life. Besides, this physical state provides a stable, natural, easily dosable ingredient which generally finds usage in many foods and pharmaceutical products such as flavoring and coloring agents. However, the dehydration of fruit juice is not a simple task. Fruit juices are sensitive to high temperature and contain highly hygroscopic ingredients such as low molecular weight sugars and organic acids (such as citric, malic, tartaric acid, etc.) that lead to problems in controlling the drying time, adhesion to dryer wall, removal of the product from the dryer, caking and subsequent handling of the product.[Citation1,Citation2,Citation3] Spray drying is a common method of dehydrating liquid foods where the moisture is quickly removed resulting in mostly amorphous (glassy) solid or a syrupy/sticky powder.[Citation4]
All the amorphous materials change from the glassy to rubbery state at a glass transition temperature (Tg) which is specific for each materials. The Tg values of soluble sugars and organic acids are low and increase with increases in molecular weight of the compounds.[Citation5,Citation6] The viscosity of the amorphous solid is very high (typically >1012 Pa.s). As the temperature increases, the viscosity of the glass decreases. Downton et al.[Citation7] reported that the critical viscosity of the amorphous solid is around 106 to 108 Pa.s when it first becomes sticky. Generally the critical viscosity of the amorphous solids is reached at temperatures around 10–20°C above the Tg value.[Citation4,Citation8] The major components of orange juice such as fructose, glucose and citric acid have very low Tg of 14, 31 and 16°C, respectively[Citation9,Citation10,Citation11] in a pure, dry state, which decrease drastically when moisture is absorbed. The low glass transition temperature of these juice components as well as their high hygroscopicity, low melting point, and high water solubility results in a highly sticky product when spray dried.[Citation12] Some of the most commonly used methods to spray dry such products are: addition of drying aid (glucose for orange juice by Brennan et al.;[Citation1] sodium chloride for tomato syrup by Lazar et al.;[Citation13] soybean protein for banana by Mirzahi et al.;[Citation14] skim milk powder for orange juice by Rao and Gupta;[Citation15] and maltodextrins for orange juice, black currant and honey by Bhandari et al.;[Citation16,Citation17] scrapping of surface of dryer (for tomato juice by Karatas and Esin[Citation18]); and drying aid with cooling of the drying chamber walls.[Citation16] The major limitations of the use of drying aid are the subsequent change in the product properties and the cost. The last two methods require specialized equipments and labor intensive. Drying aids or additives are high molecular weight compounds such as maltodextrins, that has higher Tg values and raises the Tg value of feed and the subsequent powder. Maltodextrin with a dextrose equivalent of 6 (DE 6) is the most common drying aid for spray drying of fruit juice.[Citation16,Citation17,Citation19] Maltodextrin with DE6 is preferred for this study. Tg value of DE6 is not available but DE5 is reported to have a Tg value of 188°C.[Citation6]
The Tg value of the amorphous materials is generally measured by Differential Scanning Calorimetry (DSC).[Citation20] The measurement in DSC is based on the change in heat capacity of the material. A static mechanical technique, the Thermal Mechanical Compression Test (TMCT), recently developed by the our group in The University of Queensland, Brisbane, Australia, is also used to measure the Tg or more specifically glass rubber transition temperature (Tg-r) of the spray dried powders. The TMCT is based on the principle that an amorphous sample under compression and temperature ramping suddenly transforms from a glasssy state to rubbery state which can be manifested by a sudden displacement of compression probe.[Citation21,Citation22]
Considering the difficulty in obtaining orange juice powders, the major objective of this study is to determine the amount of maltodextrin required as a drying aid to successfully spray dry the orange juice concentrate. An effort has been made to relate the product recovery with the glass transition temperature of the product. The Tg values of the spray dried orange juice/maltodextrin powders obtained from DSC will be compared with TMCT values.
MATERIALS AND METHODS
Materials
Valencia orange juice concentrate (7:1) manufactured by Orchy™, Australia, was purchased from DKM Consulting Pty., Ltd., Brisbane, Australia. The basic composition of the juice concentration is given in the . The TSS of juice concentrate was further analyzed by Leica AR200 digital refractometer (Leica Microsystem, Inc., New York, USA) and acidity of juice by titrimetric method.[Citation23] Maltodextrin with DE 6 (Glucidex 6) was obtained from Roquette Freres, France.
Table 1 Physiochemical properties of Orchy™ orange juice concentrateFootnote 1
Spray Drying
The total soluble solid (TSS) of the orange juice (OJ) concentrate was measured as 55.7°Brix. Maltodextrin had 7.7% moisture which was taken into consideration during formulation of the mixture. Different proportions of orange juice concentrate (based on TSS) and maltodextrin (on dry basis) at 50:50, 40:60, 35:65, 30:70, and 25:75 (orange juice:maltodextrin), by weight, were chosen for spray drying. A 1000 g of orange juice and maltodextrin solution with 30°Brix was prepared. At first maltodextrin solution was prepared in warm water (about 50°C) with constant stirring followed by addition of measured amount of orange juice concentrate.
Anhydro Lab 1 Rotary Atomizer Spray-dryer (Copenhagen, Denmark) with a co-current air flow was used for spray drying. The speed of atomizer was set at 20,000 rpm for all the drying trials. Distilled water was pumped into the dryer at a set flow rate to achieve the inlet/outlet temperatures of 160°C and 65°C, respectively. The dryer was run at this condition for about 10 min before the feed was introduced. The temperature of feed was maintained at about 50°C. The condition of atmosphere surrounding was 20°C dry bulb, 16°C wet bulb and corresponding relative humidity of 67%. The product was collected in a pre-weighed, insulated glass bottle connected at the end of cyclone collector. The amounts of the powder collected in glass bottle (cyclone recovery) and manually sweeping walls of spray dryer and switching the fan on to collect the powder from the cyclone (sweep recovery) were also recorded. Total recovery was calculated by adding cyclone and sweep recoveries. Powder recovery was chosen as a measure of spray drying performance as it is easily measured with reproducible results.[Citation17] The powders collected from cyclone after spray drying were immediately vacuum packed in Cryovac® plastic bag and stored in a dry chamber containing silica gel. The experiment was repeated twice for each combination of orange juice concentrate and maltodextrin.
Assessment of Physiochemical Properties
The color characteristics (L, a and b) of all spray-dried powders were measured quantitatively by a Chroma Meter CR-400/410 (Konica Minolta Sensing Inc., Tokyo, Japan). The Chroma Meter was calibrated with a white standard plate before actual color measurement. In this system, L value indicates lightness, +a value indicates redness and −a to greenness, +b value indicates yellow and −b to blueness. Five replicate measurements were performed for each sample.
About one gram of orange juice powder was dissolved in 10 g of distilled water and the percent acidity (w/w), as citric acid, was measured by acid-base titration using 0.1N sodium hydroxide.[Citation23] The moisture content of the freshly spray dried powders was measured by AOAC method 927.05.[Citation24] Water activity of the powders was measured by using an AquaLab 3 Water Activity Meter (Decagon Devices, Inc., Pullman, USA) at 25°C.
The particle size and size distribution of the spray dried powders was measured using a Malvern Laser Diffraction Particle Size Analyzer with a 100 mm lens (Malvern Mastersizer B, from Malvern Instruments Co. Worcestshire, UK). Isopropanol was used as a dispersing medium for all powders. Mechanical stirring was applied to ensure better dispersion and particle distribution. Five replicates of each measurement were taken. Bulk density of the powders was determined by weighing 20 g of sample into a 100 ml graduated cylinder. The cylinder with sample was then loosely clamped in a stand and gently dropped 10 times onto a rubber mat from a distance of 15 cm. The volume of the sample was noted and the result is present as g/cm3.
Glass Transition Temperature (Tg)
A Differential Scanning Calorimeter or DSC (Pyris 1 equipped with Intracooler II, Perkin Elmer 7, CT, USA) was used to determine the glass transition temperature (Tg) of all spray dried powders. The purge gas used was dry nitrogen (20 ml/min). Although onset and end values for Tg samples were calculated in each DSC thermogram, only the Tg value determined at half the extrapolated change in specific heat (ΔCp) between the glassy state and the rubbery state were reported in this study. Indium and zinc (Perkin Elmer standards) were used for temperature and heat flow calibration. An empty aluminum pan was used as a reference. Five to ten mg samples were scanned in a hermetically sealed 50 μL DSC aluminum pans (Perkin Elmers). All analyses were done in triplicate. The rate of thermal scanning was carried out in the following order, unless described otherwise: (1) isothermal at −20°C for 1 minute; (2) heat scanning from −20°C to temperature just over predetermined apparent Tg at 10°C/min; (3) cooling rapidly −20°C at 50°C/min; and (4) heat scanning from −20°C to 200°C at 10°C/min.
A heating rate of 10°C was chosen as a standard. A second scanning of sample was used in this method to reduce the enthalpy relaxation of the amorphous powder which appears in the first scan, thereby enhancing the accuracy of Tg measurement on DSC thermogram. The transfer of samples from container to DSC pan was done in a sealed “Dry box” containing silica gel with regular N2 flushing, to avoid moisture absorption by the sample.
Measurement of Glass-Rubber Transition (Tg-r)
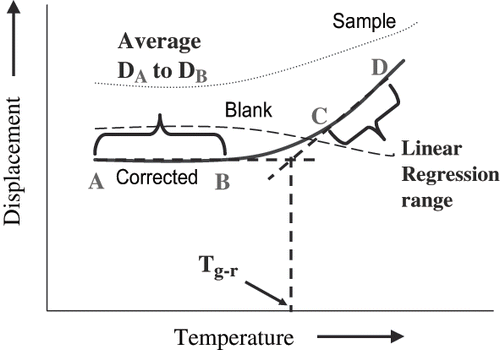
The Thermal Mechanical Compression Test (TMCT) device developed in the School of Land and Food Sciences, The University of Queensland, St. Lucia, Australia, was used to measure the glass rubber transition (Tg-r) of amorphous powders. The principle, design, process optimization and application for food powders in details are given elsewhere,[Citation21] only a brief description is given here. TMCT device consist of a thermally controlled sample cell made up of an aluminum block (50 × 50 × 25 mm) with a round engraved section (37 × 5 mm) to hold the powder. The sample cell was inserted with 4 heating elements, which were connected to the heater controller. Three K type thermocouples were inserted into different sections of sample cell and connected to temperature data logger. The sample holder was connected to a texture analyzer TA-XT2 (Stable Microsystem, UK) fitted with a 35-mm cylindrical aluminum probe. Since the test was based on the thermal scanning and compression of food powder by the probe, the effect of thermal expansion due to sample holder had to be determined. Maltodextrin (DE6) which has a high Tg value (Tg > 180°C), physically and chemically stable, and has characteristics of a typical food powder, was chosen for expansion correction. The test was carried out in relaxation mode (constant force applied). A time of 300s was allowed for the maltodextrin under the probe, at compression force of 50N, to be stabilized before thermal scanning. The sample holder with maltodextrin was heat scanned from 25°C to 180°C at 30°C/min and data was recorded (Blank value). When the amorphous powder was heat scanned, at the glass transition temperature the powder became soft and rubbery causing the downward displacement of probe. The magnitude of expansion by the sample cell (Blank value) was subtracted from the displacement of probe during thermal compression of sample to get the corrected data. The glass rubber transition temperature (Tg-r) was determined by performing linear regression of corrected data (). Linear regression was done at data points at the linear regions below the glass rubber transition temperature (region A to B) and extrapolated above the Tg-r (region C to D). Similarly, extrapolation of linear regression data above Tg-r was performed. The intersection of the two linear lines is taken as the Tg-r point.
Statistical Analysis
Three to five replicate experiments were carried out for each determination. Data were analyzed using the Analysis of Variance (ANOVA) by Statgraphics package (Statistical Graphics Corporation, 1993, Manguistics Inc., USA). The multiple range tests with Least Significant Difference, with significance level 0.05, were applied to the results to test the significance.
RESULTS AND DISCUSSION
Physiochemical Properties of Orange Juice/maltodextrin Powders
The water activity and moisture content of freshly spray dried powders were measured to evaluate the physical condition of the powder. shows the wetness of the powders was high as indicated by water activity values of 0.30–0.40 and moisture content of 4.3–4.5%. The spray drying was carried out at relatively cold and wet condition, 20°C dry and 16°C wet bulb with corresponding RH of 67%. The psychrometric calculation, based on above atmospheric condition and 160/65°C inlet and outlet temperatures, showed outlet air had 29% RH. The spray dried powders were further dried in a vacuum oven at 70°C for overnight and then desiccated in a P2O5 environment for a week to desiccate the powders close to the 0% moisture. The aim of drying was to compare the glass transition temperature and glass-rubber transition temperatures of these powders in anhydrous condition.
Table 2 Moisture and acidity of the orange juice/maltodextrin powdersFootnote 1 , Footnote 2
As mentioned before that the moisture content of all spray dried powders was high. There was no definite trend in change of aw values with the change in the formulation of the products. The moisture content and aw of orange juice:maltodextrin (50:50) was not determined as there was very little product (cyclone) recovered during spray drying. These powders were highly sticky and get caked within 24 h of storage (in vacuum packed plastic bag). The presence of reasonably high amount of highly hygroscopic sugars and organic acids in this juice powder together with high moisture might have rendered the collapse of the structure resulting into the caking of the powder. The orange juice:maltodextrin (50:50) powders obtained from sweep recovery, however, were less sticky and free flowing. Therefore, this powder was considered for further analysis instead of the one obtained from the cyclone collector. All desiccated powders had some residual moisture and aw level as high as 0.1. The aw of powders rich in orange juice were higher as the sugars and acids in juice rich powders are more hygroscopic.
also shows the acidity of the spray dried orange juice/maltodextrin powders. The powders contained significant amount of citric acid and as expected the acidity in the powder increased linearly with increase in the proportion of orange juice concentrate in the mixture. Citric acid due to its highly hygroscopic nature and lower Tg value of 16°C[Citation11] also contribute to the spray drying behavior of the orange juice and maltodextrin mixtures.
Particle Size, Bulk Density and Color Parameters of the Powders
The Sauter mean (volume-surface) diameter was chosen to express the particle size as it is the most commonly used to report the particle size of spray dried powders.[Citation25] The particle size diameter of orange juice powder containing 50% maltodextrin was significantly higher (p > 0.05) than the other 4 combinations (). In fact, there was no significant difference (p > 0.05) in particle size of powders between 60 to 75% maltodextrins that suggests that there is no effect of orange juice or maltodextrin concentration on particles. The 50% orange juice powder contained a high amount of acids and sugars which render the individual particles more hygroscopic acid sticky. The average particle size is almost similar to the orange juice/maltodextrin powders spray dried under similar conditions as reported by Chegini and Ghobadian.[Citation19]
Table 3 Particle size and color characteristics of orange juice/maltodextrin powdersFootnote 1 , Footnote 2
The color of the juice powder is important as it determines the color of the reconstituted juice. The color of orange juice concentrate was yellow, whereas maltodextrin powder was white; therefore, the color profile of these 2 mixtures was expected to differ with change in their proportions. shows a significant (p > 0.05) increase in lightness (L) value when the proportion of maltodextrin in the formulation increased. Obviously, the powder with 50% orange juice solid was the darkest one. There was no significant (p > 0.05) difference in a value for powders with 60 to 75% maltodextrin whereas 50% maltodextrin sample was significantly (p > 0.05) more red. All the powders appeared pale yellow in color as indicated by +b values. The yellowness of the powder gradually but significantly (p > 0.05) increased when the amount of orange juice increased in the mixture. This was also reasonable and coherent with the lightness of powder.
shows bulk densities of orange juice/maltodextrin powders at different ratios. The bulk density of the powders containing 50% maltodextrin was significantly (p > 0.05) higher than the rest of the combinations. This powder contained highest level of soluble sugars and acids. The sticky or less free flowing nature of the powder might have rendered higher bulk density. The bulk density of powders from the present study is lower than spray dried orange juice powders (with maltodextrin, glucose and methylcellulose as additives) as reported by Chegini and Ghobadian.[Citation19] They reported bulk densities of spray dried orange juice ranging from 0.34 to 0.95 g/cm3, depending on the inlet temperature, atomizer speed and feed rate.
Relating Glass Transition Temperature with Spray Drying Recovery
Various studies have shown that the surface stickiness of an amorphous powder is closely related with the glass transition temperature.[Citation7,Citation26,Citation27] Attempts have also been made to develop a direct relationship between glass rubber transition and stickiness during spray drying process.[Citation6,Citation28] In this study, we have investigated the relationship between Tg and recovery of the powder during spray drying. The recovery of the spray dried orange juice and maltodextrin powder in cyclone separator is given in . The cyclone recovery of the powder was very low at 22% when orange juice:maltodextrin ratio was 50:50. In fact no powder was recovered in cyclone separator as the product collected was highly sticky mass and firmly stuck inside the cyclone collector (glass container). A large amount of the orange juice powder, however, was recovered from inside wall of the spray dryer by manual sweeping (67% sweep recovery). Boonyai[Citation29] also reported the cake formation when the freshly spray dried apple juice:maltodextrin (50:50) powder exposed to aw as low as 0.23 (moisture content 3%) at room temperature. Increase in maltodextrin level from 50 to 60 parts resulted in a significant increase in product recovery—up to 78%. Further increase in maltodextrin level only slightly increased in the product recovery, to about 83%. This study suggested that under the given spray drying conditions, the maximum orange juice concentrate can be dried in conjunction with a maltodextrin (DE6) is 40%.
Figure 2 Product recoveries and glass transition temperatures (Tg) of anhydrous orange juice powders containing various percentages of maltodextrin (on dry basis).
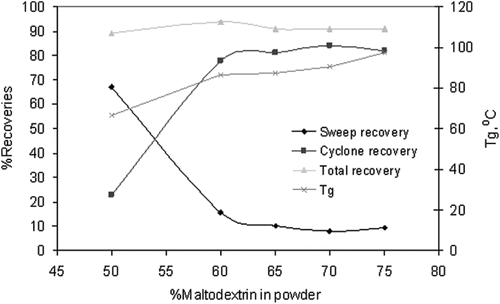
shows the addition of maltodextrin in orange juice significantly increased the Tg values of the amorphous fractions in the mixtures that are rich in low Tg components such as fructose, glucose, citric acid, malic acid etc., however, the increase in Tg was not linear. The Tg value of anhydrous orange juice:maltodextrin (50:50) powder was 66.4°C. The cyclone recovery of this powder was very low which can be explained based on glass transition temperature and out-let air concept. Bhandari et al.[Citation4] reported that the recovery of sugar rich product during spray drying is a direct function of temperature and moisture of the out-let air. Towards the end of the spray drying, the particle temperature may reach close to the outlet air temperature. Stickiness may occur if the Tg value of the materials in particle is less than 20°C of the outlet air. We have found that the powders (caked) containing 50% orange juice and maltodextrin mixture from cyclone had a high moisture content (>4%). Considering a Tg of 66.4°C for anhydrous powder (from sweeping), the product collected at cyclone must have Tg value less than 40°C due to high moisture. Caking had occurred as the outlet air temperature of the spray dryer (65°C) was much higher (>20°C) than the Tg of the product.
Table 4 Glass transition temperature (Tg) and glass-rubber transition (Tg-r) of orange juice/maltodextrin powdersFootnote 1 , Footnote 2 , Footnote 3
Initially, there was huge jump in Tg from 66.4 to 86.4°C with only 10% increase in maltodextrin concentration (from orange juice:maltodextrin 50:50 to 40:60). Further addition of maltodextrin in the mixture, however, failed to achieve such a rise in Tg value, only about 11°C raise in Tg value when maltodextrin increased to 15% level (60 to 75 part). Roos and Karel[Citation6] reported that the Tg values of the binary system (maltodextrin/sucrose) increases with increasing maltodextin concentration, however, the increase is not linear and largely depend on the concentration each component. shows the increase in cyclone recovery of the powders with increase in Tg values. There was a big jump in Tg value of powders when the concentration of maltodextrin increased from 50 to 60% which is accompanied by sudden jump in cyclone recovery from 23 to 78%. Further increase in maltodextrin did not increase the recovery due to plateau effect: the spray dried powders attained glass transition temperature much higher than outlet temperature of the spray dryer.
Bhandari et al.[Citation17] reported a successful spray drying of pineapple juice: maltodextrin (DE6) with a recovery of 59%. Like pineapple juice, citric acid is the major (0.6%, w/v) and malic acid (0.13% w/v) is the minor type of organic acid present in pineapple juice.[Citation30] The higher amount of citric acid present in concentrated orange juice, about 5.25–6.25% (w/v) compared to 3.5% (w/v) in concentrated pineapple juice as reported by Bhandari et al.,[Citation17] may have hindered the spray drying recovery of the orange juice in present study. Bhandari et al.[Citation16] reported the maximal fruit juice/maltodextrin ratio for successful spray drying of blackcurrant, apricot and raspberry are 65/35, 60/40 and 55/45, respectively. They also found that besides the amount of drying aid, the recovery of the powder is largely affected by the inlet/outlet temperatures, drying chamber temperature, solid concentration in feed, DE of maltodextrin, type of sugar and acidity of the juice.
Glass-Rubber Transition (Tg-r)
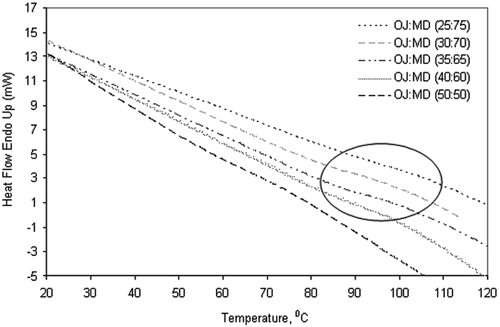
At glass transition temperature, the free volume and the mobility of the molecules in the amorphous matrix increase rapidly. DSC measures the endothermal change in the apparent specific heat during this stage.[Citation31] Unlike smaller molecules, in the large molecules this endothermal change cannot be detected easily, particularly in anhydrous state.[Citation22] However, water which has plasticization effect on all amorphous materials lowers the Tg value and facilitates the measurement of Tg.[Citation8] In this study the increase in maltodextrin proportion from 60 to 75% in orange juice/maltodextrin mixture showed relatively lesser increase in Tg value compared to 50 to 60% increase in maltodextrin (). Considering very high Tg of anhydrous DE6, higher Tg (than 97°C) was expected of anhydrous orange juice:maltodextrin mixture (25:75). Roos and Karel[Citation6] predicted a Tg value of about 130°C for maltodextrin:sucrose mixture (75:25), considering Tg values of 188 and 57°C for anhydrous maltodextrin and sucrose, respectively. Based on this, it is considered that DSC may have under-estimated actual Tg value of the orange juice and maltodextrin mixtures. Therefore, the glass transition behavior of this system was also studied by the thermal mechanical compression test (TMCT) method as glass-rubber transition temperature (Tg-r).
Figure 3 DSC thermogram showing the glass transition temperatures (Tg) of spray dried orange juice (OJ) and maltodextrin (MD) powders at various proportions (circle shows lesser increase in Tg value of OJ:MD mixtures at ratios from 40:60 to 25:75, as compared to that of 50:50).
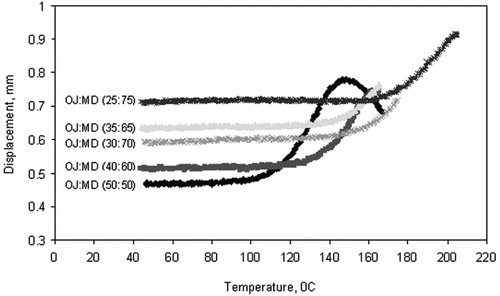
compares the Tg-r values measured by TMCT and Tg values from DSC for all the powders. The result is also presented in as temperature versus probe displacement plot. As expected, the Tg-r values were relatively higher than the Tg value (DSC). DSC measures the sudden change in enthalpy and heat capacity when an amorphous solid is in the state of transition towards the rubbery state. Whereas TMCT detects the exact point where mechanical change has taken place due to transition of the material into rubbery state. It is based on the surface property of the test material and giving the value closest to the stickiness point. The flow of material or stickiness occurs at temperatures several degrees higher than the Tg, a phenomenon, also reported by several other researchers.[Citation3,Citation4,Citation6] This study shows that TMCT gives better estimation of stickiness point than DSC. Our previous study showed that TMCT (Tg-r) and DSC (Tg) measurements give glass transition temperatures of 117°C and 97.8°C for lactose, 137°C and 98°C for lactose:hydrolyzed whey protein isolate (HWPI) (2:3) and 145°C and 93°C for skim milk powder, respectively.[Citation22,Citation32] The Tg-r value for lactose was about 20°C higher than Tg value. This means TMCT gives a value very close to the stickiness point of lactose, Tg + 20°C, as recommended by Roos and Karel.[Citation6] However, incorporation of protein in lactose (or milk powder in general) remarkably increases the TMCT (Tg-r) value of the system whereas DSC (Tg) value remained very close to lactose. These results showed that DSC does not accurately measures the effect of proteins on the glass transition temperature of the lactose.
Figure 4 TMCT thermogram showing the increasing trend of glass-rubber transition temperatures (Tg-r) of spray dried orange juice (OJ) and maltodextrin (MD) powders with increase in MD concentration.
The difference between two methods of glass transition temperatures found to increase with increasing maltodextrin content of the orange juice powder, ranging from 40 to 64°C. DSC gives better estimation of Tg values when the material has higher sugar and acid concentrations. However, at higher maltodextrin concentration, the ability of DSC to accurately measure the Tg is diminished as less defined enthalpic shift takes place in macromolecules. Besides the endothermic relaxation peak associated with Tg became broader with increase in maltodextrin concentration in the mixtures (). Similar peak broadening was noticed when protein concentration increased in hydrolyzed whey protein isolate and lactose mixtures.[Citation22] It is likely that the Tg value of the mixture might have been underestimated by DSC when maltodextrin concentration was high. The TMCT gave almost equal response to both low and high maltodextrin system. This study showed that TMCT could measure the phase transition behavior of amorphous system that has high molecular weight component.
During a thermal compression test, the relaxation of the sugars and acids due to phase transition is not clearly detected as it is absorbed into the larger protein or maltodextrin molecules. The Tg-r values of the orange juice powders ranged from 106°C for 50% maltodextrin to 161°C for 75% maltodextrin containing juice powder. The Tg-r value of the orange juice powder appeared to be largely contributed by maltodextrin component. The TMCT found to give highly repeatable results within 2°C, and the detection of transition is very easy and less subjective than DSC. Besides, it is easy to construct and much cheaper than DSC. Although TMCT is highly promising instrument for characterization of amorphous powders, further study is needed to conclude the effect of high molecular compounds on Tg-r and Tg values of sugars. The compatibility of the two (or more) components in the mixture and their rate of change in heat capacity during heat scanning or mechanical change during compression are two major issues when computing glass transition temperature.
CONCLUSIONS
An optimum concentration of drying aid as maltodextrin (DE6) to spray dry orange juice concentrate and their relationship with glass transition temperatures (Tg) was established. The use of 50% maltodextrin did not help in spray drying of the orange juice and as the product obtained was highly sticky. The product recovery increased with increasing maltodextrin concentration (>60%) suggesting that such a level of additive addition is required under the drying conditions used in our study to have a successful spray drying. The moisture contents of the spray dried products were relatively high, about 4.5% which may warrant increase in outlet temperature of spray dryer. Maltodextrin decreased the lightness, redness and yellowness parameters of the orange juice. The use of maltodextrin significantly increased the Tg values of the powders which are positively correlated to the product recovery. Thermal Mechanical Compression Test (TMCT) gave much higher Tg values compared to DSC suggesting phase transition data is a function of the measuring technique.
REFERENCES
- Brennan , J.G. , Herrera , J. and Jowitt , R. 1971 . A Study of Some of the Factors Affecting the Spray Drying of Concentrated Orange Juice, on a Laboratory Scale . J. Food Technol. , 6 : 295 – 307 .
- Papadakis , S.E. and Bahu , R.E. 1992 . Sticky Issue of Drying . Drying Technol. , 10 : 817 – 837 .
- Chuy , L.E. and Labuza , T.P. 1994 . Caking and Stickiness of Dairy-based Food Powders as Related to Glass Transition . J. Food Sci. , 59 : 43 – 46 .
- Bhandari , B.R. , Data , N. and Howes , T. 1997 . Problems Associated with Spray Drying of Sugar-rich Foods . Drying Technol. , 15 : 671 – 684 .
- Levine , H. and Slade , L. 1990 . “ Cryostabilization Technology: Thermoanalytical Evaluation of Food Ingredients and Systems in Thermal Analysis of Foods ” . In Thermal Analysis of Foods , Edited by: Ma , C.Y. and Hawalkar , V.R. London : Elsevier Applied Science .
- Roos , Y. and Karel , M. 1991 . Phase-transitions of Mixtures of Amorphous Polysaccharides and Sugars . Biotechnol. Prog. , 7 ( 1 ) : 49 – 53 .
- Downton , G.E. , Flores-Luna , J.L and King , C.J. 1982 . Mechanism of Stickiness in Hygroscopic Amorphous Powders . Indust. Eng. Chem. Funda. , 21 : 447 – 451 .
- Roos , Y. and Karel , M. 1991 . Plasticizing Effect of Water on Thermal-behaviour and Crystallization of Amorphous Food Models . J. Food Sci. , 56 : 38 – 43 .
- Roos , Y and Karel , M. 1991 . Water and Molecular-weight Effects on Glass Transitions in Amorphous Carbohydrates and Carbohydrate Solutions . J. Food Sci. , 56 : 1676 – 1681 .
- Roos , Y. 1993 . Melting and Glass Transition of Low Molecular Weight Carbohydrates . Carbohydrate Res. , 238 : 39 – 48 .
- Troung , V. 2003 . Modelling of the Glass Transition Temperature of Sugar-Rich Foods and Its Relation to Spray Drying of Such Products , Brisbane, , Australia : PhD Thesis. The University of Queensland .
- Adhikari , B. , Howes , T. , Bhandari , B.R. and Truong , V. 2003 . Characterization of the Surface Stickiness of Fructose–maltodextrin Solutions during Drying . Drying Technol. , 21 : 17 – 34 .
- Lazar , W.E. , Brown , A.H. , Smith , G.S. , Wong , F.F. and Lindquist , F.E. 1956 . Experimental Production of Tomato Powder by Spray Drying . Food Technol. , : 129 – 134 .
- Mirzahi , S. , Berk , Z and Motoki , M. 1967 . Effect of Crystallinity on the Glass Transition Temperature of Starch . Cereal Science Today , : 322 – 325 .
- Rao , R.H.G. and Gupta , P.M. 2002 . Development of Spray Dried Orange Juice Blend Skim Milk Powder . Lait , 82 : 523 – 529 .
- Bhandari , B.R. , Senoussi , A. , Dumoulin , E.D. and Lebert , A. 1993 . Spray Drying of Concentrated Fruit Juices . Drying Technol. , 11 : 1081 – 1092 .
- Bhandari , B.R. , Datta , N. , Crooks , R. , Howes , T. and Rigby , S. 1997 . A Semi-empirical Approach to Optimize the Quantity of Drying Aids Required to Spray Dry Sugar Rich Foods . Drying Technol. , 15 : 2509 – 2525 .
- Karatas , S. and Esin , A.A. 1990 . A Laboratory Scraped Surface Drying Chamber for Spray Drying of Tomato Paste . Lebensm Wiss Technol. , 23 : 354 – 357 .
- Chegini , G.R. and Ghobadian , B. 2005 . Effect of Spray-drying Conditions on Physical Properties of Orange Juice Powder . Drying Technol. , 23 : 657 – 668 .
- Roos , Y.H. 1995 . Phase Transitions in Foods , San Diego : Academic Press .
- Boonyai , P. and Bhandari , B. 2006 . Application of Thermal Mechanical Compression Tests in Food Powder Analysis . Int. J. Food Prop. , 9 : 127 – 134 .
- Shrestha , A.K. , Adhikari , B. , Howes , T. and Bhandari , B. 2005 . In Glass Transition and Crystallization Behavior of Lactose in Spray Dried Mixtures of Hydrolyzed Whey Protein and Lactose . 4th Asia Pacific Drying Conference . Dec. 13–15 2005 , Kolkata, India. pp. 1118 – 1129 .
- Kimball , D.A. 1991 . Citrus Processing-quality Control and Technology , New York : Van Nostrand Reinhold .
- AOAC International . 1990 . “ Official Methods of Analysis of AOAC ” . Gaithersberg, MD : AOAC International .
- Masters , K. 1991 . Spray Drying Handbook , 5th , New York : Longman Scientific & Technical .
- Lloyd , R.J. , Chen , X.D. and Hargreaves , J.B. 1996 . Glass Transition and Caking of Spray-Dried Lactose . Int. J. Food Sci. and Technol. , 31 ( 4 ) : 305 – 311 .
- Chung , M.S. , Ruan , R.R. , Chen , P. , Chung , S.H. , Ahn , T.H. and Lee , K.H. 2000 . Study of Caking in Powdered Foods Using Nuclear Magnetic Resonance Spectroscopy . J. Food Sci. , 65 ( 1 ) : 134 – 138 .
- Bhandari , B.R. and Howes , T. 1999 . Implication of Glass Transition for the Drying and Stability of Dried Foods . J. Food Eng. , 40 ( 1–2 ) : 71 – 79 .
- Boonyai , P. 2005 . “ Development of New Instrumental Techniques for Measurement of Stickiness of Solid Particulate Food Materials ” . Brisbane, , Australia : PhD Thesis. The University of Queensland .
- Campo , G.D. , Berregi , I. , Caracena , R. and Santos , J.I. 2006 . Quantitative Analysis of Malic and Citric Acids in Fruit Juices Using Proton Nuclear Magnetic Resonance Spectroscopy . Analyt. Chim. Acta. , 556 ( 2 ) : 462 – 468 .
- Roos , Y.H. and Karel , M. 1990 . Differential Scanning Calorimetry Study of Phase Transition Affecting the Quality of Dehydrated Materials . Biotech. Prog. , 6 : 159 – 163 .
- Shrestha , A.K. , Adhikari , B. , Howes , T. and Bhandari , B. in press . Spray Drying of Lactose Hydrolyzed Skim Milk and Study of Water Absorption and Glass Transition Temperature Properties of the Powder . Lebensm Wiss Technol. – Food Science and Technology , DOI: 10.1016/j.lwt. 2006.11.003.