Abstract
A standardized red orange extract (Red Orange Complex, ROC) has been evaluated for its potential protective effect on ethanol- and indomethacin-induced gastric lesions in rats. Oral administration of the ROC significantly prevented the development of gastric lesions in these experimental models. In addition, the effect of the ROC on the spontaneous healing of acetylsalicylic acid (ASA) ulcers in rats was examined. The spontaneous healing of gastric ulcer was significantly accelerated by repeated administration of the ROC. The histological condition of the mucosa, examined by scanning electronic microscopy (SEM), confirmed the in vivo data. The results, on the whole, indicate that the ROC possesses favourable antiulcer and cytoprotective properties in rat.
This study was performed according to the international principles for laboratory animal use and care (EEC Directive of 1986; 86/609/EEC).
INTRODUCTION
Gastric acid secretion is a complex, continuous process controlled by multiple central and peripheral factors. Each factor contributes to a common final event, the secretion of H+ by parietal cells. Neuronal, paracrine, and endocrine factors play important roles in the regulation of acid secretion. The stomach protects itself from damage of gastric acid through several mechanisms such as the presence of intercellular tight junctions between the gastric epithelial cells, the production of prostaglandins in the gastric mucosa, and the secretion of bicarbonate ions into the mucin layer. Prostaglandins E2 and I2 inhibit gastric acid secretion by direct effect on parietal cell mediated by EP3 receptors. In addition, prostaglandins enhance mucosal blood flow and stimulate secretion of mucus and bicarbonate.[Citation1,Citation2,Citation3]
It is generally accepted that gastric ulcer is due to an imbalance between mucosal defense factors (bicarbonate, mucin, prostaglandins, nitric oxide, other peptides, and growth factors) and aggressive factors (acid and pepsin). Although patients with gastric ulcers have normal or even lower acid production than control subjects, ulcer rarely, if ever, occurs in the complete absence of acid. In these gastric ulcer patients, even the low levels of acid can produce injury, presumably due to a weakened mucosal defense and a reduced bicarbonate production. Both Helicobacter pylori (H. pylori) and exogenous agents, such as nonsteroidal antinflammatory drugs (NSAIDs), interact with these factors in complex ways, leading to an ulcer diathesis. The H. pylori infection may lead to impaired production of somatostatin by gastric D cells resulting in increased gastrin production, increased acid output as well as impaired duodenal bicarbonate production. The effects of NSAIDs are mediated systemically, and vertical element is the suppression of the constitutive form of selective cyclooxygenase COX-1 in the mucosa and the consequent reduction in cytoprotective prostaglandins.[Citation4] Impressive advances have been made in the pharmacological treatment of acid-peptic disorders. These progresses have been made possible largely by the availability of the proton pump inhibitors and the discovery of H. pylori and its role in acid-peptic disorders.[Citation5] Another, even if indirect, contribution has been provided by the new selective COX-2 inhibitors, which are expected to dissociate antiphlogistic activity from gastrointestinal toxicity of NSAID-induced ulcers.[Citation6] New drug discovery in this area may overcome diarrhea, headache, drawsiness, fatigue, and muscular pain produced by H2-receptors antagonists;[Citation7] diarrhea with or without abdominal pain produced by misoprostol, constipation and alluminum overload by sucralfate; decreased absorption of vitamin B12, nausea, abdominal pain, constipation, myopathy, arthralgias, headaches by proton pump inhibitors.[Citation8,Citation9,Citation10,Citation11,Citation12,Citation13]
For centuries, several plants containing polyphenols (particularly flavonoids) have been used to treat gastrointestinal disorders, including gastric ulcer.[Citation14] In fact, a number of flavonoids is able to protect against gastric damage induced in different experimental models by various necrotizing agents. Three mechanisms have been suggested to be involved in their protective action.[Citation15] Flavonoids are good antioxidant agents, and oxidative stress plays a role in ulcer formation. Moreover, some flavonoids have shown to increase the mucosal content of prostaglandins. Finally, flavonoids can preserve capillary integrity, that is vital to the normal function of mucous membranes. The aim of this article was to evaluate if a standardized extract of red orange juice (Red Orange Complex, ROC) possesses a gastroprotective effect in rat models of gastric ulcer. Several experimental models were selected: absolute ethanol-induced gastric lesions (with or without subcutaneous (s.c.) indomethacin pretreatment), indomethacin-induced gastric erosions, acetylsalicylic acid (ASA)-induced ulcer. The ulcer index was evaluated and the ultrastructure modifications of gastric mucosa were observed by scanning electronic microscopy (SEM). The antiulcer activity of the ROC was compared with that of two well-known antiulcer drugs: sucralfate and ranitidine.
Experiments were carried out in compliance with the ethical provisions enforced by the European Union (1986; 86/609 CEE) and authorized by the National Committee of the Italian Ministry of Health for in vivo experimentation.
MATERIAL AND METHODS
Drugs
Absolute ethanol, sucralfate, indomethacin, ranitidine, glutaraldehyde, sodium cacodylate, and osmium tetroxide were purchased from Sigma (Milan, Italy). Acetylsalicylic acid was purchased from Fluka (Milan, Italy). The ROC was kindly supplied by Bionap (Rome, Italy). It was obtained from the juice of three pigmented Citrus sinensis varieties (Moro, Tarocco, Sanguinello) with the following polyphenolic composition: anthocyanins (cyanidin-3-glucoside) 3.1%, hydroxycinnamic acids (caffeic, cumaric, ferulic, and sinapic acid) 2.07%, flavanone glycosides (narirutin, hesperedin) 8.1% and ascorbic acid 5.0%. Briefly, the juice was filtered using 0.2 μm paper filter in order to remove any impurities and then was applied to an XAD-16 column (Rohm and Haas, Philadelphia, PA, USA). The resins were eluted with an ethanol/water solution (50:50) then ethanol was removed by evaporation and the aqueous residue was spray-dried. The dried extract, stored at 4°C in the dark, was freshly dissolved in distilled water just before administration to the animals (0.2–0.4 g kg−1 b.w.).
Animals
Adult Sprague-Dawley rats (Rattus norvegicus, Harlan Italia, Milan, Italy) of both sex with approximately the same age, weighing 180 ± 20 g, were used after acclimatisation for a week in standard conditions (23 ± 2 °C; 60% humidity; 12 hours light-dark cycle) and fed ad libitum on a standard diet (MIL GLP, Morini diets, Italy). The animals were allocated into groups of five rats each. The distribution of animals in groups and the treatment were all randomized. Twenty-four hours prior ulcer induction, the animals were deprived of food and kept in cages with raised mesh bottoms to prevent coprophagia; they were allowed free access to drinking water until 2 hours before the experiments.
Acute and Subacute Toxicity
Acute toxicity of the ROC was evaluated in male and female rats (5 rats/group) according to the method of Lorke.[Citation16] Doses ranging from 0.5 g kg−1 to 4 g kg−1 were given to the animals as single dose p.o. Control animals received the same volume of distilled water (10 ml kg−1 b.w.). The animals were observed over a forty-eight hour period for the onset of clinical or toxicological symptoms. Mortality, if any, was determined over a period of 14 days.
Subacute toxicity of the ROC (0.4 g kg−1 p.o. daily for 30 days) was evaluated in male and female rats (5 rats/group) according to the method of Lu and Kacew.[Citation17] The criteria for toxic effects were mortality, body weight gain, relative liver and kidney weights.
Ethanol-induced Gastric Lesions, Drug Treatment, and Macroscopic Examination
The experiments were performed according to the method of Robert et al.[Citation18] The ROC (0.2–0.4 g kg−1) or sucralfate (0.7 g kg−1) were given p.o. 1 hour before the oral administration of absolute ethanol (1 ml/rat). Control animals received the same volume of vehicle (distilled water; 10 ml kg−1 b.w.). The rats (5 rats/group), anaesthetized with pentobarbital, and killed 1 hour after the necrotizing agent administration. The stomach of each animal was immediately removed, opened along the greater curvature and rinsed with physiological saline; then, it was pinned on plastic polymer plates, and macroscopically examined for the presence of lesions in the glandular portion. In general, such stretching of the stomach does not seriously affect the size of ulcers. The ulcerations was measured by an observer unaware of which treatment the animals received. The various specimens were evaluated with a binocular magnifier. The length (mm) of each lesion was measured; the lesion index (LI) of each stomach was the sum of all lesion lengths arbitrarily divided into 5 score degrees,[Citation19] where: 1 = 1–50 mm; 2 = 51–100 mm; 3 = 101–150 mm; 4 = 151–200 mm; 5 > 201 mm. The inhibition rate was calculated as follows:
Ethanol-induced Gastric Lesions in Indomethacin-pretreated Rats: Determination of the Role of Prostaglandins in the Cytoprotection Afforded by the ROC
To study the role of endogenous prostaglandins on ethanol-induced gastric lesions, the animals were randomly divided in three groups (5 rats/group). Indomethacin (suspended in 1% carboxymethylcellulose into distilled water) was administered s.c. (0.01 g kg−1; 1 ml kg−1 b.w.) followed 30 minutes later by the respective oral treatment (distilled water and ROC 0.2–0.4 g kg−1; 10 ml kg−1 b.w.), and after a further 60 minutes by absolute ethanol (1 ml/rat p.o.).[Citation20] The animals were sacrificed 1 hour later and the stomach was removed and subjected to the same procedures above described. The ulcerative lesion index (LI) was calculated according to EquationEq. (1).[Citation19]
Indomethacin-induced Gastric Ulcers, Drug Treatment, and Macroscopic Examination
The ulcerogenic procedure described by Lee et al.[Citation21] was employed with light modifications. Indomethacin was suspended in 1% carboxymethylcellulose in distilled water. The ROC (0.2–0.4 g kg−1) or ranitidine (0.1 g kg−1) were administered p.o. 1 hour before indomethacin (0.05 g kg−1, 1 ml kg−1 b.w. s.c.). Control animals received the same volume of vehicle (distilled water; 10 ml kg−1 b.w.). The rats (5 rats/group), anaesthetized with pentobarbital, were killed 5 hours after the necrotizing agent; the stomach was removed and underwent the same procedures described previously. Each gastric ulcer was rated on an arbitrary 0 to 6 point scale.[Citation22] This scale takes into consideration both number and size (mm) of the ulcers, where: 0 = no ulcer; 0.5 = punctiform ulcer (Ø ≤ 2 mm) or filiform ulcer (length ≤ 5 mm); 2 = punctiform ulcer (Ø > 2 mm ≤ 5 mm) or filiform ulcer (length > 5 mm ≤ 10 mm); 4 = punctiform ulcer (Ø > 5 mm ≤ 10 mm) or filiform ulcer (length > 10 mm); 6 = punctiform ulcer (Ø > 10 mm). The ulceration index (UI) of each stomach was the sum of its scores. The inhibition rate was calculated as follows:
Ulcer Healing Studies: Acetylsalicylic Acid (ASA)-induced Ulcer in Rat, Drug Treatment, and Macroscopic Examination
To examine the effect of the ROC on spontaneous ulcer healing, acetylsalicylic acid (ASA, dissolved in 2 ml 150 mM HCl) was suspended in 1% carboxy methyl cellulose (CMC)/distilled water, and was administered orally to rats at the dose of 0.2 g kg−1 body weight (10 ml kg−1 b.w.) for 3 consecutive days to induce ulcer in the experimental animals. The extract was orally given to rats at the doses of 0.2 and 0.4 g kg−1 once a day (9:00 AM) for 7 consecutive days beginning on the 4th day. The same volume of distilled water, used as a vehicle, was orally administered to rats once a day for the same period (10 ml kg−1 b.w.).[Citation23] The rats were randomly divided in five groups of five animals each, according to the respective treatment ():
-
Group I (vehicle) received distilled water (10 ml kg−1 b.w. p.o.) once daily, for 10 consecutive days. On 11th day rats were sacrificed;
-
Group II (ASA) received ASA (0.2 g kg−1 b.w., p.o.) once daily, for 3 consecutive days. On 4th day rats were sacrificed;
-
Group III (ASA + vehicle) received ASA (0.2 g kg−1 b.w., p.o.) once daily, for 3 consecutive days and vehicle (distilled water; 10 ml kg−1 b.w., p.o.) for the following 7 consecutive days. On 11th day rats were sacrificed;
-
Group IV (ASA + ROC 0.2 g kg−1 ) received ASA (0.2 g kg−1 b.w., p.o.) once daily for 3 consecutive days and the ROC (0.2 g kg−1 b.w., p.o.) for the following 7 consecutive days. On 11th day rats were sacrificed;
-
Group V (ASA + ROC 0.4 g kg−1 ) received ASA (0.2 g kg−1 b.w., p.o.) once daily for 3 consecutive days and the ROC (0.4 g kg−1 b.w., p.o.) for the following 7 consecutive days. On 11th day rats were sacrificed.
Table 1 Ulcer healing study protocol
The animals were maintained on normal pellet diet and water was provided ad libitum. Twenty four hours after the last treatment, the animals were sacrificed by means of penthobarbital administration. The abdomen was cut open through a midline incision; the stomach was cut, opened along the greater curvature and the length of each ulcer was measured and scored on an arbitrary 0–6 point scale (2).[Citation22] The ulcer index (UI) of each stomach was the sum of its scores.
Histological Examination
Histological analysis was carried out to determine the effects of the ROC (0.4 g kg−1) on ulcerated damaged gastric mucosa. After macroscopic examination of the stomach, tissue samples were harvested in lesion sites and were prepared for scanning electronic microscopy (SEM). The tissues were fixed in 2.5% glutaraldehyde prepared in 0.1 M cacodylate buffer at room temperature (pH 7.3) added with 2% sucrose overnight at 4°C. After 2 hours, the samples were postfixed in 1% osmium tetroxide (OsO4) in sodium cacodylate buffer (0.1 M) for an additional 1 hour. Samples were dehydrated through graded ethanol concentrations, critical point-dried in CO2 (CPD 030 Bal-Tec device, Balzers, Liechtenstein) and gold-coated by sputtering (SCD 040 Bal-Tec device). The samples were examined with a Cambridge Stereoscan 360 scanning electron microscope (Cambridge Instruments, Cambridge,U.K.).
Statistical Analysis
Data are expressed as mean ± SEM per each experimental group and analyzed using Kruskal-Wallis followed by Dunn's test (Statistical Software SigmaStat 2.03, Jandel Scientific Software Corporation, Chicago IL, USA). A P value < 0.05 was considered statistically significant.
RESULTS
Acute and Subacute Toxicity
No deaths or any toxic symptoms were recorded up to 4 g kg−1 b.w. of the ROC in the observation period (). Moreover, no deaths or any toxic symptom were recorded up to 30 days of treatments with 0.4 g kg−1. These data demonstrated that the ROC may be considered non-toxic.
Table 2 Acute oral toxicity in rats
Experimental Gastric Ulcers
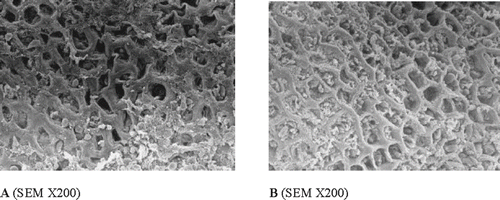
The intragastric ethanol administration produced extensive necrotic hemorrhagic lesions (more or less thickened) in the glandular portion of the mucosa, but little, if any effect was observed in the forestomach mucosa (). Oral pretreatment with the ROC (0.4 g kg−1) significantly reduced the extent of ethanol-induced mucosal necrosis and maintained the rats stomach mucosa in a nearly normal status. In fact, in animals pre-treated with the ROC, a significant inhibition of EtOH mucosal injury was detected (LI = 3 ± 0.58 in control group; LI = 1 ± 0.82 in the ROC 0.4 g kg−1 pretreated rat group; P < 0.05). It is remarkable that, at the dose of 0.4 g kg−1, the protection against ethanol injury produced by ROC was greater than that induced by 0.7 g kg−1 of sucralfate, a clinically effective drug (). This protective effect was confirmed by scanning electron microscopy (SEM) that showed significant epithelial damage in EtOH group and minimal damage in ROC group ().
Figure 1 Gross anatomy of gastric mucosa: (A) normal rat, (B) EtOH-treated rat, and (C) indomethacin-treated rat.
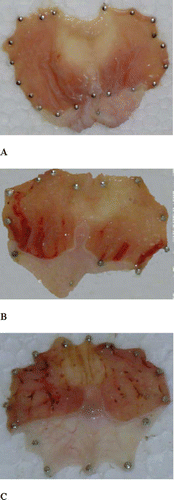
Table 3 Effect of the ROC and sucralfate on absolute EtOH-induced gastric lesions in rats
Figure 2 Effect of the ROC (0.4 g kg−1 b.w.; B) on absolute EtOH-induced gastric mucosal lesions (A) in rats. The extract proved to exert a partial protective effect versus ethanol-induced ulcer. In fact, the gastric epithelium showed a quite preserved morphology even though inflammatory infiltrates were visible (B).
The protective activity of the ROC was considerably reduced in ethanol induced ulcer model with previous administration of indomethacin (inhibition of the ulcerative lesion index only by 22.5%) (). Moreover, in indomethacin-pretreated rats, SEM did not revealed marked differences between the experimental groups, indicating that inhibition of prostaglandin synthesis by indomethacin reduced the preventive efficancy of the ROC in the ethanol ulcer ().
Figure 3 Effect of the ROC (0.4 g kg−1 b.w.; B) on the ulceration induced by absolute EtOH (A) in indomethacin-pretreated rats. When rats were treated with both indometacine and ethanol (A), the gastric mucosa showed wide areas of remarkable alterations with loss of the epithelial layer, exhibition of the lamina propria, and presence of inflammatory infiltration. SEM did not reveal marked differences between the experimental groups (B).
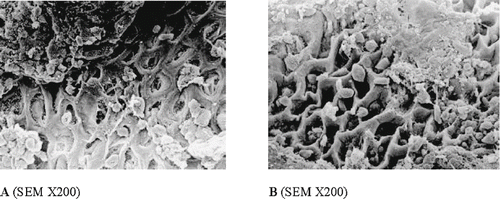
Table 4 Effect of the ROC on the ulceration induced by absolute EtOH in indomethacin-pretreated rats
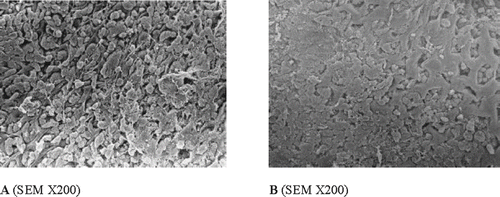
Subcutaneous administration of indomethacin (0.05 g kg−1) resulted in the production of punctiform and filiform gastric ulcers (UI = 27.1 ± 3.64), predominantly in the glandular portion of the stomach and few or none in the antrum (). The pretreatment of rats with the ROC (0.4 g kg−1) significantly protected against the development of indomethacin-induced gastric ulcers (UI = 13.5 ± 1.08). The effect of ROC was comparable with that of ranitidine, which, however, showed the highest level of gastric protection (). This protective effect was confirmed by SEM () that showed a good mucosal morphology of gastric tissue in the ROC pre-treated group.
Table 5 Effect of the ROC and ranitidine on indomethacin-induced gastric ulcers in rats
Figure 4 Effect of the ROC (0.4 g kg−1 b.w.; B) on indomethacin-induced gastric ulcers (A) in rats. In the control group, scanning electron micrograph (A) indicates that surface topography was extremely disrupted with epithelial desquamation In the ROC-treated group (B), scanning electron micrograph indicates a normal topography with very slight degree of epithelial degeneration.
In a subset of experiments we examined the effect of the ROC on spontaneous ulcer healing. Treatment with the ROC (Group IV–V) produced a significant decrease in the extension of ulceration produced by ASA (). The UI after 3 days of ASA administration (Group II; ASA) was 15 ± 3.3. The UI of control rats (Group III; ASA + vehicle), significantly decreased to 4.2 ± 0.5 the following 7 days because the ulcers tend to heal naturally. The UI after 7 days of treatment with the ROC was 4.1 ± 0.5 (Group IV; ASA + ROC 0.2 g kg−1) and 1.6 ± 1.5 (Group V; ASA + ROC 0.4 g kg−1). SEM confirmed in vivo results on gastric ulcers induced by aspirin (). In fact, in the group treated with the ROC focal areas of disepithelization, with preservation of normal morphology were evident. Only limited and modest signs of inflammation could be detected. These data demonstrated that ROC accelerated the healing of ASA induced ulcers after the treatment for 7 days.
Table 6 Effect of the ROC (0.2–0.4 g kg−1 b.w., p.o.) on gastric ulcers induced by aspirin (ASA; 0.2 g kg−1 b.w., p.o.) in rats
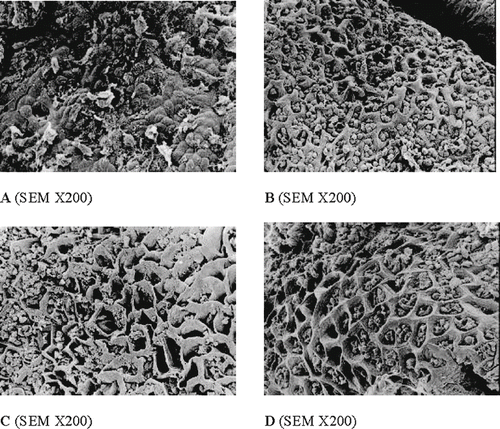
Figure 5 Effect of the ROC (0.4 g kg−1 b.w., p.o.) on gastric ulcers induced by aspirin (ASA; 0.2 g kg−1 b.w., p.o.) in rats. A: vehicle (distilled water) for 10 days; B: ASA for 3 days; C: ASA for 3 days and vehicle (distilled water) for the following 7 days; and D: ASA for 3 days and the ROC for the following 7 days. In the group treated with the ROC (D) focal areas of disepithelization, with preservation of normal morphology were evident.
DISCUSSION
Rat gastric mucosal damage induced by ethanol and indometachin has widely been used to investigate gastroprotective effect of medicinal plants. In these models, cytoprotection is assessed in terms of the absence or reduction in macroscopically visible lesions. The results confirm that both necrotizing agents, EtOH and indomethacin, produced severe gastric damage. The extensive gastric damage induced by EtOH is related to an oxidant action, increase lipid peroxidation and generation of free-radicals.[Citation24] The mechanisms of indomethacin-induced stomach ulcers include inhibition of prostaglandin biosynthesis, reduction in gastric mucosal blood flow, and interference with restitution and tissue repair.[Citation25] Healing of gastric ulcers is a very complex event and involves epithelial cell migration and proliferation, reconstruction of gastric glands, angiogenesis, and production of granulation tissue.[Citation26] Various endogenous factors are known to contribute to ulcer healing, including prostaglandins,[Citation27] nitric oxide,[Citation28] as well as several growth factors.[Citation29,Citation30] Acetylsalicylic acid is a well known breaker of the gastric mucosal barrier,[Citation31] the administration of this NSAID resulted in the production of gastric ulcers mainly in the glandular segment of the stomach. The reason is attributed principally to inhibition of biosynthesis of ‘cytoprotective prostaglandins’, by inhibition of cyclooxygenase pathway of arachidonic acid metabolism, resulting in overproduction of leukotrienes and other products of 5-lipoxygenase pathway.[Citation32] 100% of the animals in the ASA group showed gastric ulceration and the majority of the ulcers was gastric erosion, that is multiple, dotted or elongated superficial haemorrhagic mucosal lesion that do not penetrate the muscularis mucosae.
The present study demonstrates that the ROC, with the well-known antioxidant polyphenols, exhibits both gastric cytoprotective and gastric ulcer healing actions. Moreover, the ROC is effective against gastric damage induced by different agents in rats. The data obtained do not indicate, however, which is the specific mechanism responsible for its antiulcer activity. Flavonoids were found to act on blood vessels and are necessary for the maintenance of normal vascular permeability.[Citation33] Bonina et al.[Citation34] studied a systematic valuation of the in vitro antioxidant activity of ROC. Results obtained in 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) and peroxidation induced acid unilamellar vesicles (LP-LUV) tests demonstrated the strong antioxidant properties of ROC, with a clear relationship between ROC scavenger efficiency and its content in antioxidant compounds. These results suggest that the ROC protects against necrotizing gastric damage as well as ulcer healing properties are in agreement with the in vitro antioxidant effect. Pohle et al.[Citation35] demonstrated that NSAID-induced gastric damage was accompanied by increase in free radical generation. This increase was significantly suppressed by vitamin C, suggesting that the mechanism may involve the ability of ascorbic acid to scanvenger free radical. It is well known that vitamin C is secreted into the gastric lumen of normal subjects and that it significantly declines in patients with peptic ulcer,[Citation36] in patients with H. pylori- associated gastritis,[Citation37] and in subjects with NSAID.[Citation35] This suggests that vitamin C in ROC may be usefull to elevate the level of this vitamin in gastric lumen and this fact can contribute to antioxidant protection of ROC against NSAID-induced gastric damage.
It is well known that the activation of mucus-alkaline secretion and mucosal microcirculation prevent mucosal injury of experimental gastric ulcers. It has been reported that nitric oxides and endogenous prostanoids play a crucial role in mucosal microcirculation[Citation38] and mucus synthesis.[Citation39] Nitric oxide is destroyed by an O2 −-radical-generating system. Therefore ROC may activate the gastric mucosal defensive factors mentionated above, which, in turn, leads to gastric cytoprotective and ulcer-healing actions of the ROC by inhibiting inactivation of nitric oxide by O2 −.
Gastrointestinal disorders are one of the most important causes of morbidity and pharmaceutical products employed for these diseases can produce adverse effects. Thus, natural compounds that possess antiulcer activity can be of particular importance. One of the most important side effects of conventional anti-inflammatory drugs is their ulcerogenic activity. Flavonoids were found to be good anti-inflammatory compounds[Citation40] and were also able to protect the gastric mucosa against a variety of ulcerogenic agents.[Citation41] It could be concluded, in relation to its low toxicity and the properties reported, that the ROC could have a therapeutic potential, ideal for treatment of gastrointestinal diseases. The present findings demonstrated that ROC has a very good tollerability and gives excellent gastric protection, very likely as a results of the antioxidant/radical scavenger activity of its active ingredients.
ACKNOWLEDGMENTS
The authors are thankful to Enrico and Enrica Sovena Fondation for financial support of part of this work. The authors also gratefully acknowledge the contribution of Rossana Di Mario for her care and attention in animal maintenance.
REFERENCES
- Takeuchi , K. , Aihara , E. , Hayashi , M. and Sasaki , Y. 2005 . Role of Prostaglandin E Receptor Subtypes in Gastroduodenal HCO3-secretion . Med. Chem. , 1 ( 4 ) : 395 – 403 .
- Takeuchi , K. , Kato , S. , Ogawa , Y. , Kanatsu , K. and Umeda , M. 2001 . Role of Endogenous Prostacyclin in Gastric Ulcerogenic and Healing Responses—A Study Using IP-Receptor Knockout Mice . J. Physiol. Paris , 95 ( 1–6 ) : 75 – 80 .
- Sachs , G. , Kaunitz , J. , Mendlein , J. and Wallmark , B. 1989 . “ Biochemistry of Gastric Acid Secretion ” . In Handbook of Physiology, Section 6: The Gastrointestinal System, Vol. 3: Salivary, Gastric and Hepatobiliary Secretion , Edited by: Forte , J.G. 229 – 254 . Bethesda : American Physiological Society .
- Brzozowski , T. , Konturek , P.C. , Konturek , S.J. , Sliwowski , Z. , Pajdo , R. , Drozdowicz , D. , Ptak , A. and Hahn , E.G. 2001 . Classic NSAID and Selective Cyclooxygenase (COX)-1 and COX-2 Inhibitors in Healing of Chronic Gastric Ulcers . Microsc. Res. Tech. , 53 ( 5 ) : 343 – 353 .
- Balli , F. , Pancaldi , M.E. and Di Biase , A.R. 1998 . Helicobacter Pylori. Morphology, Biochemistry, Antigenic Profile and Correlated Diseases . Pediatr. Med. Chir. , 20 ( 5 ) : 323 – 328 .
- Jacobsen , R.B. and Phillips , B.B. 2004 . Reducing Clinically Significant Gastrointestinal Toxicity Associated with Nonsteroidal Anti-inflammatory Drugs . Ann. Pharmacother. , 38 ( 9 ) : 1469 – 1481 .
- Rampello , L. and Nicoletti , G. 1990 . The H2-antagonist Therapy Withdrawal Syndrome: The Possible Role of Hyperprolactinemia . Medicina (Firenze) , 10 ( 3 ) : 294 – 296 .
- Rostom , A. , Dube , C. , Wells , G. , Tugwell , P. , Welch , V. , Jolicoeur , E. and McGowan , J. 2000 . Prevention of NSAID-induced Gastroduodenal Ulcers . Cochrane Database Syst. Rev. , 4 : CD002296
- Zimmerman , T.W. 1984 . Problems Associated with Medical Treatment of Peptic Ulcer Disease . Am. J. Med. , 77 ( 5B ) : 51 – 56 .
- Mukherjee , S. 2003 . Diarrhea Associated with Lansoprazole . J. Gastroenterol. Hepatol. , 18 ( 5 ) : 602 – 603 .
- Poole , P. 2001 . Pantoprazole . Am. J. Health Syst. Pharm. , 58 ( 11 ) : 999 – 1008 .
- Ruscin , J.M. , Page , R.L. and Valuck , R.J. 2002 . Vitamin B12Deficiency Associated with Histamine(2)-receptor Antagonists and a Proton-pump Inhibitor . Ann. Pharmacother. , 36 ( 5 ) : 812 – 816 .
- Clark , D.W. and Strandell , J. 2006 . Myopathy Including Polymyositis: A Likely Class Adverse Effect of Proton Pump Inhibitors? . Eur. J. Clin. Pharmacol. , 62 ( 6 ) : 473 – 479 .
- Borrelli , F. and Izzo , A.A. 2000 . The Plant Kingdom as a Source of Anti-ulcer Remedies . Phytother. Res. , 14 ( 8 ) : 581 – 591 .
- Harborne , J.B. and Williams , C.A. 2000 . Advances in Flavonoid Research Since 1992 . Phytochemistry , 55 ( 6 ) : 481 – 504 .
- Lorke , D. 1983 . A New Approach to Practical Acute Toxicity Testing . Arch. Toxicol. , 54 ( 4 ) : 275 – 287 .
- Lu , F.C. and Kacew , S. 2002 . Lu's Basic Toxicology , 4th , 73 – 88 . New York : Taylor & Francis .
- Robert , A. , Nezamis , J.E. , Lancaster , C. and Hanchar , A.J. 1979 . Cytoprotection by Prostaglandins in Rats. Prevention of Gastric Necrosis Produced by Alcohol, HCl, NaOH, Hypertonic NaCl, and Thermal Injury . Gastroenterology , 77 ( 3 ) : 433 – 443 .
- Okabe , S. , Takata , Y. , Takeuchi , K. , Naganuma , T. and Takagi , K. 1976 . Effects of Carbenoxolone Na on Acute and Chronic Gastric Ulcer Models in Experimental Animals . Am. J. Dig. Dis. , 21 ( 8 ) : 618 – 625 .
- Martin , M.J. , Marhuenda , E. , Perez-Guerrero , C. and Franco , J.M. 1994 . Antiulcer Effect of Naringin on Gastric Lesions Induced by Ethanol in Rats . Pharmacology , 49 ( 3 ) : 144 – 150 .
- Lee , Y.H. , Mollison , K.W. and Cheng , W.D. 1971 . The effects of Anti-ulcer Agents on Indomethacin-induced Gastric Ulceration in the Rat . Arch. Int. Pharmacodyn. Ther. , 192 ( 2 ) : 370 – 377 .
- Magistretti , M.J. , Conti , M. and Cristoni , A. 1988 . Antiulcer Activity of an Anthocyanidin From Vaccinium Myrtillus . Arzneimittelforschung , 38 ( 5 ) : 686 – 690 .
- Galati , E.M. , Mondello , M.R. , D'Aquino , A. , Miceli , N. , Sanogo , R. , Tzakou , O. and Manforte , M.T. 2000 . Effects of Teucrium Divaricatum Heldr. ssp. Divaricatum Decoction on Experimental Ulcer in Rats . J. Ethnopharmacol. , 72 ( 1–2 ) : 337 – 342 .
- Terano , A. , Hiraishi , H. , Ota , S. , Shiga , J. and Sugimoto , T. 1989 . Role of Superoxide and Hydroxyl Radicals in Rat Gastric Mucosal Injury Induced by Ethanol . Gastroenterol. Jpn. , 24 ( 5 ) : 488 – 493 .
- Valentik , M. and Visnovsky , P. 1978 . Mechanism of the Ulcerogenic Effects of Phenylbutazone and Indomethacin . Cesk. Gastroenterol. Vyz. , 32 ( 8 ) : 494 – 500 .
- Tarnawski , A. 2000 . “ Cellular Mechanisms of Gastric Ulcer Healing ” . In The Stomach , Edited by: Domschke , W. and Konturek , S.J. 177 – 192 . Berlin : Springer Verlag .
- Arakawa , T. , Higuchi , K. , Fukuda , T. , Fujiwara , Y. , Kobayashi , K. and Kuroki , T. 1998 . Prostaglandins in the Stomach: An Update . J. Clin. Gastroenterol. , 27 ( 1 ) : S1 – 11 .
- Brzozowski , T. , Konturek , S.J. , Sliwowski , Z. , Drozdowicz , D. , Zaczek , M. and Kedra , D. 1997 . Role of L-arginine, a Substrate for Nitric Oxide-synthase, in Gastroprotection and Ulcer Healing . J. Gastroenterol. , 32 ( 4 ) : 442 – 452 .
- Pohle , T. , Shahin , M. , Domschke , W. and Konturek , J.W. 1999 . Effect of Basic Fibroblast Growth Factor on Gastric Ulcer Healing and its Own mRNA Expression . Aliment Pharmacol. Ther. , 13 ( 11 ) : 1543 – 1551 .
- Konturek , P.C. , Konturek , S.J. , Brzozowski , T. and Ernst , H. 1995 . Epidermal Growth Factor and Transforming Growth Factor-alpha: Role in Protection and Healing of Gastric Mucosal Lesions . Eur. J. Gastroenterol. Hepatol. , 7 ( 10 ) : 933 – 937 .
- Devenport , H.W. 1967 . Salycilate Damage to the Gastric Mucosal Barrier . New Engl. J. Med. , 276 : 1307 – 1312 .
- Rainsford , K.D. 1987 . The Effects of 5-lipoxygenase Inhibitors and Leukotriene Antagonists on the Development of Gastric Lesions Induced by Nonsteroidal Antiinflammatory Drugs in Mice . Agents Actions , 21 ( 3–4 ) : 316 – 319 .
- Mochizuki , M. , Kajiya , K. , Terao , J. , Kaji , K. , Kumazawa , S. , Nakayama , T. and Shimoi , K. 2004 . Effect of Quercetin Conjugates on Vascular Permeability and Expression of Adhesion Molecules . Biofactors , 22 ( 1–4 ) : 201 – 204 .
- Bonina , F. , Saija , A. , Tomaino , A. , Lo Cascio , R. , Rapisarda , P. and Dederen , J.C. 1998 . In vitro Antioxidant Activity and in vivo Photoprotective Effect of a Red Orange Extract . Int. J. Cosm. Sci. , 20 : 331 – 342 .
- Pohle , T. , Brzozowski , T. , Becker , J.C. , van der Voort , I.R. , Markmann , A. , Konturek , S.J. , Moniczewski , A. , Domschke , W. and Konturek , J.W. 2001 . Role of Reactive Oxygen Metabolites in Aspirin-induced Gastric Damage in Humans: Gastroprotection by Vitamin C . Aliment Pharmacol. Ther. , 15 : 677 – 687 .
- O'Connor , H.J. , Schorah , C.J. , Habibzedah , N. , Axon , A.T.R. and Cocel , R. 1989 . Vitamin C Concentration in the Human Stomach: Relation to Gastric pH, Gastroduodenal Disease, and Possible Sources . Gut , 30 : 436 – 442 .
- Ruiz , B. , Rood , J.C. and Fontham , E.T.H. 1994 . Vitamin C Concentration in Gastric Juice Before and After Anti-Helicobacter pyloriTreatment . Am. J. Gastroenterol. , 89 : 533 – 539 .
- Tepperman , B.L. and Whittle , B.J.L. 1992 . Endogenous Nitric Oxide and Sensory Neuropeptides Interact in the Modulation of the Rat Gastric Microcirculation . Br. J. Pharmacol. , 105 : 171 – 175 .
- Takao , Y. , Shimamoto , C. , Hazama , K. , Umegaki , E. , Yoshizumi , M. , Tahashi , Y. , Sasaki , S. , Hirata , I. and Katsu , K. 1997 . The Effects of Nitric Oxide on Mucin Synthesis in the Isolated Human Colonic Cells Culture System . Ulcer Res. , 24 : 230 – 232 .
- Ferrandiz , M.L. and Alcaraz , M.J. 1991 . Anti-inflammatory Activity and Inhibition of Arachidonic Acid Metabolism by Flavonoids . Agent Actions , 32 ( 3–4 ) : 283 – 288 .
- Parmar , N.S. and Ghosh , M.N. 1981 . In Flavonoids and Bioflavonoids, 6thHungarian Bioflavonoids Symposium , Edited by: Farkas , L. , Gabor , N. , Kallai , F. and Wagner , H. 513 – 516 . Amsterdam : Elsevier .